Short-Term Supplementation of Sodium Nitrate vs. Sodium Chloride Increases Homoarginine Synthesis in Young Men Independent of Exercise
Abstract
1. Introduction
2. Results
2.1. Baseline Plasma Concentrations
2.2. Effect of Nitrate Supplementation on Plasma Nitrate and Nitrite Concentrations
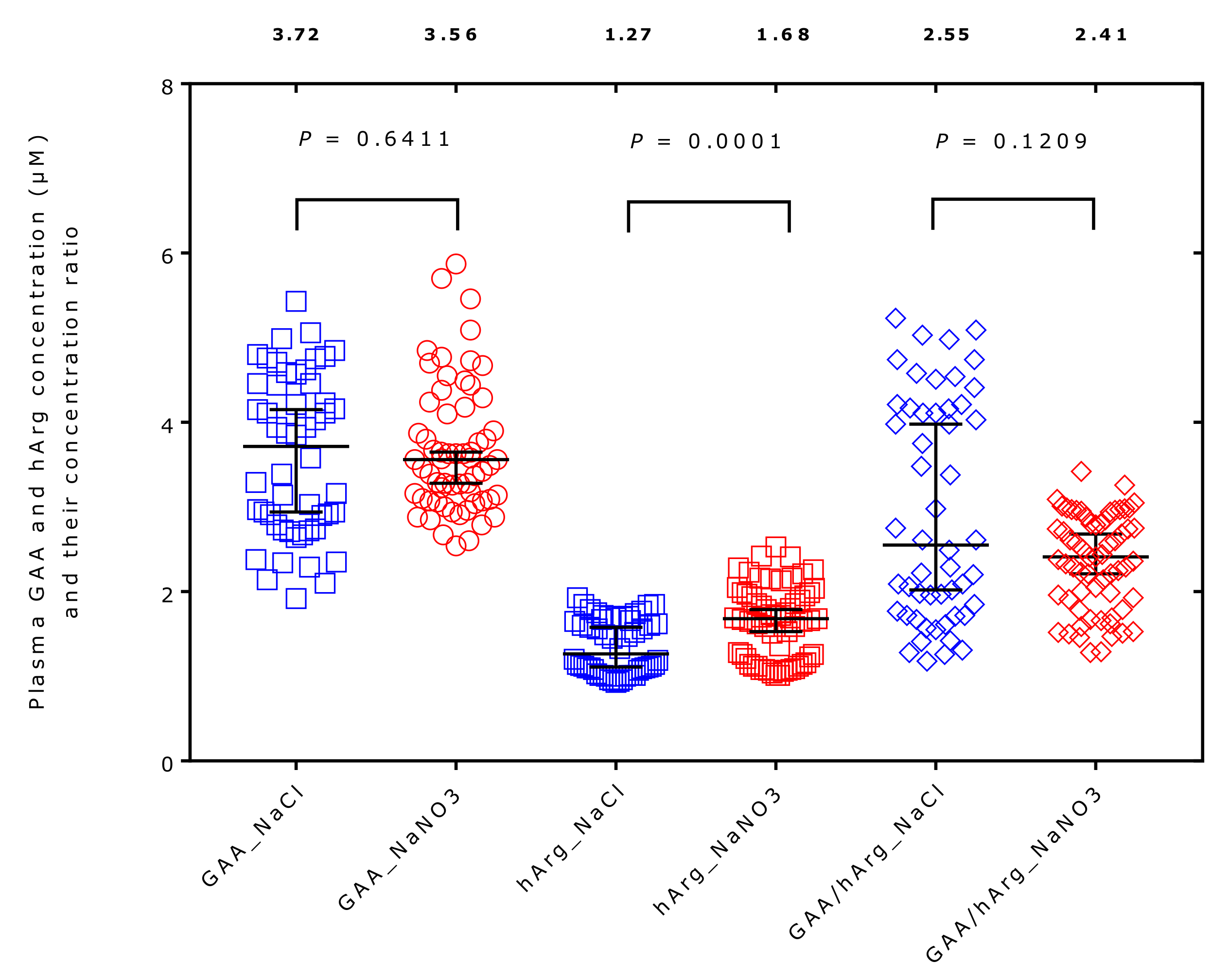
2.3. Effect of Supplementation on Plasma Amino Acids Concentrations
2.4. Effect of Supplementation on the Equilibrium Constants of the AGAT-Catalyzed Reactions Producing Guanidinoacetate and Homoarginine
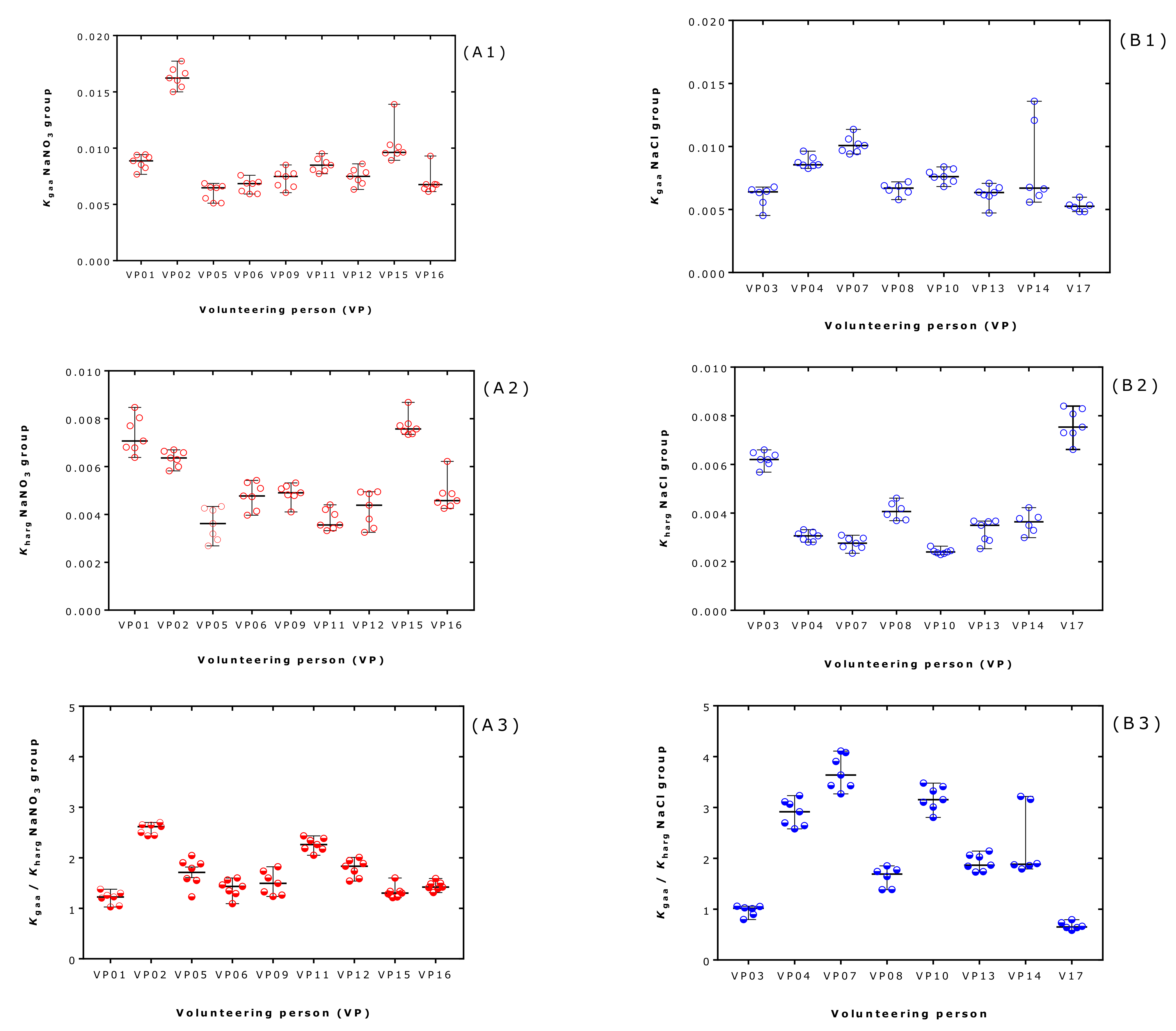
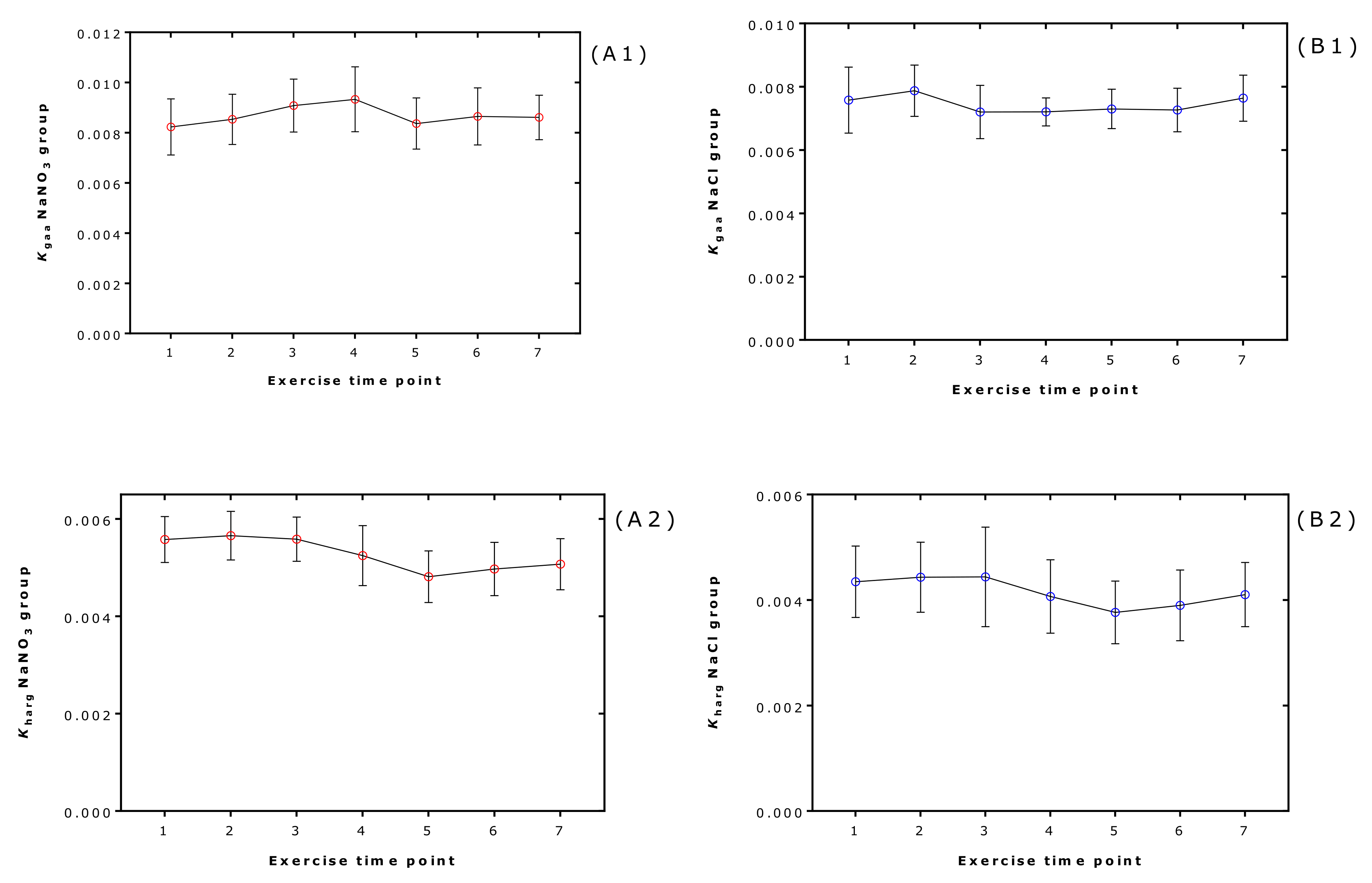
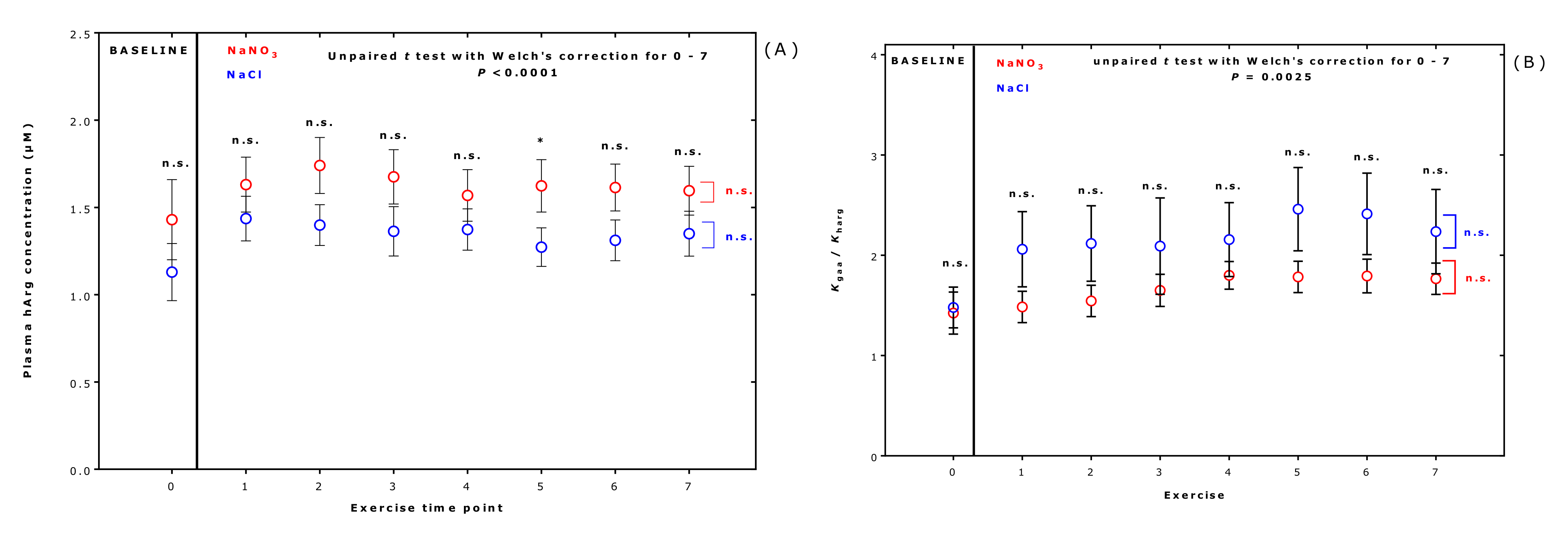
2.5. Effect of Supplementation and Exercise on the Equilibrium Constants in Each Volunteer and at Each Exercise Timepoint
2.6. Effects of Supplementation and Exercise on Plasma Creatinine in Each Volunteer and at Each Exercise Timepoint
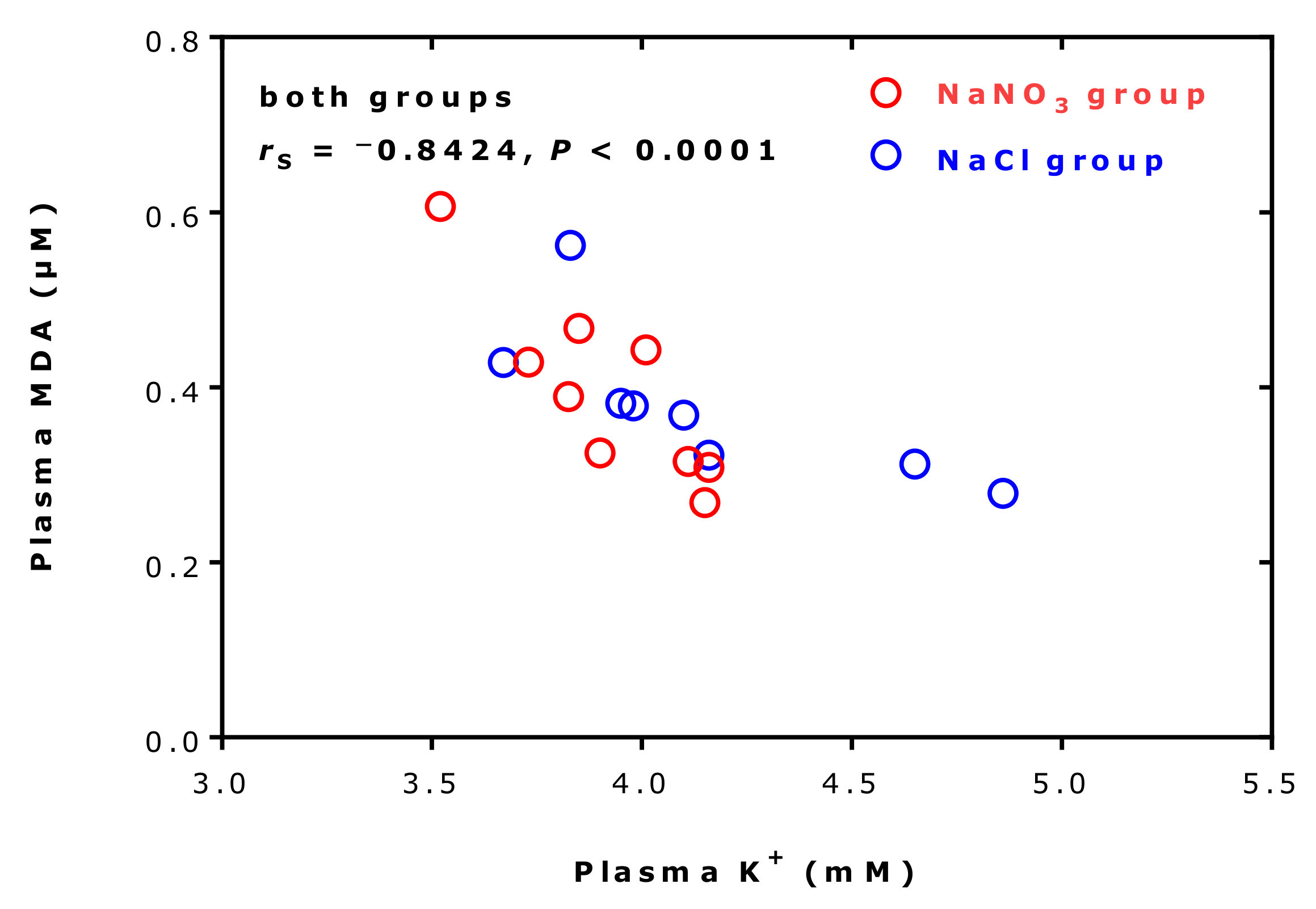
