Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis
Abstract
:1. Introduction
2. Results
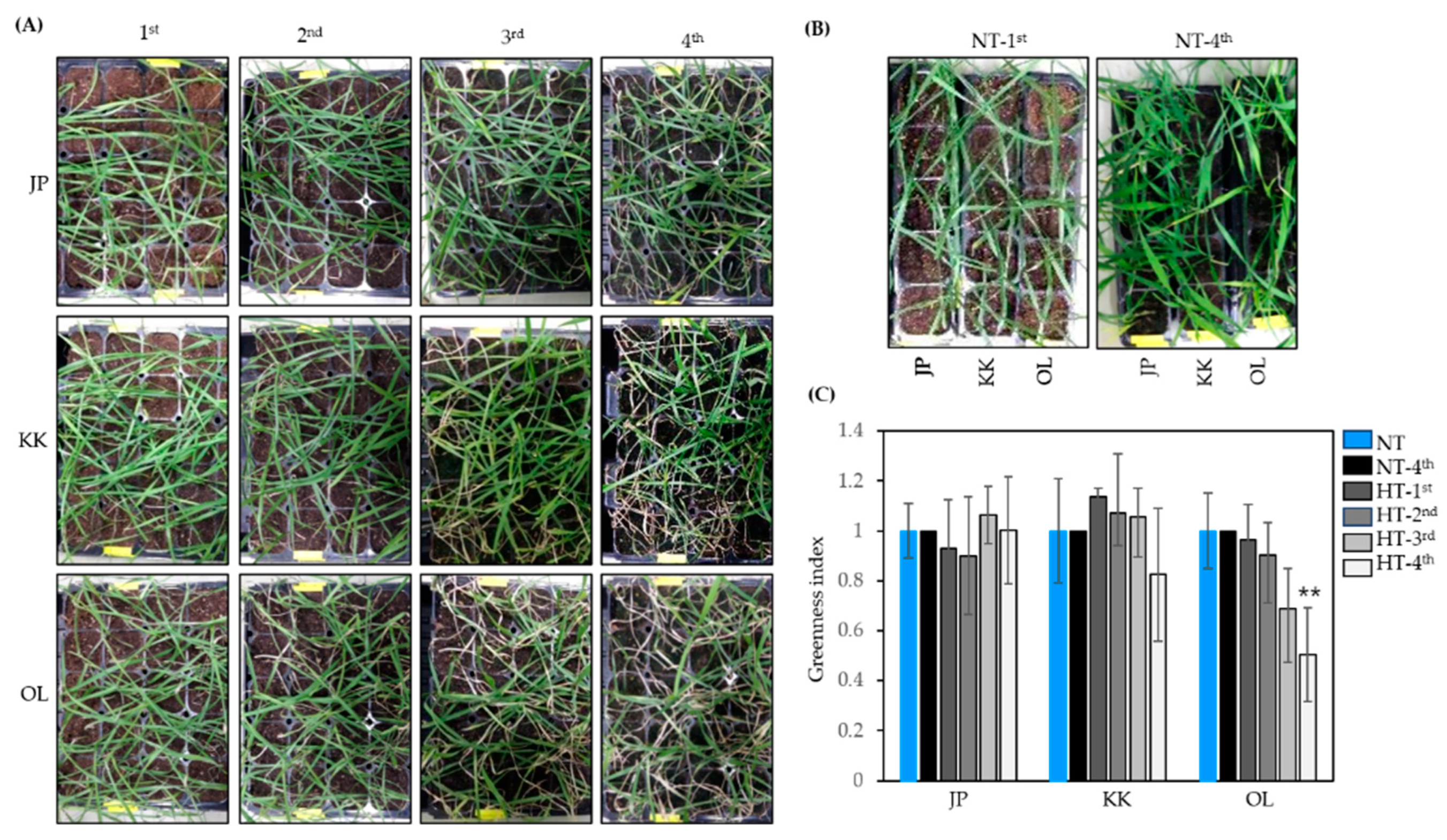
2.1. Evaluation of the Heat-Stress Response in the Wheat Varieties Tested
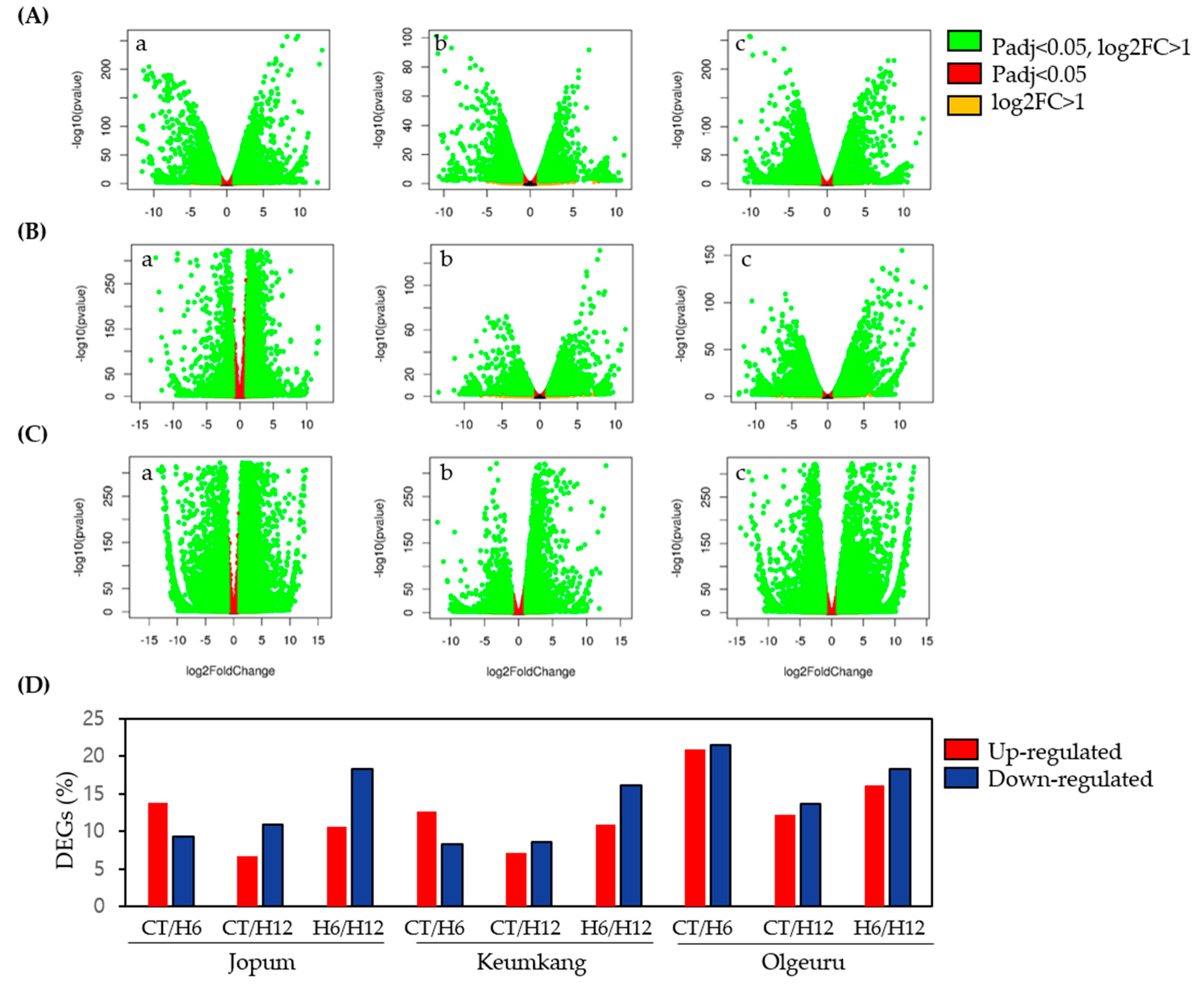
2.2. Differential Expression and Cluster Analyses of Inducible Genes by RNA-Seq
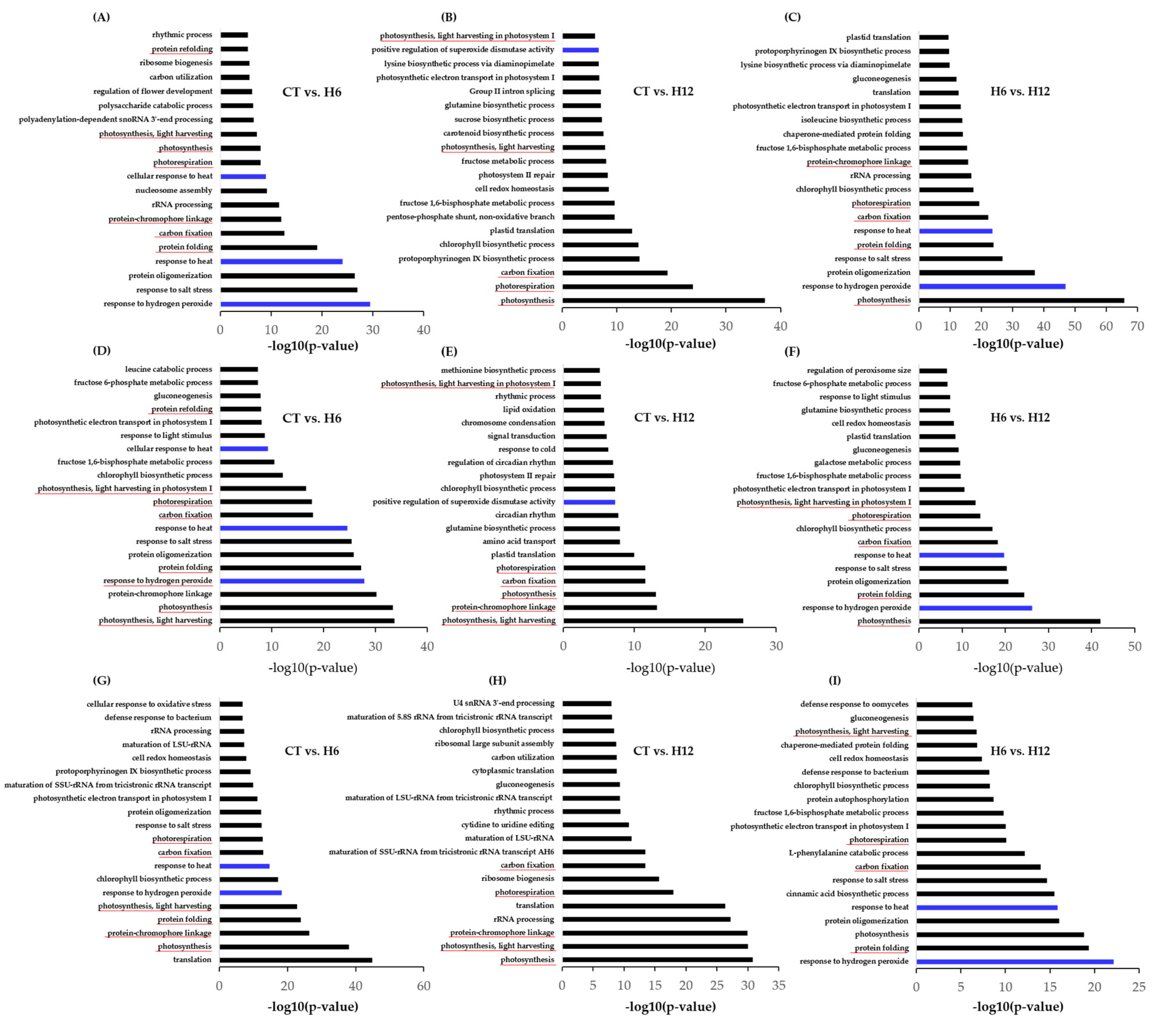
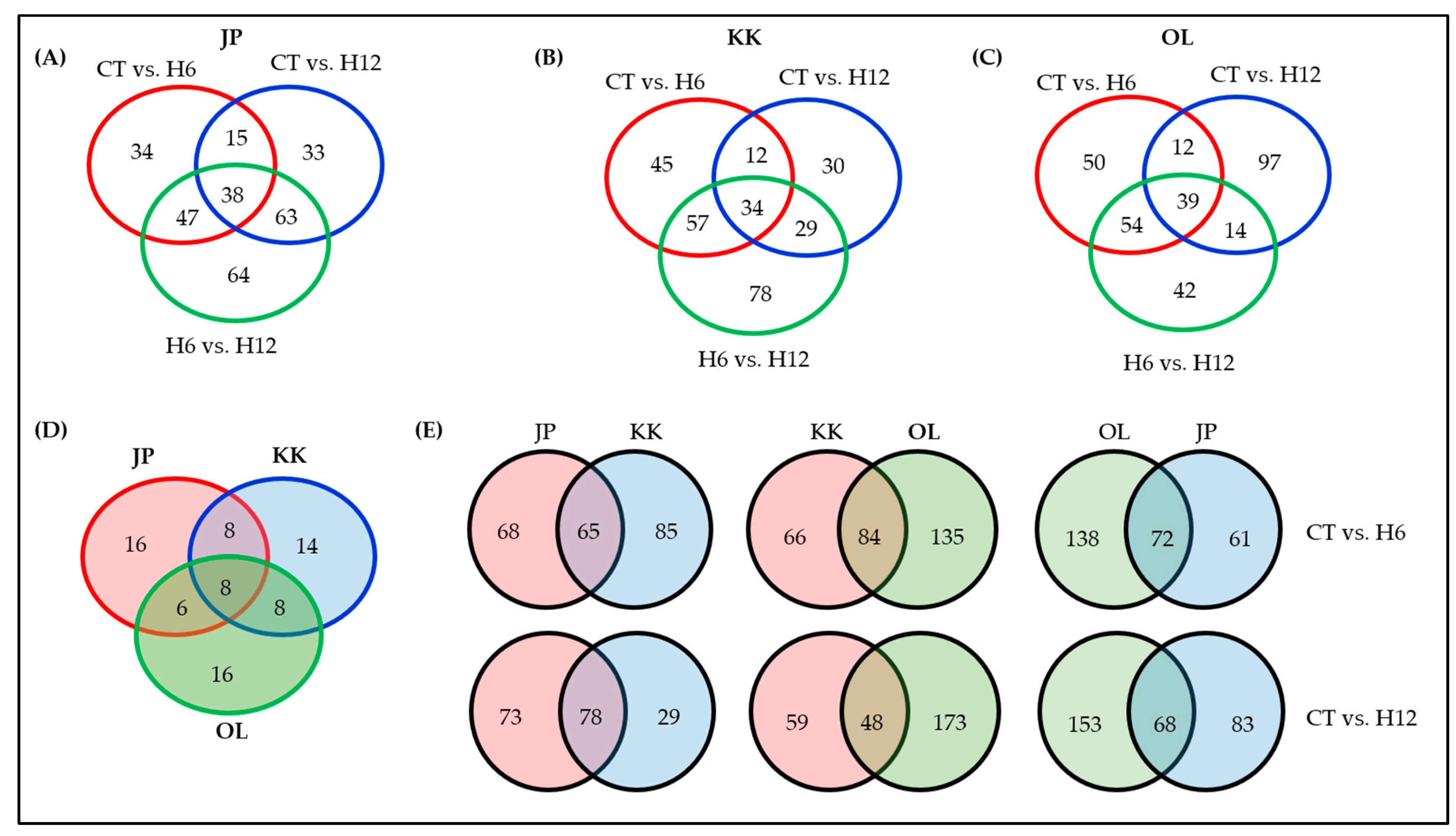
2.3. Functional Annotation of the DEGs and Shared and Specific Biological Processes (BPs)
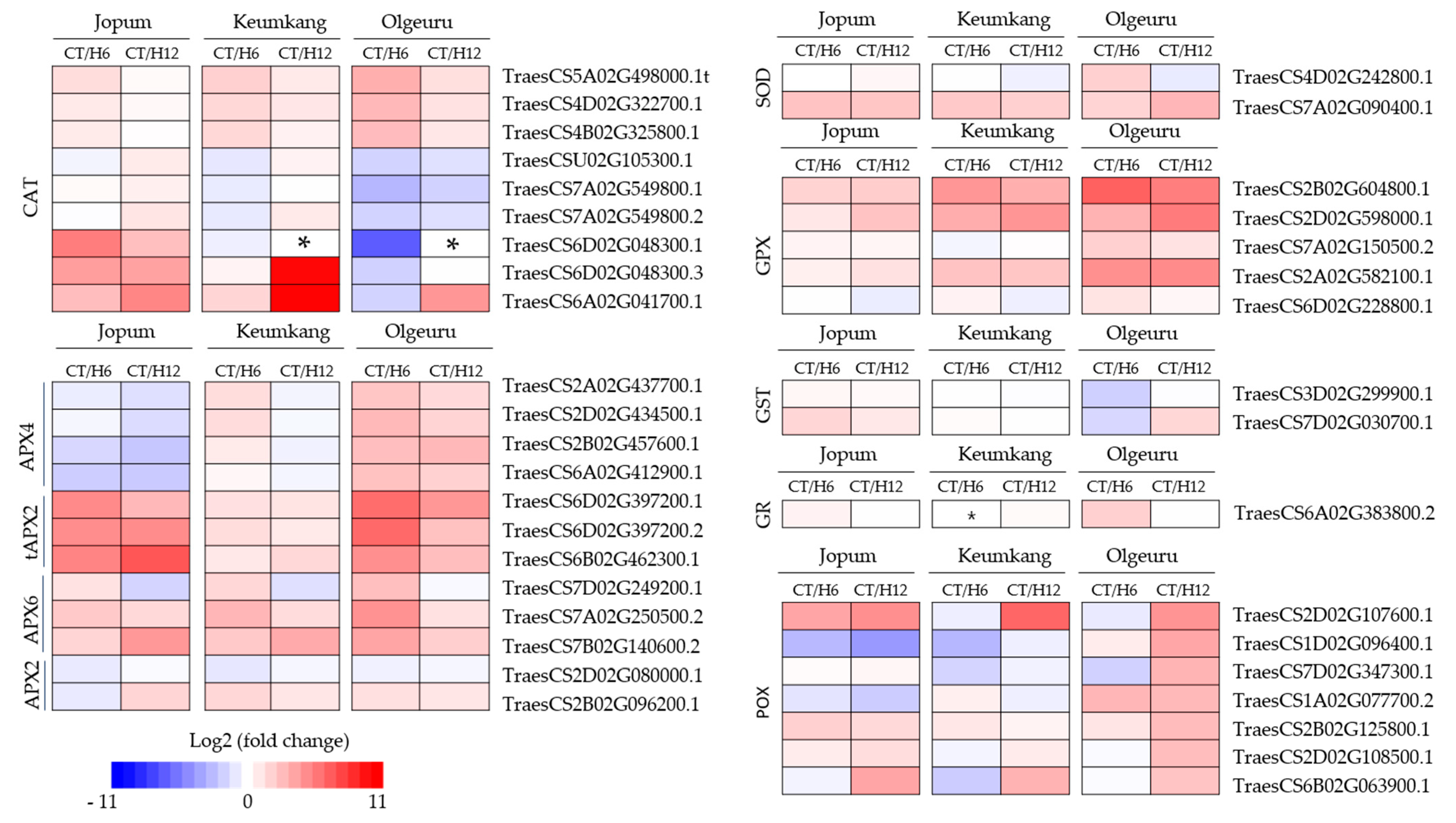
2.4. Expression of Antioxidant Enzyme-Related Genes and ROS Detoxification
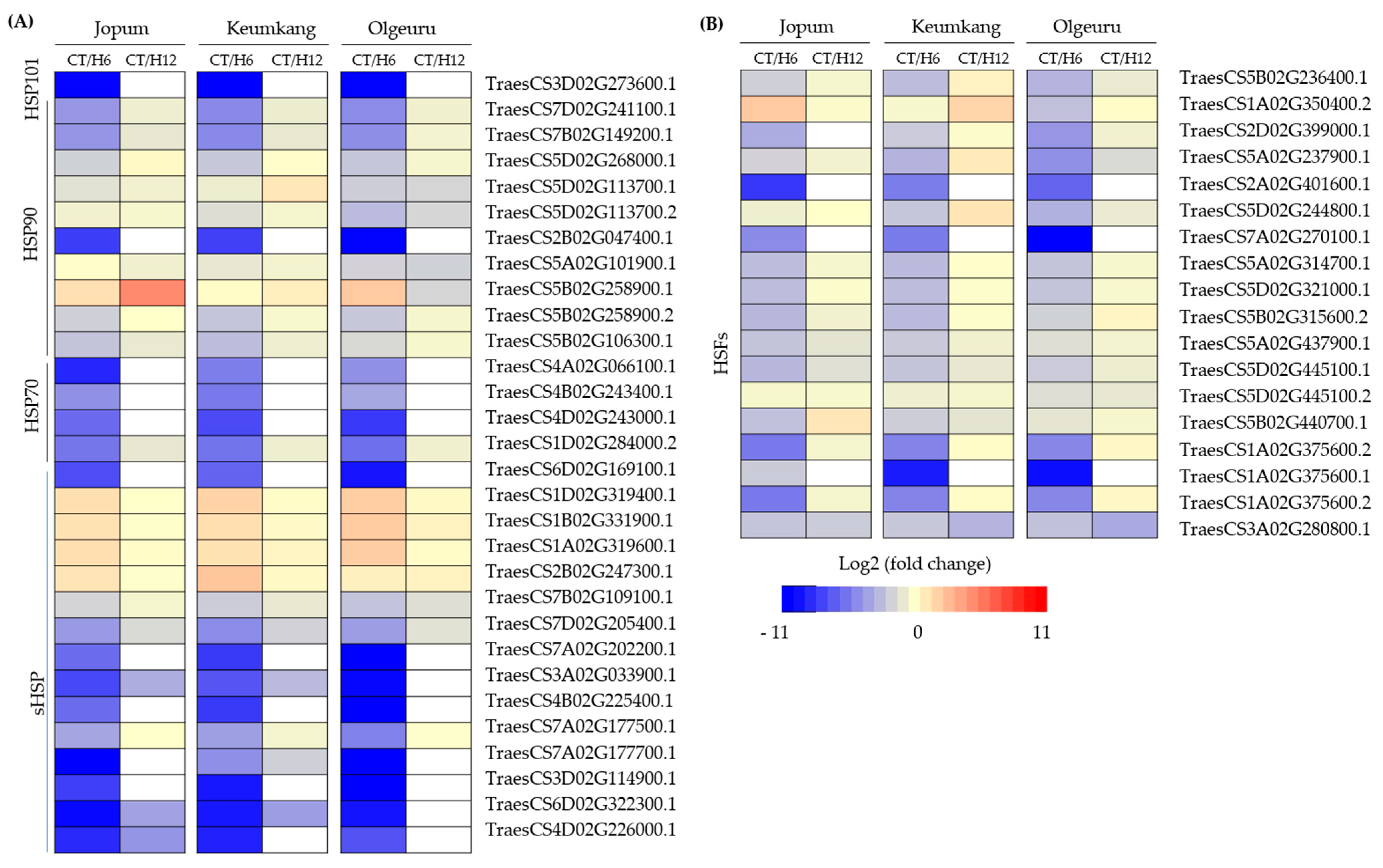
2.5. Dynamic Expression of Transcription Factors and Heat Shock Proteins
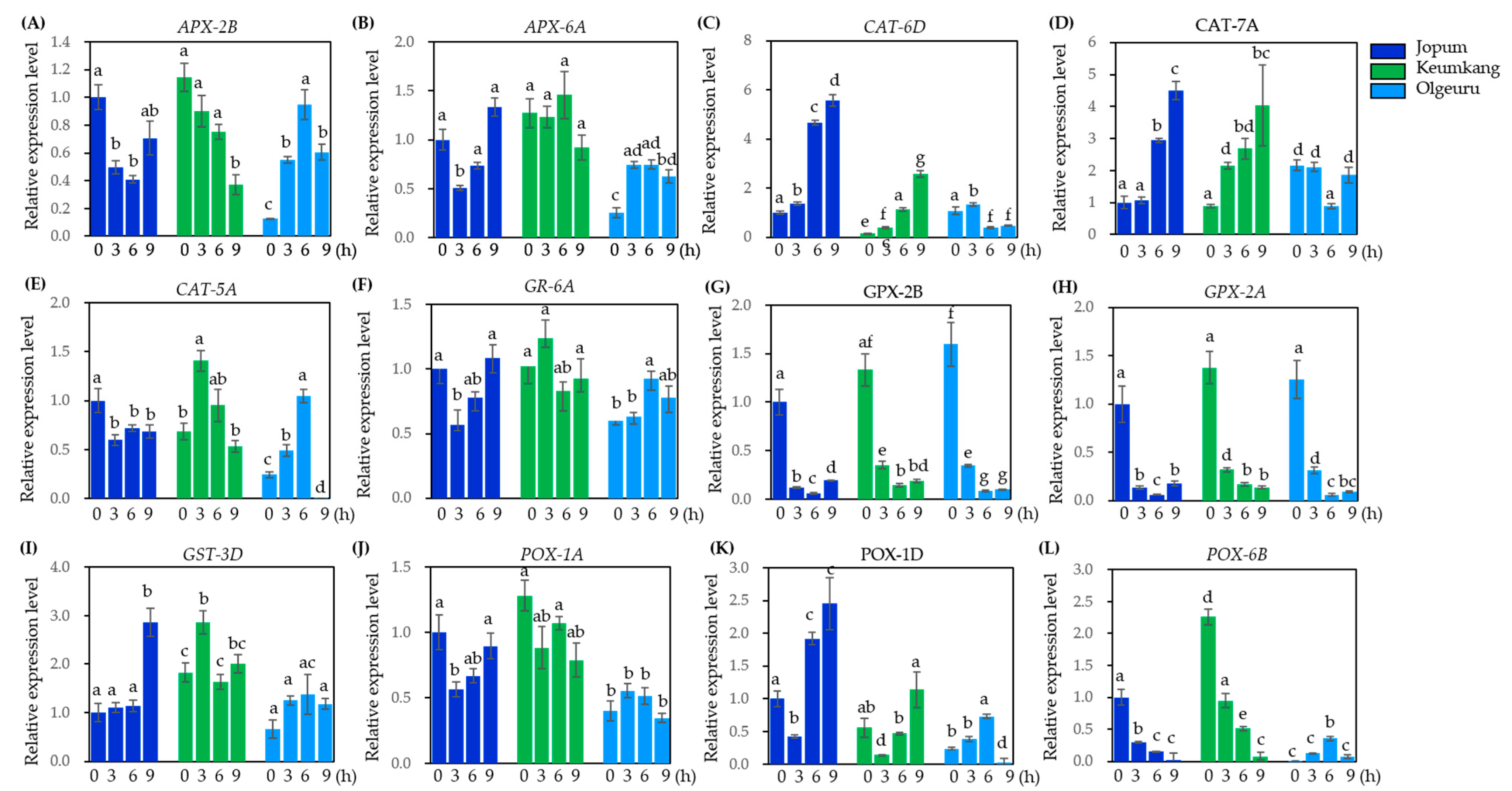
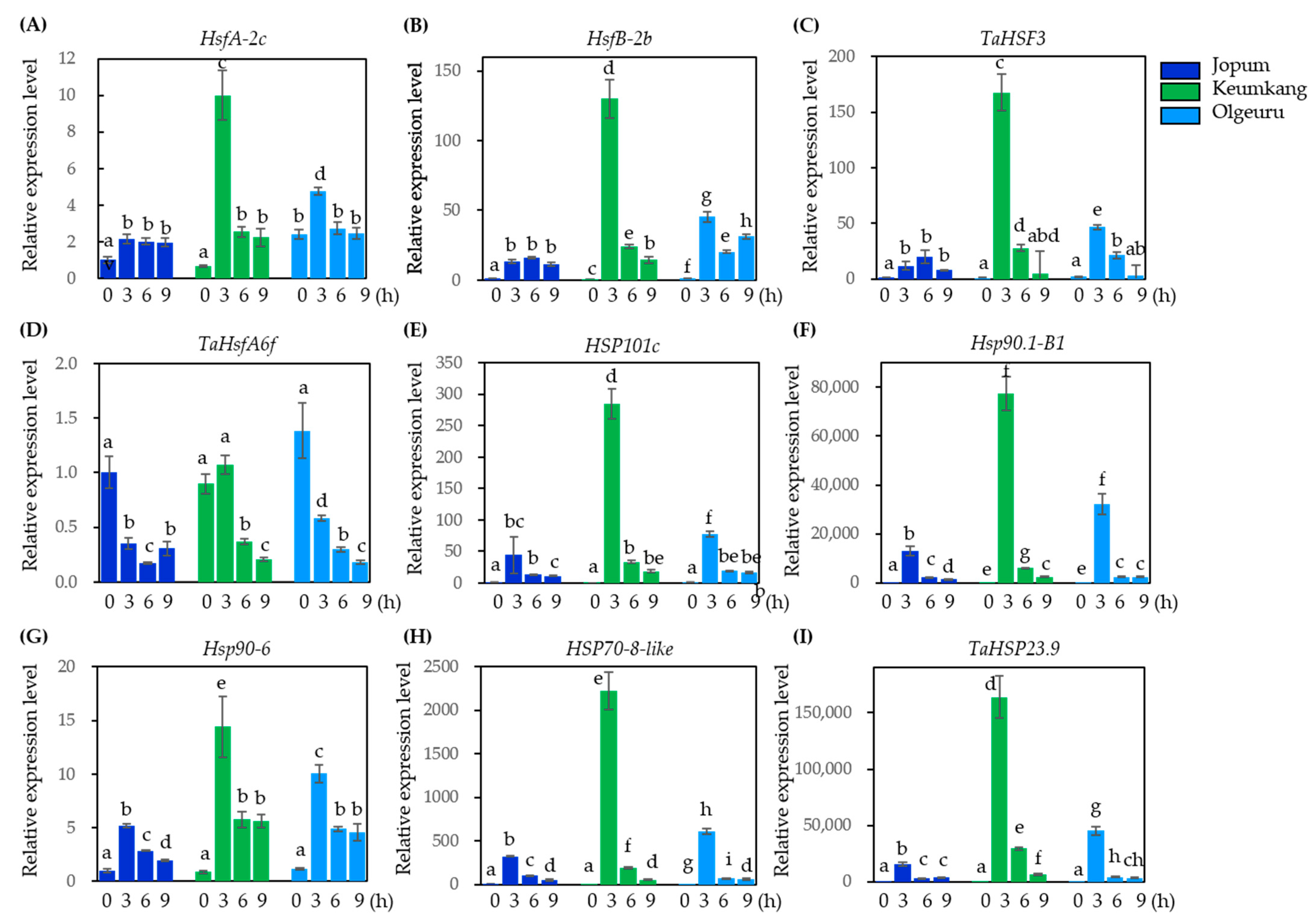
2.6. Quantitative Real-Time PCR Analysis to Validate Gene Expression Levels Found by RNA-Seq Analysis
3. Discussion
4. Materials and Methods
4.1. Evaluation of Heat Stress in Three Wheat Varieties
4.2. RNA Preparation and Quality Control for RNA-Seq Transcriptional Profiling
