Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation
Abstract
1. Introduction
2. Results
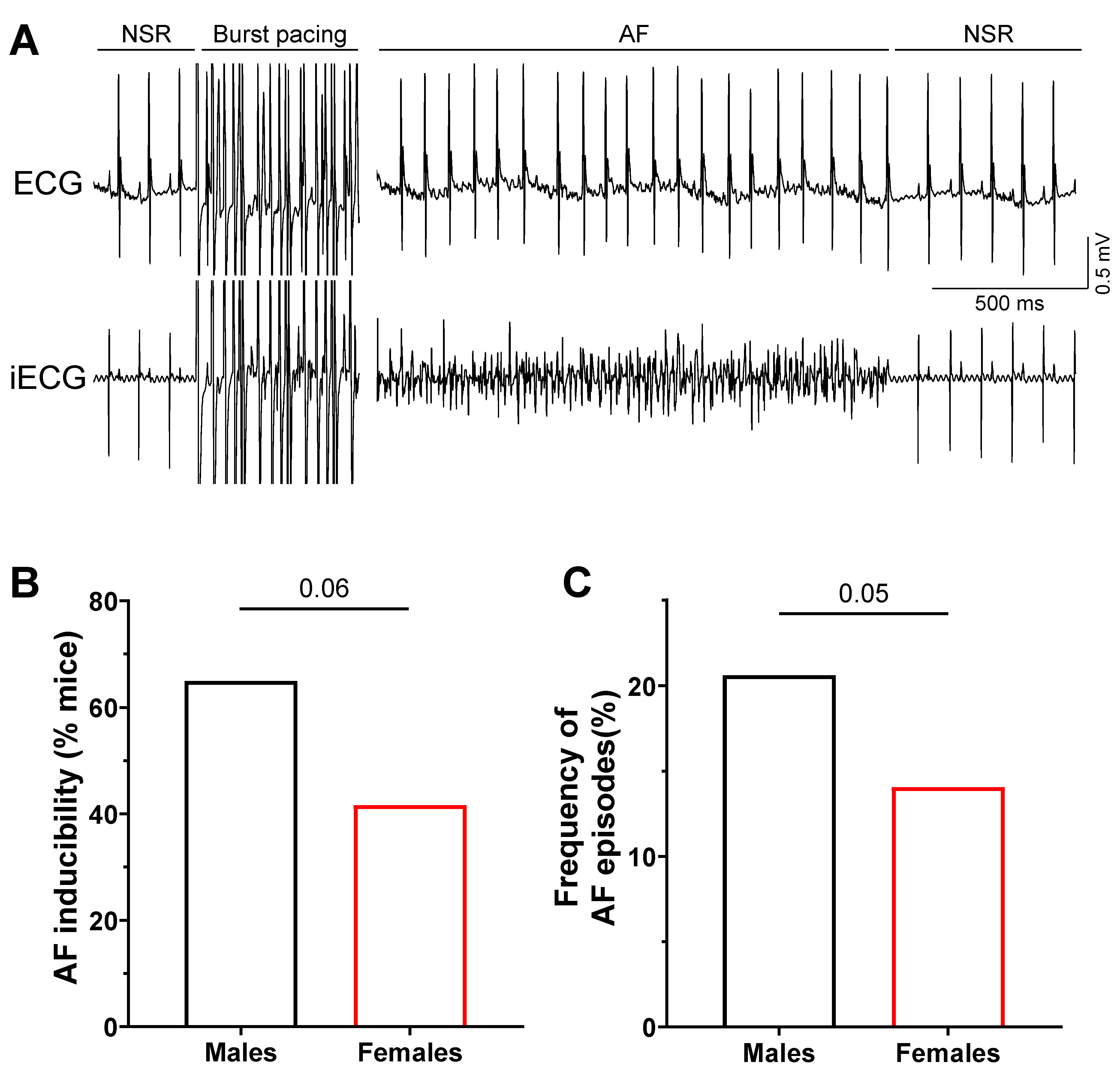
2.1. Increased Susceptibility to AF in Male Mice
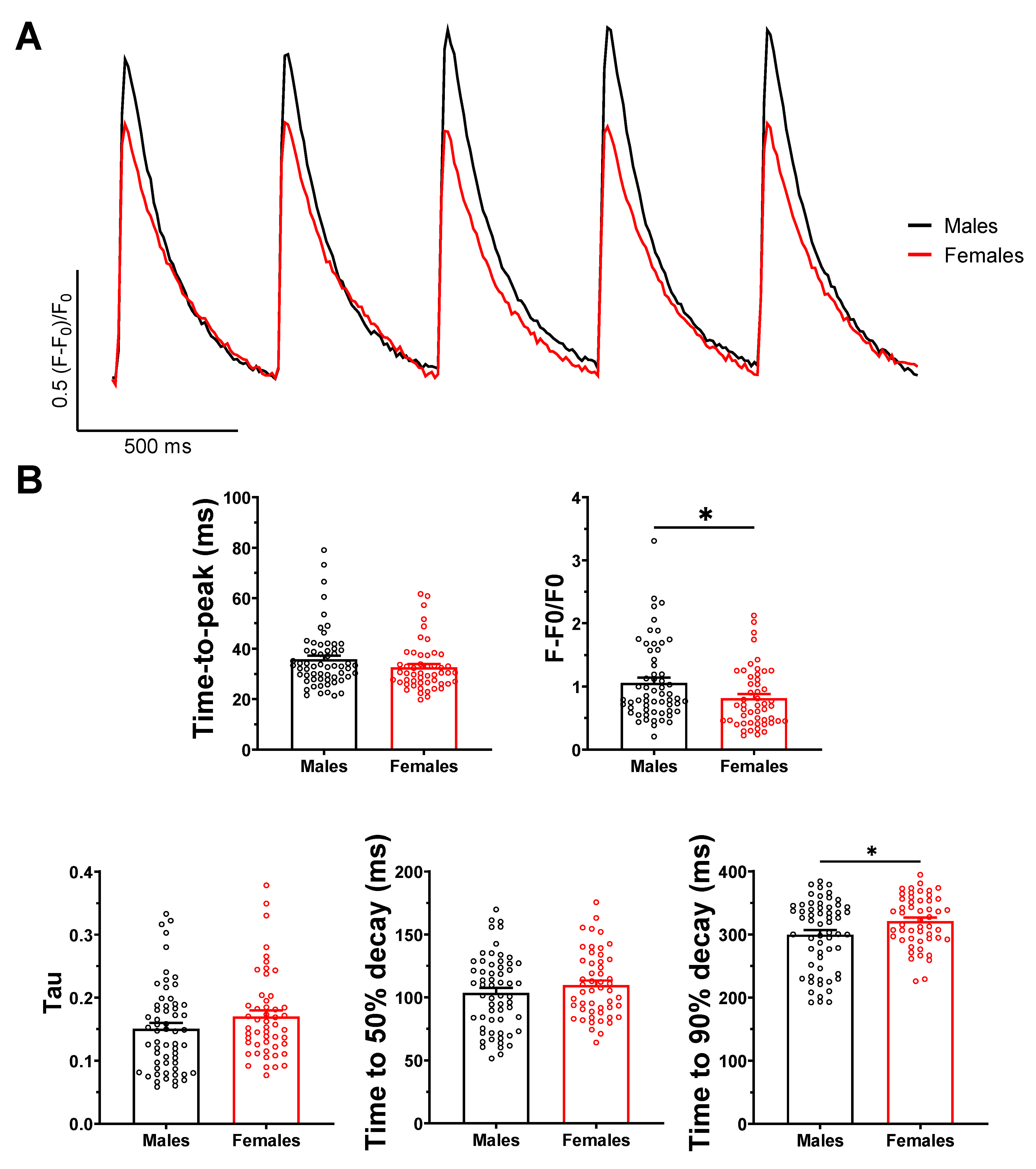
2.2. Ca2+ Transient Amplitude Is Larger in Male Than Female Atrial Myocytes
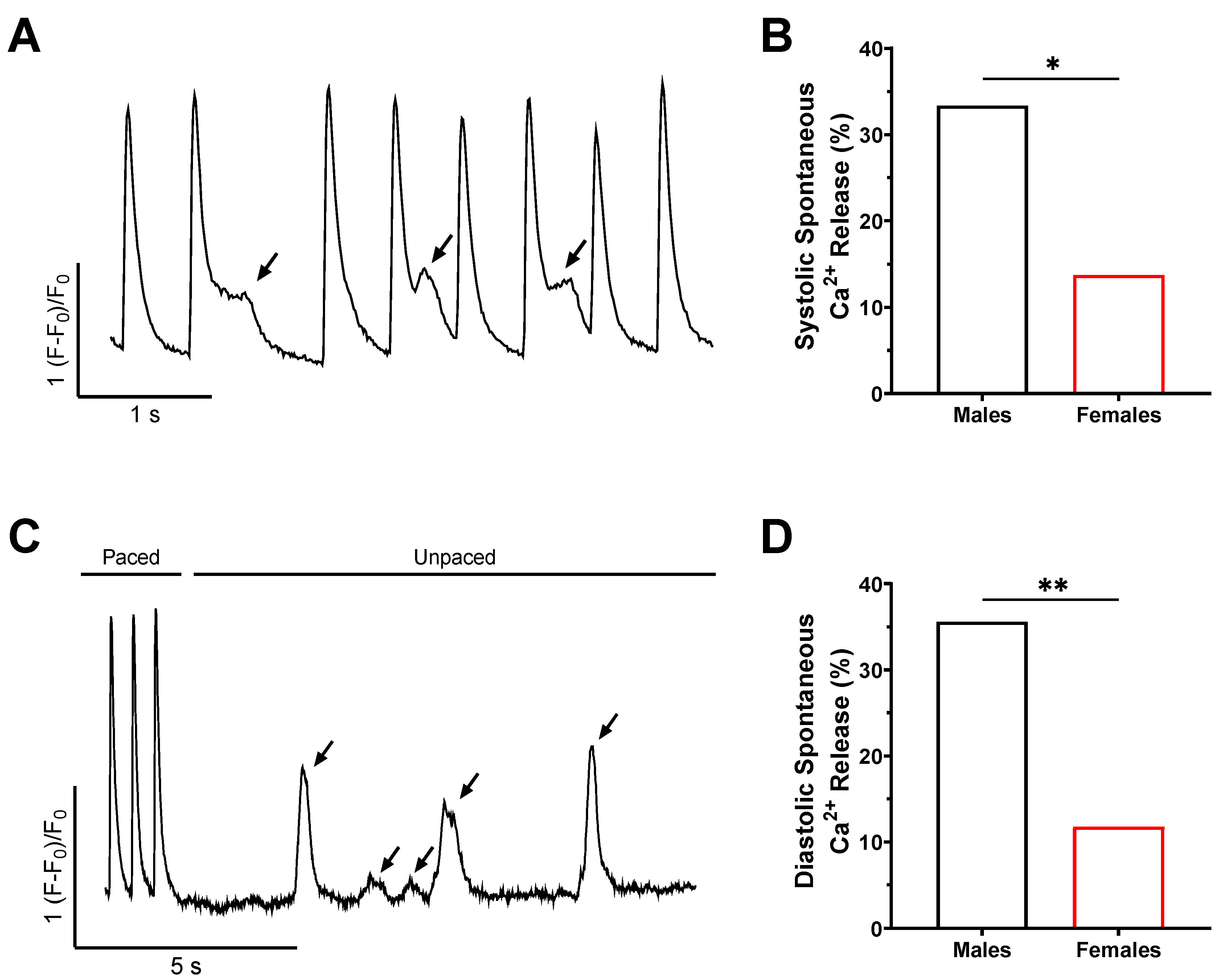
2.3. The Frequency of Spontaneous Ca2+ Release Is Higher in Male Mice
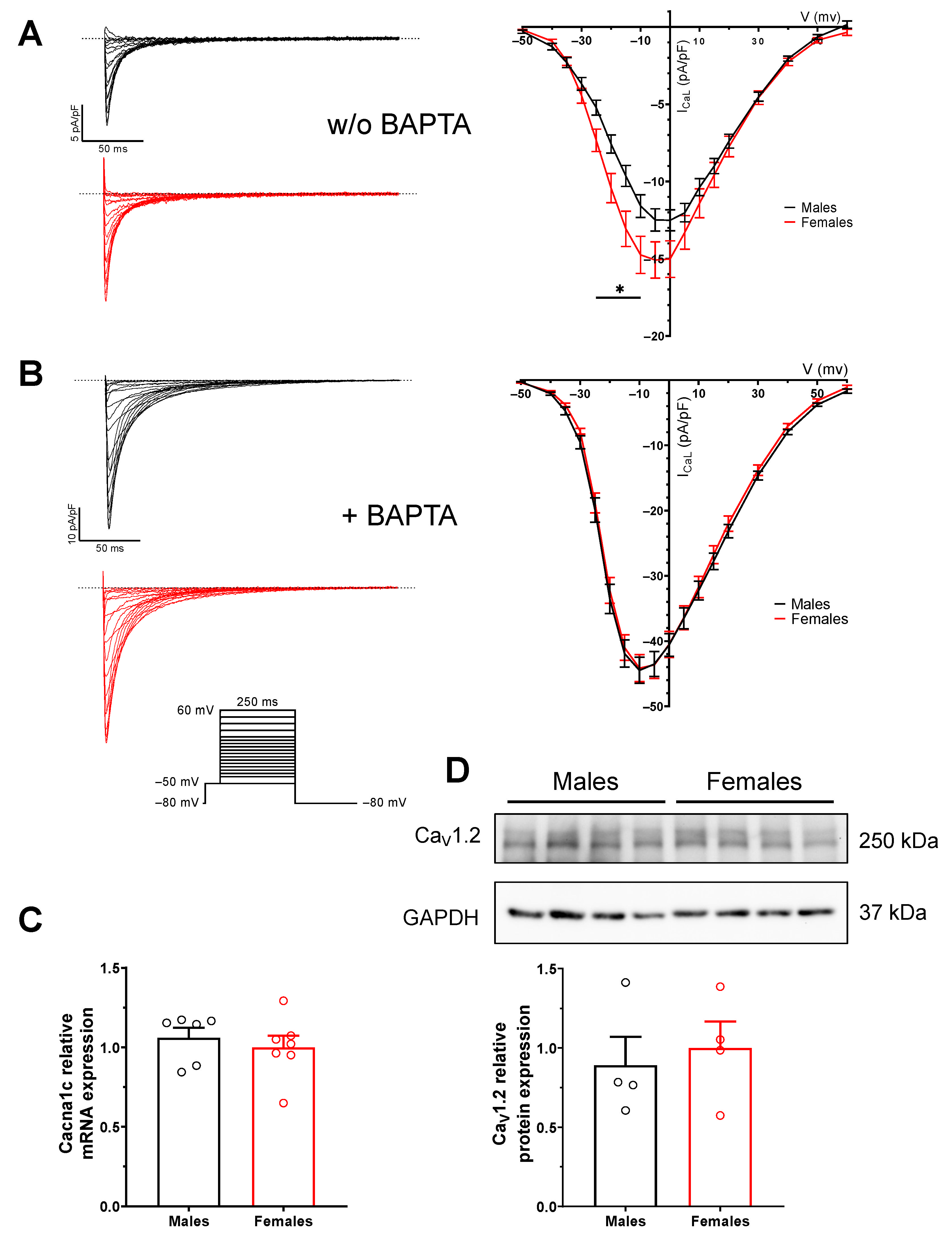
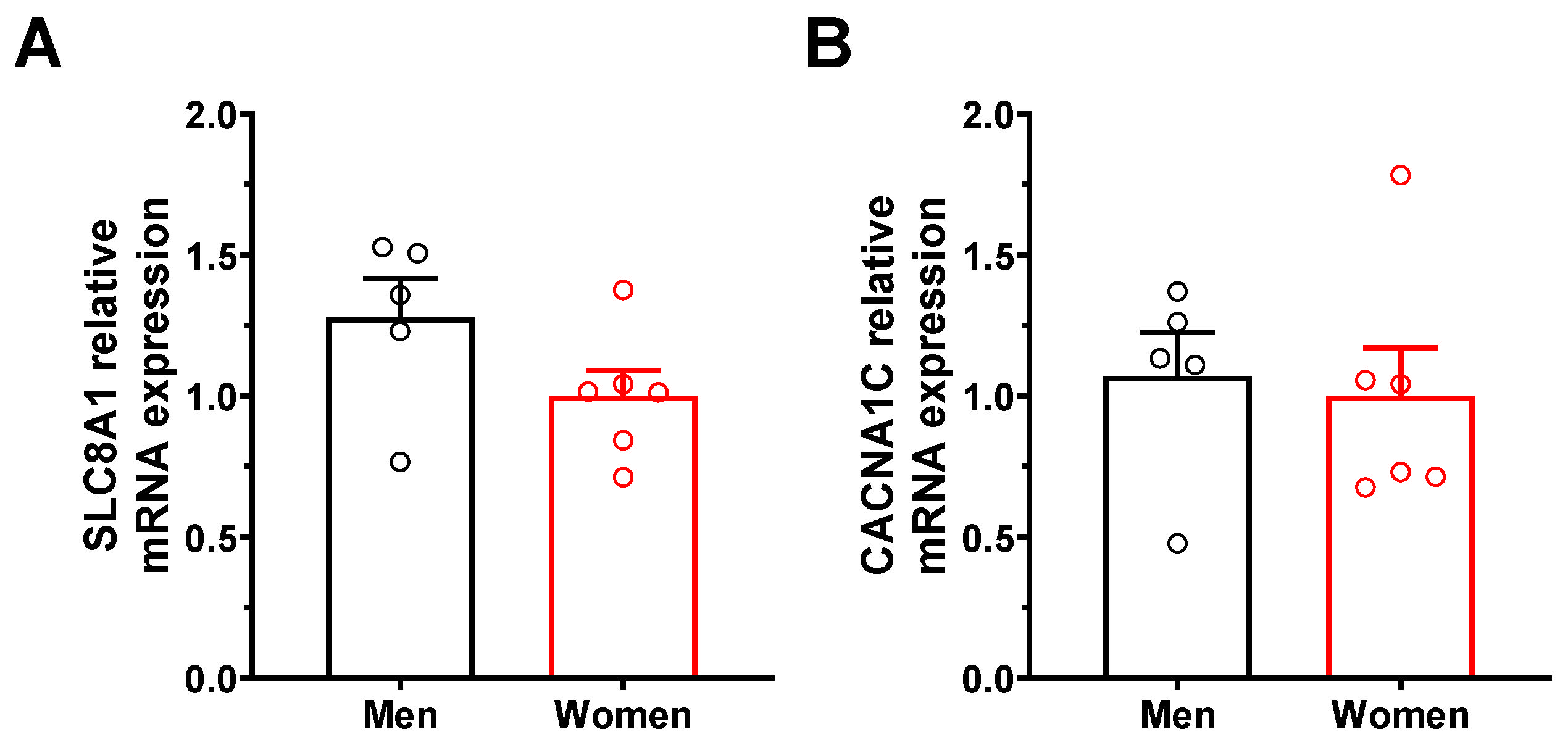
2.4. Enhanced NCX1 Function and Expression in Male Mice
2.5. Sex Difference in L-Type Ca2+ Current (ICaL) Is Due to Difference in Intracellular Calcium
2.6. RyR2 Gene Expression Is Increased in Female Atria
2.7. Increased NCX1 Gene Expression in Human Atrial Tissues
2.8. NCX1 Gene Expression Is Unchanged in Orchiectomized (ORC) and Ovariectomized (OVX) Mice
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Electrophysiological Programmed Stimulation (EPS) Studies
4.3. Isolation of Mouse Atrial Myocytes
4.4. Voltage Clamp Experiments
4.5. Calcium Transients
4.6. qPCR
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H.; Zheng, Z.-J.; et al. Worldwide Epidemiology of Atrial Fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S.M. Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Wann, L.S.; Curtis, A.B.; Ellenbogen, K.A.; Estes, N.A.; Ezekowitz, M.D.; Jackman, W.M.; January, C.T.; Lowe, J.E.; Page, R.L.; Slotwiner, D.J.; et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2013, 127, 1916–1926. [Google Scholar] [PubMed]
