Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Establishing the Y. lipolytica as a Phospholipid-Producing Platform Relative to Different Carbon Sources
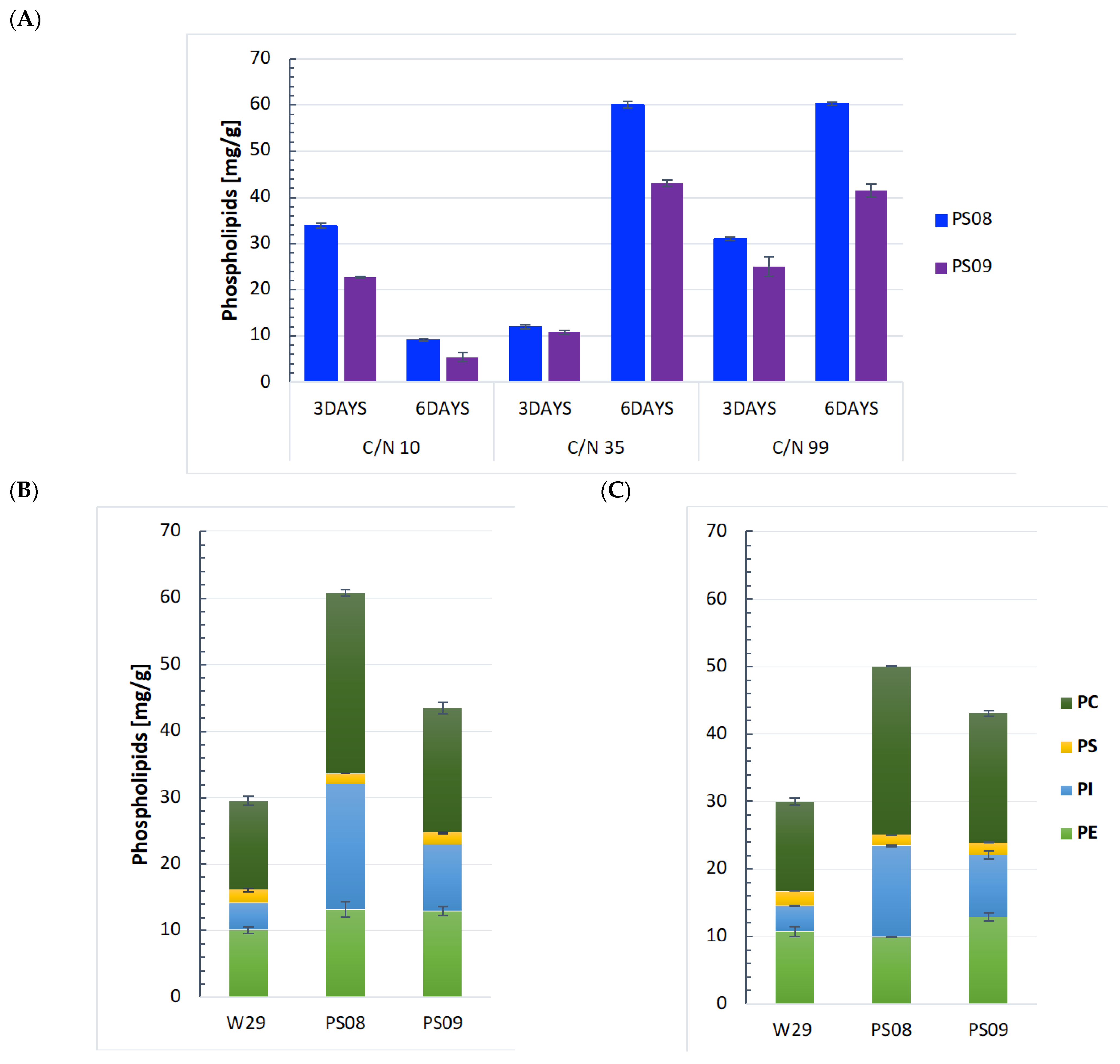
2.2. Enhancing Phospholipid Production by Overexpression of DGK1 and GUT1 Genes
2.3. Optimization of Flask Culture Conditions
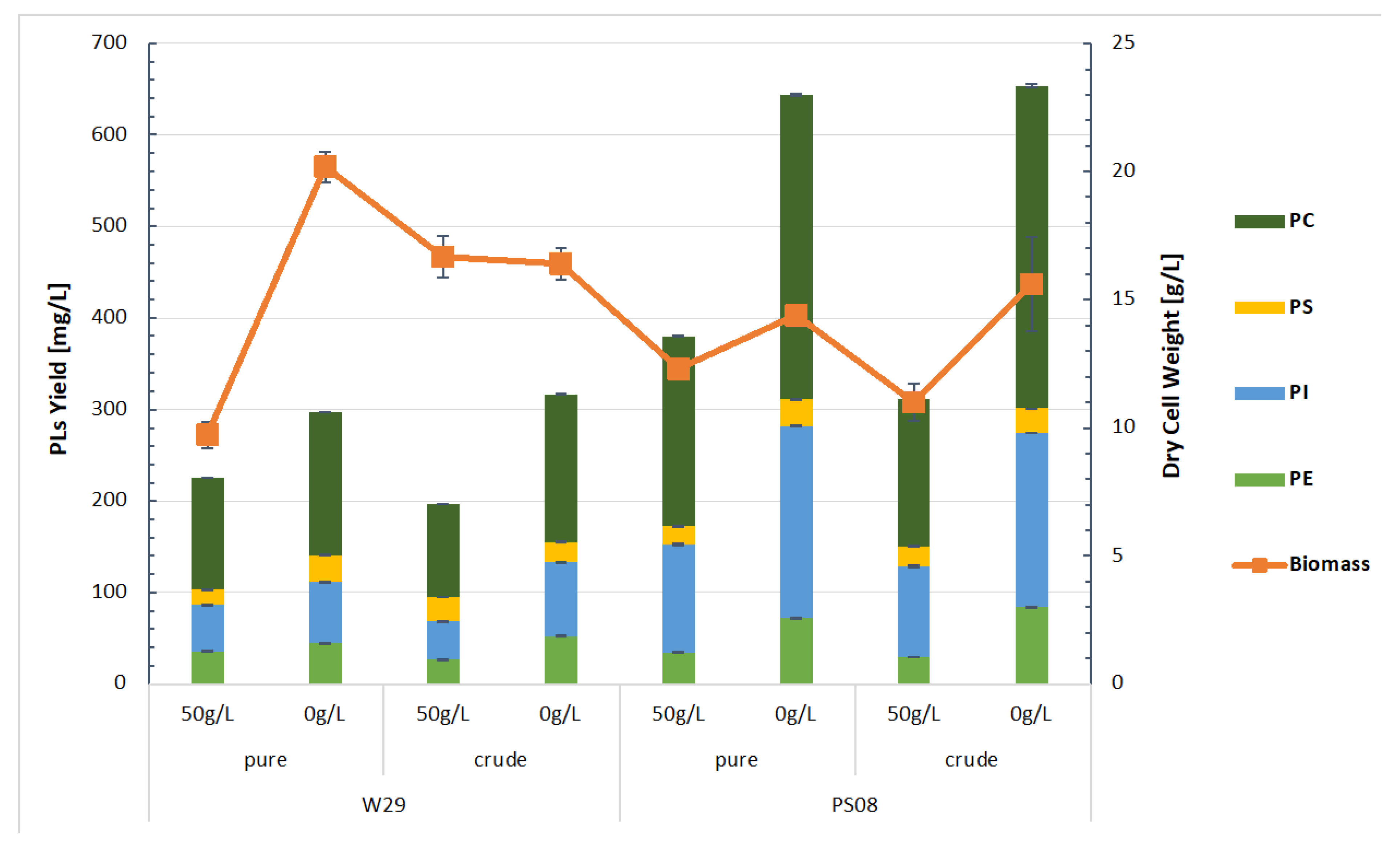
2.4. Production of Phospholipids on Bioreactor Scale
3. Materials and Methods
3.1. Strains, Media, and Culture Conditions
3.2. Plasmid Preparation
3.3. Cloning and Transformation Protocols
3.4. Flask Cultivation
3.5. Bioreactor Cultures
3.6. Lipids Extraction
3.7. Quantification of Phospholipids
3.8. Quantification of Fatty Acids
3.9. Thin-Layer Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Parchem, K.; Bartoszek, A. Fosfolipidy oraz produkty ich hydrolizy jako żywieniowe czynniki prewencyjne w chorobach cywilizacyjnych. Adv. Hyg. Exp. Med. 2016, 70, 1343–1361. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Gong, R.; Xue, X.; Guan, X.; Song, L.; Guo, X.; Xiao, D. Improved ethyl caproate production of Chinese liquor yeast by overexpressing fatty acid synthesis genes with OPI1 deletion. J. Ind. Microbiol. Biotechnol. 2016, 43, 1261–1270. [Google Scholar]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.S. Phospholipids from marine source: Extractions and forthcoming industrial applications. J. Funct. Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, T.; Campbell, K.; Nielsen, J.; Chen, Y. Modular pathway rewiring of yeast for amino acid production. Methods Enzymol. 2018, 608, 417–439. [Google Scholar]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef]
- Wang, J.; Ledesma-Amaro, R.; Wei, Y.; Ji, B.; Ji, X.J. Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica–a review. Bioresour. Technol. 2020, 313, 123707. [Google Scholar]
- Yook, S.D.; Kim, J.; Gong, G.; Ko, J.K.; Um, Y.; Han, S.O.; Lee, S.M. High-yield lipid production from lignocellulosic biomass using engineered xylose-utilizing Yarrowia lipolytica. GCB Bioenergy 2020, 12, 670–679. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar]
- Hardman, D.; McFalls, D.; Fakas, S. Characterization of phosphatidic acid phosphatase activity in the oleaginous yeast Yarrowia lipolytica and its role in lipid biosynthesis. Yeast 2017, 34, 83–91. [Google Scholar] [CrossRef]