4.3. cDNA Library Preparation and Illumina Sequencing
4.4. Differentially-Expressed Gene (DEG) Identification
4.5. Cluster Analysis, GO Enrichment Analysis of DEGs, and Heatmap
4.6. Validation of Gene Expression Profile by qRT-PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asseng, S.; Foster, I.A.N.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rotter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Shew, A.M.; Tack, J.B.; Nalley, L.L.; Chaminuka, P. Yield reduction under climate warming varies among wheat cultivars in South Africa. Nat. Commun. 2020, 11, 4408. [Google Scholar] [CrossRef]
- Poudel, P.B.; Poudel, M.R. Heat stress effects and tolerance in wheat: A review. J. Biol. Today’s World 2020, 9, 1–6. [Google Scholar]
- Balla, K.; Karsai, I.; Bencze, S.; Veisz, O. Germination ability and seedling vigour in the progeny of heat-stressed wheat plants. Acta Agron. Hung. 2012, 60, 299–308. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 6, 225–232. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Savicka, M.; Skute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Vensel, W.H.; Tanakam, C.K.; Whitehand, L.; Altenbach, S.B. Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J. Cereal Sci. 2009, 49, 12–23. [Google Scholar] [CrossRef]
- Pradhan, G.P.; Prasad, P.V. Evaluation of wheat chromosome translocation lines for high temperature stress tolerance at grain filling stage. PLoS ONE 2015, 10, e0116620. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, R.; Tiwari, R.; Kumar, R.; Gupta, V. Wheat Responses and Tolerance to Terminal Heat Stress: A Review. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 149–173. [Google Scholar]
- Nahar, K.; Ahamed, K.U.; Fujita, M. Phenological variation and its relation with yield in several wheat (Triticum aestivum L.) cultivars under normal and late sowing mediated heat stress condition. Not. Sci. Biol. 2010, 2, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Sareen, S.; Saini, M. Assessing genetic variation for heat stress tolerance in Indian bread wheat genotypes using morphophysiological traits and molecular markers. Plant Genet. Resour. 