- Friberg, L.; Bergfeldt, L. Atrial fibrillation prevalence revisited. J. Intern. Med. 2013, 274, 461–468. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Vargas, E.R.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- Kamel, H.; Okin, P.M.; Elkind, M.S.V.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef]
- Peters, R.W.; Gold, M.R. The influence of gender on arrhythmias. Cardiol. Rev. 2004, 12, 97–105. [Google Scholar] [CrossRef]
- Rivero, A.; Curtis, A.B. Sex differences in arrhythmias. Curr. Opin. Cardiol. 2010, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. EP Eur. 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.E.; Deiß, S.; Dilling-Boer, D.; Didenko, M.; Eriksson, U.; Nedios, S.; Ng, F.S.; Roca Luque, I.; Sanchez Borque, P.; Vernooy, K.; et al. Mechanisms of sex differences in atrial fibrillation: Role of hormones and differences in electrophysiology, structure, function, and remodelling. EP Eur. 2019, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Westerman, S.; Wenger, N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. Curr. Cardiol. Rev. 2019, 15, 136–144. [Google Scholar] [CrossRef]
- Greiser, M.; Schotten, U. Dynamic remodeling of intracellular Ca2+ signaling during atrial fibrillation. J. Mol. Cell. Cardiol. 2013, 58, 134–142. [Google Scholar] [CrossRef]
- Dobrev, D.; Carlsson, L.; Nattel, S. Novel molecular targets for atrial fibrillation therapy. Nat. Rev. Drug Discov. 2012, 11, 275–291. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 8. [Google Scholar] [CrossRef]
- Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 2002, 415, 219–226. [Google Scholar] [CrossRef]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial Remodeling and Atrial Fibrillation: Mechanisms and Implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Greiser, M.; Lederer, W.J.; Schotten, U. Alterations of atrial Ca2+ handling as cause and consequence of atrial fibrillation. Cardiovasc. Res. 2011, 89, 722–733. [Google Scholar] [CrossRef][Green Version]
- Bögeholz, N.; Pauls, P.; Bauer, B.K.; Schulte, J.S.; Dechering, D.G.; Frommeyer, G.; Kirchhefer, U.; Goldhaber, J.I.; Müller, F.U.; Eckardt, L.; et al. Suppression of Early and Late Afterdepolarizations by Heterozygous Knockout of the Na+/Ca2+ Exchanger in a Murine Model. Circ Arrhythmia Electrophysiol. 2015, 8, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Pauls, P.; Kaese, S.; Schulte, J.S.; Lemoine, M.D.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Seidl, M.D.; Kirchhefer, U.; et al. Triggered activity in atrial myocytes is influenced by Na+/Ca2+ exchanger activity in genetically altered mice. J. Mol. Cell. Cardiol. 2016, 101, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation–contraction coupling. Pflugers. Arch. 2013, 465, 747–763. [Google Scholar] [CrossRef]
- Farrell, S.R.; Ross, J.L.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H36–H45. [Google Scholar] [CrossRef]
- Leblanc, N.; Chartier, D.; Gosselin, H.; Rouleau, J.L. Age and gender differences in excitation-contraction coupling of the rat ventricle. J. Physiol. 1998, 511, 533–548. [Google Scholar] [CrossRef]