- Loewen, C.J.R.; Gaspar, M.L.; Jesch, S.A.; Delon, C.; Ktistakis, N.T.; Henry, S.A.; Levine, T.P. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science 2004, 304, 1644–1647. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Shen, Y.; Hou, J.; Bao, X. Increasing malonyl-CoA derived product through controlling the transcription regulators of phospholipid synthesis in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 905–912. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Athenstaedt, K. YALI0E32769g (DGA1) and YALI0E16797g (LRO1) encode major triacylglycerol synthases of the oleaginous yeast Yarrowia lipolytica. Biochim. Biophys. Acta 2011, 1811, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Fakas, S. Lipid biosynthesis in yeasts: A comparison of the lipid biosynthetic pathway between the model nonoleaginous yeast Saccharomyces cerevisiae and the model oleaginous yeast Yarrowia lipolytica. Eng. Life Sci. 2017, 17, 292–302. [Google Scholar] [CrossRef]
- Fakas, S.; Konstantinou, C.; Carman, G.M. DGK1-encoded diacylglycerol kinase activity is required for phospholipid synthesis during growth resumption from stationary phase in Saccharomyces cerevisiae. J. Biol. Chem. 2011, 286, 1464–1474. [Google Scholar] [CrossRef]
- Nicaud, J.M.; Madzak, C.; van den Broek, P.; Gysler, C.; Duboc, P.; Niederberger, P.; Gaillardin, C. Protein expression and secretion in the yeast Yarrowia lipolytica. FEMS Yeast Res. 2002, 2, 371–379. [Google Scholar]
- Liu, Y.; Liu, Q.; Krivoruchko, A.; Khoomrung, S.; Nielsen, J. Engineering yeast phospholipid metabolism for de novo oleoylethanolamide production. Nat. Chem. Biol. 2020, 16, 197–205. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Gajdos, P.; Nicaud, J.M.; Certik, M. Glycerol conversion into a single cell oil by engineered Yarrowia lipolytica. Eng. Life Sci. 2017, 17, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Mirończuk, A.M. The influence of transketolase on lipid biosynthesis in the yeast Yarrowia lipolytica. Microb. Cell Fact. 2020, 19, 138. [Google Scholar] [CrossRef]