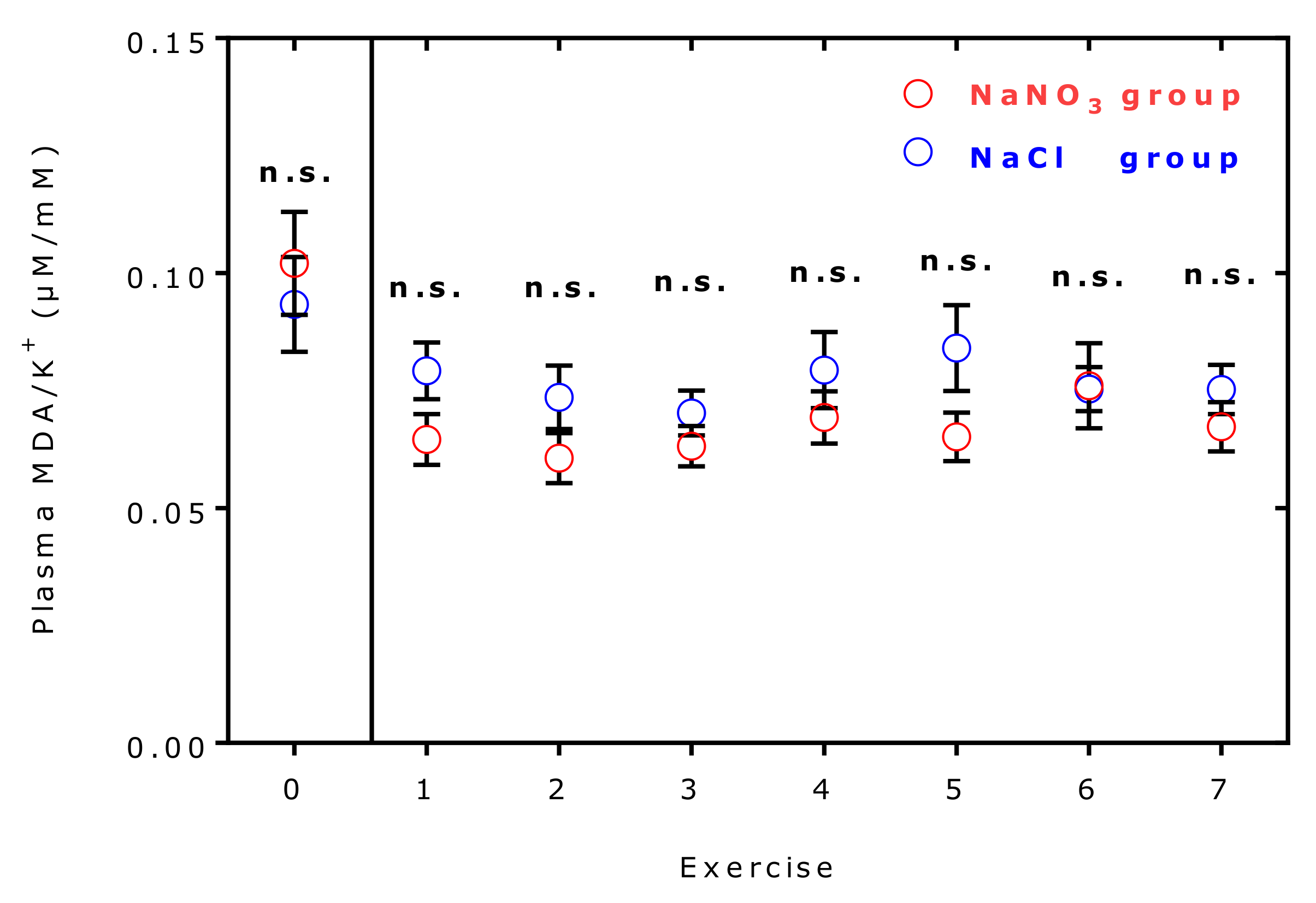
2.7. Effects of Supplementation and Exercise on Plasma Malondialdehyde and Potassium Ions
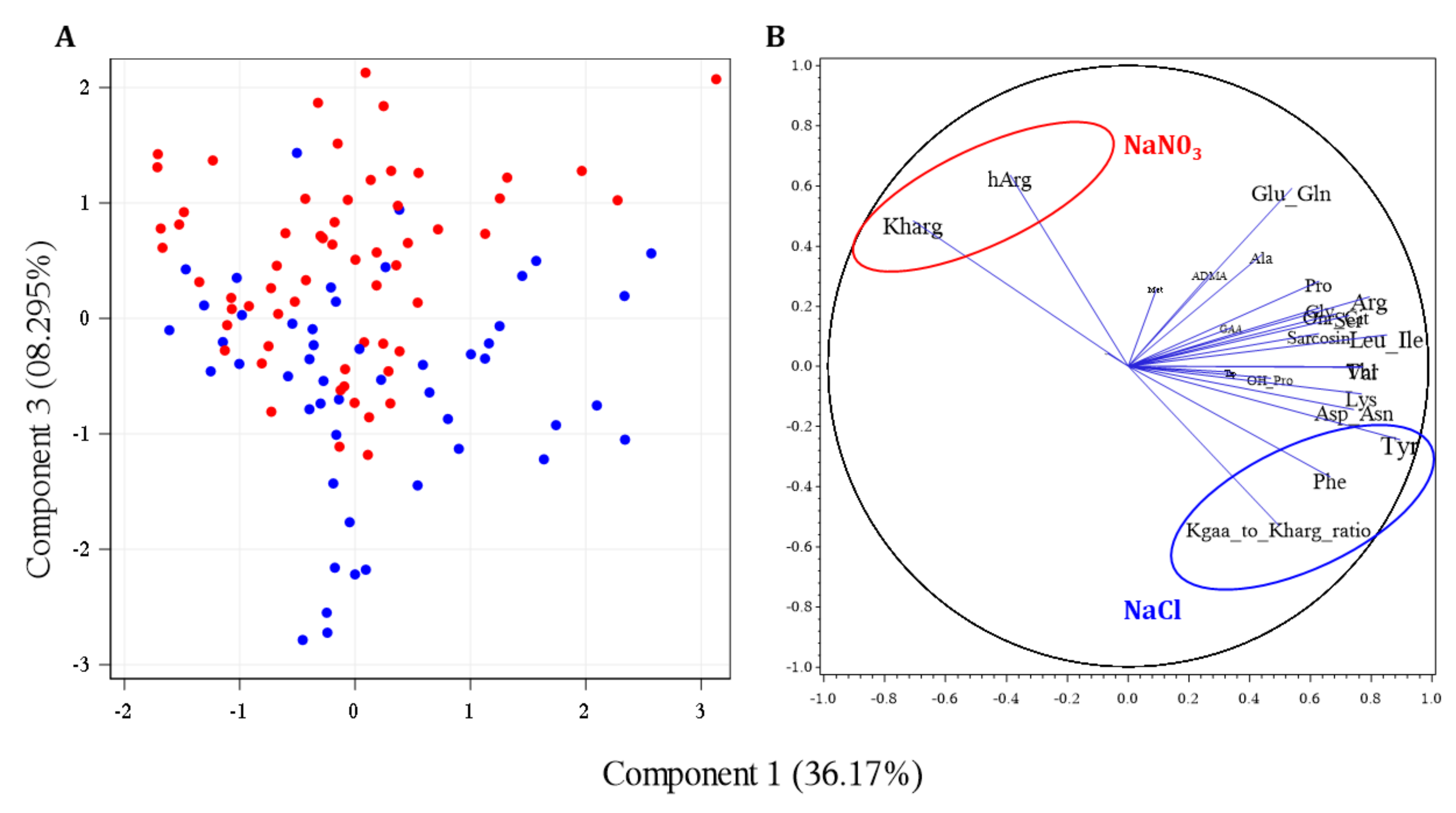
2.8. Multivariate Analyses
3. Discussion
4. Materials and Methods
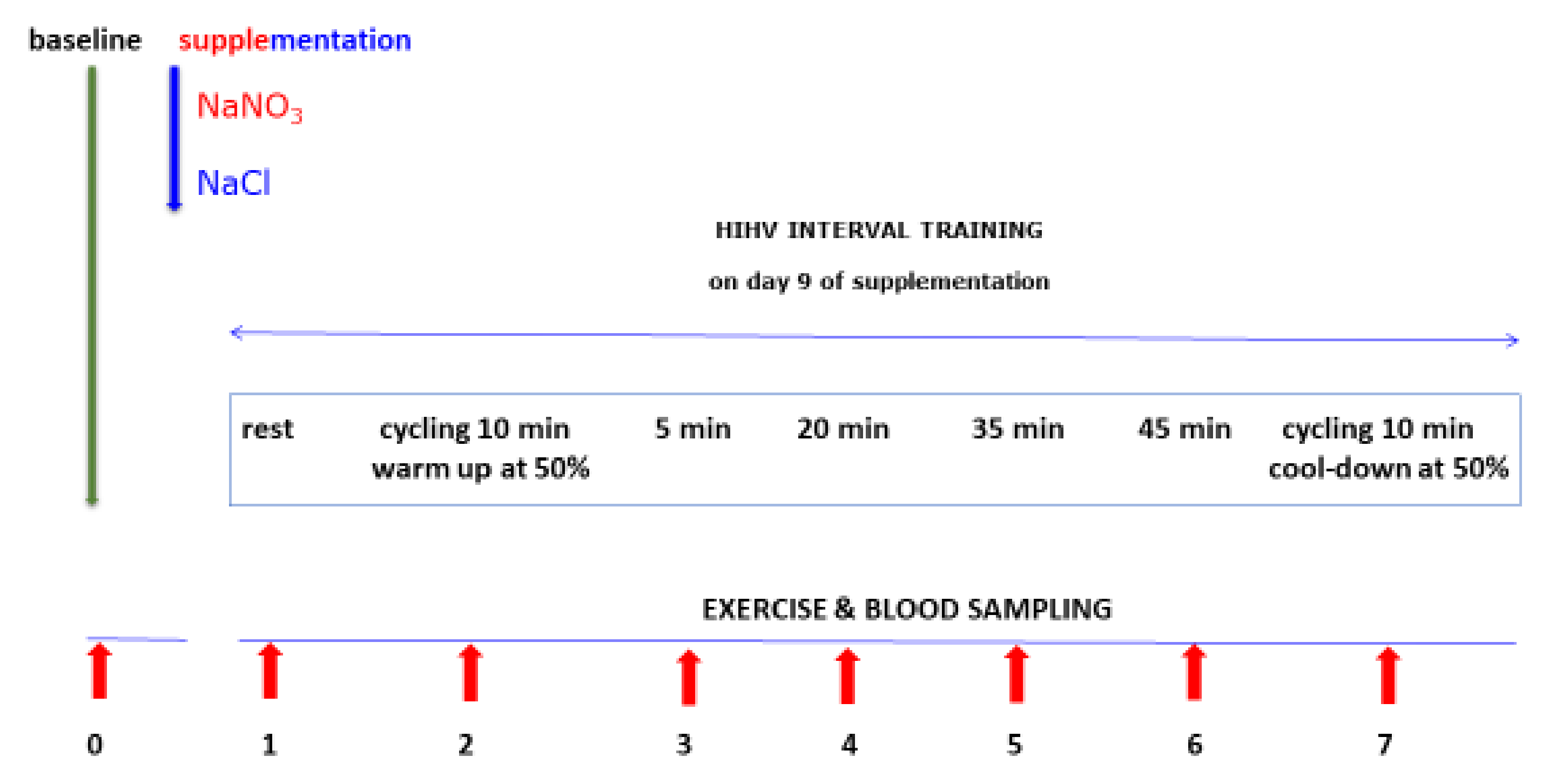
4.1. Study Design and Subjects
4.2. Sodium Nitrate and Sodium Chloride Supplementation
4.3. Physical Exercise and Blood Sampling
4.4. Biochemical GC–MS Analyses in Plasma Samples
4.5. Calculation of Equilibrium Constants
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humm, A.; Fritsche, E.; Steinbacher, S.; Huber, R. Crystal structure and mechanism of human L-arginine:glycine amidinotransferase: A mitochondrial enzyme involved in creatine biosynthesis. EMBO J. 1997, 16, 3373–3385. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Wu, G. Homoarginine, arginine, and relatives: Analysis, metabolism, transport, physiology, and pathology. Amino Acids 2015, 47, 1697–1702. [Google Scholar] [CrossRef]