- Wasserstrom, J.A.; Kapur, S.; Jones, S.; Faruque, T.; Sharma, R.; Kelly, J.E.; Pappas, A.; Ho, W.; Kadish, A.H.; Aistrup, G.L. Characteristics of intracellular Ca2+ cycling in intact rat heart: A comparison of sex differences. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1895–H1904. [Google Scholar] [CrossRef][Green Version]
- Curl, C.L.; Wendt, I.R.; Kotsanas, G. Effects of gender on intracellular. Pflugers. Arch. 2001, 441, 709–716. [Google Scholar] [CrossRef]
- Chu, S.; Sutherland, K.; Beck, J.; Kowalski, J.; Goldspink, P.; Schwertz, D. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci. 2005, 76, 2735–2749. [Google Scholar] [CrossRef]
- Yaras, N.; Tuncay, E.; Purali, N.; Sahinoglu, B.; Vassort, G.; Turan, B. Sex-related effects on diabetes-induced alterations in calcium release in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3584–H3592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyata, A.; Zipes, D.P.; Hall, S.; Rubart, M. KB-R7943 Prevents Acute, Atrial Fibrillation–Induced Shortening of Atrial Refractoriness in Anesthetized Dogs. Circulation 2002, 106, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+ Leak and Increased Na+-Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients With Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, N.; Steenman, M.; Lamirault, G.; Le Meur, N.; Le Bouter, S.; Lande, G.; Léger, J.; Charpentier, F.; Christ, T.; Dobrev, D.; et al. Human Atrial Ion Channel and Transporter Subunit Gene-Expression Remodeling Associated With Valvular Heart Disease and Atrial Fibrillation. Circulation 2005, 112, 471–481. [Google Scholar] [CrossRef]