2017, 15, 539–547. [Google Scholar] [CrossRef]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defence. J. Biol. Sci. 2010, 6, 565–572. [Google Scholar] [CrossRef]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef]
- Kaur, V.; Behl, R. Grain yield in wheat as affected by short periods of high temperature, drought and their interaction during pre- and postanthesis stages. Cereal Res. Commun. 2010, 38, 514–520. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Cheema, S.A.; Wahid, A. Differential response of wheat cultivars to terminal heat stress. Int. J. Agric. Biol. 2013, 15, 1354–1358. [Google Scholar]
- Huang, B.; Rachmilevitch, S.; Xu, J. Root carbon and protein metabolism associated with heat tolerance. J. Exp. Bot. 2012, 63, 3455–3465. [Google Scholar] [CrossRef]
- Din, R.; Subhani, G.M.; Ahmad, N.; Hussain, M.; Rehman, A.U. Effect of temperature on development and grain formation in spring wheat. Pak. J. Bot. 2010, 42, 899–906. [Google Scholar]
- Biamonti, G.; Caceres, J.F. Cellular stress and RNA splicing. Trends Biochem. Sci. 2009, 34, 146–153. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Sharma, D.K.; Ottosen, C.O. Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ. Exp. Bot. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Mishra, S.; Jha, A.B.; Dubey, R.S. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 2011, 248, 565–577. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Pandey, G.C.; Mehta, G.; Sharma, P.; Sharma, V. Terminal heat tolerance in wheat: An overview. J. Cereal Res. 2019, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.R.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to photosystem II due to heat stress without light-driven electron flow: Involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 2012, 236, 753–761. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Xin, M.; Peng, H.; Ni, Z.; Yao, Y.; Hu, Z.; Sun, Q. Wheat responses and tolerance to high temperature. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 139–147. [Google Scholar]
