A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet
Abstract
:1. Introduction
2. Results
2.1. MMP Array Assay
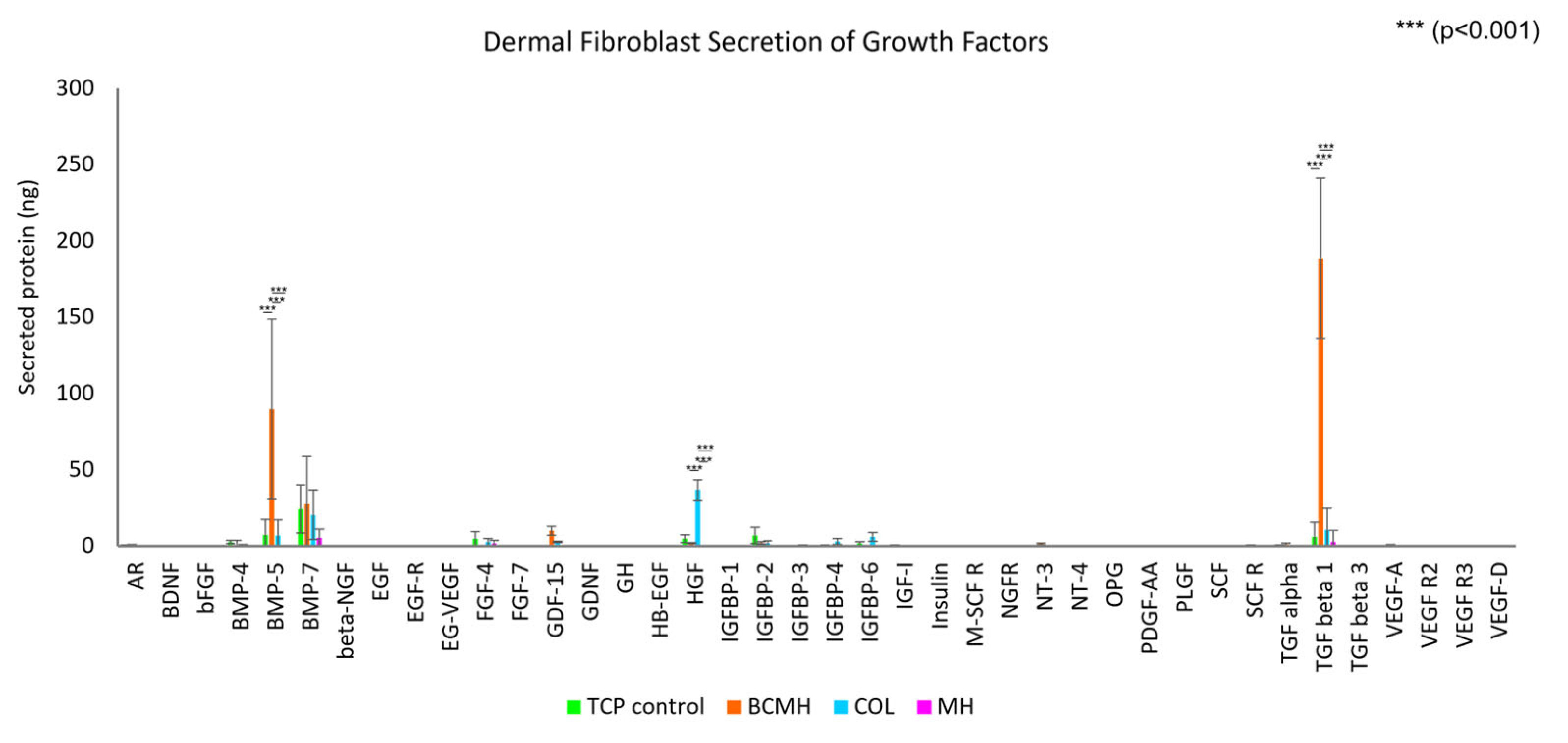
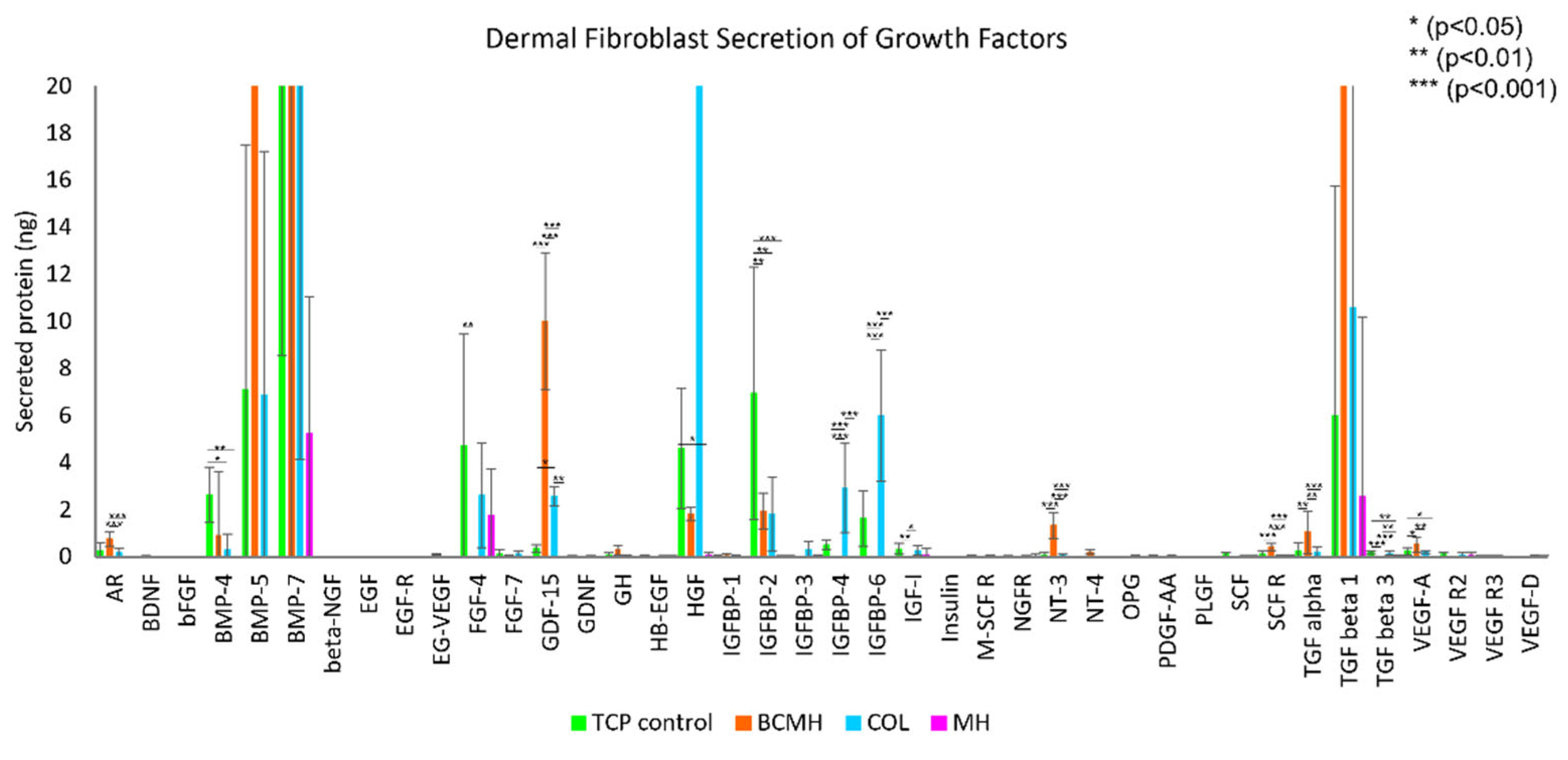
2.2. Growth Factor Array Assay
3. Discussion
3.1. MMP Array Assay
3.2. Growth Factor Array Assay
4. Materials and Methods
4.1. Materials
4.2. MMP Array Assay
4.3. Growth Factor Array Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Saarialho-Kere, U.K. Patterns of matrix metalloproteinase and TIMP expression in chronic ulcers. Arch. Dermatol. Res. 1998, 290, S47–S54. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Trocme, C.; Lardy, B.; Morel, F.; Halimi, S.; Benhamou, P.Y. Matrix metalloproteinases and diabetic foot ulcers: The ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet. Med. 2008, 25, 419–426. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Trostrup, H.; Holstein, P.; Karlsmark, T.; Moser, C.; Agren, M.S. Uncontrolled gelatin degradation in non-healing chronic wounds. J. Wound Care 2018, 27, 724–734. [Google Scholar] [CrossRef]
- Li, Z.; Guo, S.; Yao, F.; Zhang, Y.; Li, T. Increased ratio of serum matrix metalloproteinase-9 against TIMP-1 predicts poor wound healing in diabetic foot ulcers. J. Diabetes Its Complicat. 2013, 27, 380–382. [Google Scholar] [CrossRef]