- Pogwizd, S.M.; Bers, D.M. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc. Med. 2004, 14, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Li, D. Ionic Remodeling in the Heart: Pathophysiological Significance and New Therapeutic Opportunities for Atrial Fibrillation. Circ. Res. 2000, 87, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Martínez, A.; Tarifa, C.; Jiménez-Sábado, V.; Llach, A.; Godoy-Marín, H.; Colino-Lage, H.; Nolla-Colomer, C.; Casabella-Ramon, S.; Izquierdo-Castro, P.; Benítez, I.; et al. Influence of sex on intracellular calcium homoeostasis in patients with atrial fibrillation. Cardiovasc. Res. 2022, 118, 1033–1045. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; Lamorgese, M.; Rossie, S.S.; McCarthy, P.M.; Nerbonne, J.M. Atrial L-Type Ca2+ Currents and Human Atrial Fibrillation. Circ. Res. 1999, 85, 428–436. [Google Scholar] [CrossRef]
- Lai, L.-P.; Su, M.-J.; Lin, J.-L.; Lin, F.-Y.; Tsai, C.-H.; Chen, Y.-S.; Huang, S.K.S.; Tseng, Y.-Z.; Lien, W.-P. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca2+-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban. J. Am. Coll. Cardiol. 1999, 33, 1231–1237. [Google Scholar] [CrossRef]
- Brundel, B. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc. Res. 1999, 42, 443–454. [Google Scholar] [CrossRef]
- Schotten, U.; Haase, H.; Frechen, D.; Greiser, M.; Stellbrink, C.; Vazquez-Jimenez, J.F.; Morano, I.; Allessie, M.A.; Hanrath, P. The L-type Ca2+-channel subunits a1C and b2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J. Mol. Cell. Cardiol. 2003, 7, 437–443. [Google Scholar] [CrossRef]