- Park, J.O.; Liu, N.; Holinski, K.M.; Emerson, D.F.; Qiao, K.; Woolston, B.M.; Stephanopoulos, G. Synergistic substrate cofeeding stimulates reductive metabolism. Nat. Metab. 2019, 1, 643–651. [Google Scholar] [CrossRef]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Crutz-Le Coq, A.M.; Nicaud, J.M. Hexokinase—A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab. Eng. 2014, 26, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Le Dall, M.T.; Gaillardin, C.; Thonart, P.; Nicaud, J.M. New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipolytica. J. Microbiol. Methods 2003, 55, 727–737. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. [Google Scholar]
- Knittelfelder, O.L.; Kohlwein, S.D. Lipid extraction from yeast cells. Cold Spring Harb. Protoc. 2017, 2017, pdb-prot085449. [Google Scholar] [CrossRef]
- Browse, J.; McCourt, P.J.; Somerville, C.R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal. Biochem. 1986, 152, 141–145. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Kohlwein, S.D. Thin-layer chromatography to separate phospholipids and neutral lipids from yeast. Cold Spring Harb. Protoc. 2017, 2017, pdb-prot085456. [Google Scholar] [CrossRef]
- Papanikolaou, S.E.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef]
- Dulermo, R.; Brunel, F.; Dulermo, T.; Ledesma-Amaro, R.; Vion, J.; Trassaert, M.; Leplat, C. Using a vector pool containing variable-strength promoters to optimize protein production in Yarrowia lipolytica. Microb. Cell Factories 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepańska, P.; Rychlicka, M.; Moroz, P.; Janek, T.; Gliszczyńska, A.; Lazar, Z. Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol. Int. J. Mol. Sci. 2022, 23, 10737. https://doi.org/10.3390/ijms231810737
Szczepańska P, Rychlicka M, Moroz P, Janek T, Gliszczyńska A, Lazar Z. Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol. International Journal of Molecular Sciences. 2022; 23(18):10737. https://doi.org/10.3390/ijms231810737
Chicago/Turabian StyleSzczepańska, Patrycja, Magdalena Rychlicka, Paweł Moroz, Tomasz Janek, Anna Gliszczyńska, and Zbigniew Lazar. 2022. "Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol" International Journal of Molecular Sciences 23, no. 18: 10737. https://doi.org/10.3390/ijms231810737
APA StyleSzczepańska, P., Rychlicka, M., Moroz, P., Janek, T., Gliszczyńska, A., & Lazar, Z. (2022). Elevating Phospholipids Production Yarrowia lipolytica from Crude Glycerol. International Journal of Molecular Sciences, 23(18), 10737. https://doi.org/10.3390/ijms231810737