- Ibrahim, M.M.; Alsahli, A.A.; Al-Ghamdi, A.A. Cumulative abiotic stresses and their effect on the antioxidant defense system in two species of wheat, Triticum durum desf. and Triticum aestivum L. Arch. Biol. Sci. 2013, 65, 1423–1433. [Google Scholar] [CrossRef]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of heat stress tolerance in winter wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Fritz, A.K.; Boyle, D.L.; Gill, B.S. Impact of high night-time and high daytime temperature stress on winter wheat. J. Agron. Crop. Sci. 2015, 201, 206–218. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant. Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Chunduri, V.; Kaur, A.; Kaur, S.; Malhotra, N.; Kummar, A.; Kapoor, P.; Kumari, A.; Kaur, J.; et al. Genome-wide identification and characterization of Heat Shock Protein Family reveals role in development and stress conditions in Triticum aestivum L. Sci. Rep. 2020, 10, 7858. [Google Scholar] [CrossRef]
- Sung, D.Y.; Vierling, E.; Guy, C.L. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001, 126, 789–800. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Sharma, S.K.; Kala, Y.K.; Rai, G.K.; Mishra, D.C.; Grover, M.; Singh, G.P.; Pathak, H.; Rai, A.; et al. Harnessing next generation sequencing in climate change: RNA-Seq analysis of heat stress-responsive genes in wheat (Triticum aestivum L.). Omics A J. Integr. Biol. 2015, 19, 632–647. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Liu, H.; Wu, Y.; Yan, G. Wheat genotypes tolerant to heat at seedling stage tend to be also tolerant at adult stage: The possibility of early selection for heat tolerance breeding. Crop J. 2022, 10, 1006–1013. [Google Scholar] [CrossRef]
- Ko, C.S.; Oh, M.K.; Hyun, J.N.; Kim, K.H.; Kim, J.B.; Hong, M.J.; Seo, Y.W. Effect of high temperature on early stage of grain filling period in wheat (Triticum aestivum L.). Korean J. Breed. Sci. 2017, 49, 200–212. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- REVIGO. Available online: http://revigo.irb,hr/ (accessed on 3 September 2022).