- Brouillette, J.; Trepanier-Boulay, V.; Fiset, C. Effect of androgen deficiency on mouse ventricular repolarization. J. Physiol. 2003, 546, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, J.; Rivard, K.; Lizotte, E.; Fiset, C. Sex and strain differences in adult mouse cardiac repolarization: Importance of androgens. Cardiovasc. Res. 2005, 65, 148–157. [Google Scholar] [CrossRef]
- El Gebeily, G.; El Khoury, N.; Mathieu, S.; Brouillette, J.; Fiset, C. Estrogen regulation of the transient outward K(+) current involves estrogen receptor α in mouse heart. J. Mol. Cell. Cardiol. 2015, 86, 85–94. [Google Scholar] [CrossRef]
- Vizgirda, V.M.; Wahler, G.M.; Sondgeroth, K.L.; Ziolo, M.T.; Schwertz, D.W. Mechanisms of sex differences in rat cardiac myocyte response to β-adrenergic stimulation. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H256–H263. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Alber, S.; Shusterman, V.; Salama, G. Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen: Regional genomic regulation of NCX by oestrogen. J. Physiol. 2011, 589, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, G.; Papp, R.; DeFranco, D.B.; Zeng, F.; Salama, G. Oestrogen upregulates L-type Ca2+ channels via oestrogen-receptor-α by a regional genomic mechanism in female rabbit hearts: Regional genomic upregulation of ICa,L by oestrogen via ERα. J. Physiol. 2012, 590, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Bett, G.C.L.; Lis, A.; Rasmusson, R.L.; Baczkó, I.; Varró, A.; Salama, G. Genomic upregulation of cardiac Cav1.2α and NCX1 by estrogen in women. Biol. Sex Differ. 2017, 8, 26. [Google Scholar] [CrossRef]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell. Physiol. Biochem. 2009, 23, 75–86. [Google Scholar] [CrossRef]