- Watanabe, Y.; Van Pilsum, J.F.; Yokoi, I.; Mori, A. Synthesis of neuroactive guanidino compounds by rat kidney L-arginine: Glycine amidinotransferase. Life Sci. 1994, 55, 351–358. [Google Scholar] [CrossRef]
- Roche, J.; Lacombe, G.; Girard, H. On the specificity of certain bacterial deguanidases generating urea and on arginindihydrolase. Biochim. Biophys. Acta 1950, 6, 210–216. [Google Scholar] [CrossRef]
- Appleyard, G.; Woods, D.D. The pathway of creatine catabolism by Pseudomonas ovalis. J. Gen. Microbiol. 1956, 14, 351–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshimoto, T.; Oka, I.; Tsuru, D. Purification, crystallization, and some properties of creatine amidinohydrolase from Pseudomonas putida. J. Biochem. 1976, 79, 1381–1383. [Google Scholar] [CrossRef]
- Coll, M.; Knof, S.H.; Ohga, Y.; Messerschmidt, A.; Huber, R.; Moellering, H.; Rüssmann, L.; Schumacher, G. Enzymatic mechanism of creatine amidinohydrolase as deduced from crystal structures. J. Mol. Biol. 1990, 214, 597–610. [Google Scholar] [CrossRef]
- Meelua, W.; Oláh, J.; Jitonnom, J. Role of water coordination at zinc binding site and its catalytic pathway of dizinc creatininase: Insights from quantum cluster approach. J. Comput. Aided. Mol. Des. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Mellanby, E.; Twort, F.W. On the presence of beta-imidazolethylamine in the intestinal wall; with a method of isolating a bacillus from the alimentary canal which converts histidine into this substance. J. Physiol. 1912, 45, 53–60. [Google Scholar] [CrossRef]
- Twort, F.W.; Mellanby, E. On creatin-destroying bacilli in the intestine, and their isolation. J. Physiol. 1912, 44, 43–49. [Google Scholar] [CrossRef]