- Zhang, S.; Xu, Z.S.; Li, P.; Yang, L.; Wei, Y.; Chen, M.; Li, L.; Zhang, G.; Ma, Y. Overexpression of TaHSF3 in transgenic Arabidopsis enhances tolerance to extreme temperatures. Plant. Mol. Biol. Rep. 2013, 31, 688–697. [Google Scholar] [CrossRef]
- Xue, G.P.; Drenth, J.; McIntyre, C.L. TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J. Exp. Bot. 2015, 66, 1025–1039. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Dong, J.; Tian, X.; Wang, J.; Palta, J.A.; Xu, S.; Fang, Y.; Wang, Z. Over-Expression of the Heat-Responsive Wheat Gene TaHSP23.9 in Transgenic Arabidopsis Conferred tolerance to heat and salt stress. Front. Plant Sci. 2020, 11, 243. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Gard, N.K.; Maheshwari, C.; et al. Impacts, Tolerance, Adaptation, and Mitigation of Heat Stress on Wheat under Changing Climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef] [PubMed]
- Padaria, J.C.; Biswas, K.; Bhatt, D.; Raipuria, R.; Prabu, R.; Vishwakarma, H.; Singh, G.P. Transcriptional profiling of heat stress responsive genes in different developmental stages of bread wheat (Triticum aestivum L.). Indian J. Biotechnol. 2016, 15, 467–476. [Google Scholar]
- Seni, S.; Kaur, S.; Malik, P.; Yadav, I.S.; Sirohi, P.; Chauhan, H.; Kaur, A.; Chhuneja, P. Transcriptome based identification and validation of heat stress transcription factors in wheat progenitor species Aegilops speltoides. Sci. Rep. 2021, 11, 22049. [Google Scholar] [CrossRef]
- Tuteja, N.; Tiburcio, A.F.; Gill, S.S.; Tuteja, R. Improving Crop Resistance to Abiotic Stress, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Schleiff, E.; Becker, T. Common ground for protein translocation: Access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 2011, 12, 48–59. [Google Scholar] [CrossRef]
- Xue, G.P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef]
- Chauhan, H.; Khurana, N.; Agarwal, P.; Khurana, J.P.; Khurana, P. A seed preferential heat shock transcription factor from wheat provides abiotic stress tolerance and yield enhancement in transgenic Arabidopsis under heat stress environment. PLoS ONE 2013, 8, e79577. [Google Scholar]
- Poonia, A.K.; Mishra, S.K.; Sirohi, P.; Chaudhary, R.; Kanwar, M.; Germain, H.; Chauhan, H. Overexpression of wheat transcription factor (TaHsfA6b) provides thermotolerance in barley. Planta 2020, 252, 53. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246, 153135. [Google Scholar] [CrossRef]
- Bi, H.; Miao, J.; He, J.; Chen, Q.; Qian, J.; Li, H.; Xu, Y.; Ma, D.; Zhao, Y.; Tian, X.; et al. Characterization of the Wheat Heat Shock Factor TaHsfA2e-5D Conferring Heat and Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2784. [Google Scholar] [CrossRef] [PubMed]
- Korea Seed & Variety Service. Available online: http://seed.go.kr (accessed on 5 May 2022).
- Cha, J.; Lee, J.; Lee, S.; Ko, J.; Shin, D. Heading data and growth character of Korean wheat cultivars by controlling photoperiod for rapid generation advancement. Korean J. Breed. Sci. 2020, 52, 20–24. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto Interact. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Sang, W.G.; Kim, J.H.; Baek, J.K.; Kwon, D.; Ban, H.Y.; Cho, K.I.; Seo, M.C. Detection of drought stress in soybean plants using RGB-based vegetation indices. Korean J. Agric. For. Meteorol. 2021, 23, 340–348. [Google Scholar]
- Alsamadany, H. Diversity and Genetic Studies of Heat Tolerance in Wheat. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2015. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Ensembl Plants. Available online: http://plants.ensembl.org/index.html (accessed on 5 May 2022).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- DAVID. Available online: http://david.ncifcrf.gov (accessed on 3 September 2022).
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Kim, K.-M.; Sang, W.-G.; Kang, C.-S.; Choi, C. Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis. Int. J. Mol. Sci. 2022, 23, 10734. https://doi.org/10.3390/ijms231810734
Lee MH, Kim K-M, Sang W-G, Kang C-S, Choi C. Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis. International Journal of Molecular Sciences. 2022; 23(18):10734. https://doi.org/10.3390/ijms231810734
Chicago/Turabian StyleLee, Myoung Hui, Kyeong-Min Kim, Wan-Gyu Sang, Chon-Sik Kang, and Changhyun Choi. 2022. "Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis" International Journal of Molecular Sciences 23, no. 18: 10734. https://doi.org/10.3390/ijms231810734
APA StyleLee, M. H., Kim, K.-M., Sang, W.-G., Kang, C.-S., & Choi, C. (2022). Comparison of Gene Expression Changes in Three Wheat Varieties with Different Susceptibilities to Heat Stress Using RNA-Seq Analysis. International Journal of Molecular Sciences, 23(18), 10734. https://doi.org/10.3390/ijms231810734