- El Khoury, N.; Ross, J.L.; Long, V.; Thibault, S.; Ethier, N.; Fiset, C. Pregnancy and oestrogen regulate sinoatrial node calcium homeostasis and accelerate pacemaking. Cardiovasc. Res. 2018, 114, 1605–1616. [Google Scholar] [CrossRef]
- Demers, J.; Ton, A.; Huynh, F.; Thibault, S.; Ducharme, A.; Paradis, P.; Nemer, M.; Fiset, C. Atrial Electrical Remodeling in Mice With Cardiac-Specific Overexpression of Angiotensin II Type 1 Receptor. J. Am. Heart Assoc. 2022, 11, e023974. [Google Scholar] [CrossRef] [PubMed]
- Trépanier-Boulay, V.; Lupien, M.A.; St-Michel, C.; Fiset, C. Postnatal Development of Atrial Repolarization in Mouse. Cardiovasc. Res. 2004, 64, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, J.; Clark, R.B.; Giles, W.R.; Fiset, C. Functional properties of K+ currents in adult mouse ventricular myocytes. J. Physiol. 2004, 559, 777–798. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Ozdemir, S.; Bito, V.; Holemans, P.; Vinet, L.; Mercadier, J.-J.; Varro, A.; Sipido, K.R. Pharmacological Inhibition of Na/Ca Exchange Results in Increased Cellular Ca2+ Load Attributable to the Predominance of Forward Mode Block. Circ. Res. 2008, 102, 1398–1405. [Google Scholar] [CrossRef]
- Baczkó, I.; Giles, W.R.; Light, P.E. Resting Membrane Potential Regulates Na+–Ca2+ Exchange-Mediated Ca2+ Overload during Hypoxia–Reoxygenation in Rat Ventricular Myocytes. J. Physiol. 2003, 550, 889–898. [Google Scholar] [CrossRef]