- Liu, Y.; Min, D.; Bolton, T.; Nubé, V.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009, 32, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Bullen, E.C.; Longaker, M.T.; Updike, D.L.; Benton, R.; Ladin, D.; Hou, Z.; Howard, E.W. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J. Investig. Dermatol. 1995, 104, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef]
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wounds Compend. Clin. Res. Pract. 2008, 20, 347–356. [Google Scholar]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Amici, A.; Cianciosi, D.; Quiles, J.L.; Battino, M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free. Radic. Biol. Med. 2018, 126, 41–54. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Cherukuri, K.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. Manuka Honey Modulates the Inflammatory Behavior of a dHL-60 Neutrophil Model under the Cytotoxic Limit. Int. J. Biomater. 2019, 2019, 6132581. [Google Scholar] [CrossRef]
- Rodriguez, I.; Conti, T.; Bionda, N. Microenvironment Influence of a Novel Bioengineered Wound Product, APIS®: A Preliminary In Vitro Analysis of Inflammatory Marker and Growth Factor Secretion. Int. J. Biomater. 2021, 2021, 6612870. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Beck, H.N.; Drahushuk, K.; Jacoby, D.B.; Higgins, D.; Lein, P.J. Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci. 2001, 2, 12. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Sharov, A.A. BMP signaling in the control of skin development and hair follicle growth. Differentiation 2004, 72, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Strombergsson, A.; Weinstein, R.; Maloney, A.; Hendrix, C.; Liden, B.; Fridman, R. Preliminary Clinical Evaluation Using a Novel Bioengineered Wound Product to Treat Lower Extremity Ulcers. Int. J. Low. Extrem. Wounds 2020. [Google Scholar] [CrossRef] [PubMed]

| Treatment | MMP-9 (ng) | TIMP-1 (ng) | MMP-9/TIMP-1 Ratio |
|---|---|---|---|
| TCP Control | 5.38 | 55.08 | 0.098 |
| BCMH | 0.23 | 39.24 | 0.006 |
| COL | 2.74 | 41.92 | 0.065 |
| MH | 0.02 | 0.82 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, I.; Conti, T.; Bionda, N. A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet. Int. J. Mol. Sci. 2022, 23, 10670. https://doi.org/10.3390/ijms231810670
Rodriguez I, Conti T, Bionda N. A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet. International Journal of Molecular Sciences. 2022; 23(18):10670. https://doi.org/10.3390/ijms231810670
Chicago/Turabian StyleRodriguez, Isaac, Tricia Conti, and Nina Bionda. 2022. "A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet" International Journal of Molecular Sciences 23, no. 18: 10670. https://doi.org/10.3390/ijms231810670
APA StyleRodriguez, I., Conti, T., & Bionda, N. (2022). A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet. International Journal of Molecular Sciences, 23(18), 10670. https://doi.org/10.3390/ijms231810670