- Bögeholz, N.; Pauls, P.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Pott, C.; Eckardt, L.; Müller, F.U.; Schulte, J.S. Distinct Occurrence of Proarrhythmic Afterdepolarizations in Atrial Versus Ventricular Cardiomyocytes: Implications for Translational Research on Atrial Arrhythmia. Front. Pharmacol. 2018, 9, 933. [Google Scholar] [CrossRef]
- Long, V.; Fiset, C. Contribution of estrogen to the pregnancy-induced increase in cardiac automaticity. J. Mol. Cell. Cardiol. 2020, 147, 27–34. [Google Scholar] [CrossRef]
- Lizotte, E.; Tremblay, A.; Allen, B.G.; Fiset, C. Isolation and characterization of subcellular protein fractions from mouse heart. Anal. Biochem. 2005, 345, 47–54. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibault, S.; Long, V.; Fiset, C. Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10724. https://doi.org/10.3390/ijms231810724
Thibault S, Long V, Fiset C. Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. International Journal of Molecular Sciences. 2022; 23(18):10724. https://doi.org/10.3390/ijms231810724
Chicago/Turabian StyleThibault, Simon, Valérie Long, and Céline Fiset. 2022. "Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation" International Journal of Molecular Sciences 23, no. 18: 10724. https://doi.org/10.3390/ijms231810724
APA StyleThibault, S., Long, V., & Fiset, C. (2022). Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. International Journal of Molecular Sciences, 23(18), 10724. https://doi.org/10.3390/ijms231810724