- März, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Böhm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Boehm, B.O.; März, W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 2011, 97, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grübler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.Ó.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Schwedhelm, E.; Choe, C.U. L-homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Car. 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Frenay, A.R.; Kayacelebi, A.A.; Beckmann, B.; Soedamah-Muhtu, S.S.; de Borst, M.H.; van den Berg, E.; van Goor, H.; Bakker, S.J.; Tsikas, D. High urinary homoarginine excretion is associated with low rates of all-cause mortality and graft failure in renal transplant recipients. Amino Acids 2015, 47, 1827–1836. [Google Scholar] [CrossRef]
- Kayacelebi, A.A.; Minović, I.; Hanff, E.; Frenay, A.S.; de Borst, M.H.; Feelisch, M.; van Goor, H.; Bakker, S.J.L.; Tsikas, D. Low plasma homoarginine concentration is associated with high rates of all-cause mortality in renal transplant recipients. Amino Acids 2017, 49, 1193–1202. [Google Scholar] [CrossRef]
- Hanff, E.; Said, M.Y.; Kayacelebi, A.A.; Post, A.; Minovic, I.; van den Berg, E.; de Borst, M.H.; van Goor, H.; Bakker, S.J.L.; Tsikas, D. High plasma guanidinoacetate-to-homoarginine ratio is associated with high all-cause and cardiovascular mortality rate in adult renal transplant recipients. Amino Acids 2019, 51, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Kleist, C.J.; Choe, C.-U.; Atzler, D.; Schönhoff, M.; Böger, R.; Schwedhelm, E.; Wicha, S.G. Population kinetics of homoarginine and optimized supplementation for cardiovascular risk reduction. Amino Acids 2022, 54, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Clements, W.T.; Lee, S.R.; Bloomer, R.J. Nitrate ingestion: A review of the health and physical performance effects. Nutrients 2014, 6, 5224–5564. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Lost-in-Translation of Metabolic Effects of Inorganic Nitrate in Type 2 Diabetes: Is Ascorbic Acid the Answer? Int. J. Mol. Sci. 2021, 22, 4735. [Google Scholar] [CrossRef] [PubMed]
- Finkel, A.; Röhrich, M.A.; Maassen, N.; Lützow, M.; Blau, L.S.; Hanff, E.; Tsikas, D.; Maassen, M. Long-term effects of NO3− on the relationship between oxygen uptake and power after three weeks of supplemented HIHVT. J. Appl. Physiol. 2018, 125, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Bollenbach, A.; Tsikas, D. Pharmacological activation of dimethylarginine dimethylaminohydrolase (DDAH) activity by inorganic nitrate and DDAH inhibition by NG-hydroxy-L-arginine, Nω,Nω-dimethyl-L-citrulline and Nω,Nω-dimethyl-Nδ-hydroxy-L-citrulline: Results and overview. Amino Acids 2019, 51, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Kimoto, M.; Sasaoka, K. Purification and properties of a new enzyme, NG, NG-dimethylarginine dimethylaminohydrolase from rat kidney. J. Biol. Chem. 1989, 264, 10205–10209. [Google Scholar] [CrossRef]
- Murray-Rust, J.; Leiper, J.; McAlister, M.; Phelan, J.; Tilley, S.; Santa Maria, J.; Vallance, P.; McDonald, N. Structural insights into the hydrolysis of cellular nitric oxide synthase inhibitors by dimethylarginine dimethylaminohydrolase. Nat. Struct. Biol. 2001, 8, 679–683. [Google Scholar] [CrossRef]
- Linsky, T.; Fast, W. Mechanistic similarity and diversity among the guanidine-modifying members of the pentein superfamily. Biochim. Biophys. Acta 2010, 1804, 1943–1953. [Google Scholar] [CrossRef][Green Version]
- Stone, E.M.; Costello, A.L.; Tierney, D.L.; Fast, W. Substrate-assisted cysteine deprotonation in the mechanism of dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry 2006, 45, 5618–5630. [Google Scholar] [CrossRef]
- Sipilä, I. Inhibition of arginine-glycine amidinotransferase by ornithine. A possible mechanism for the muscular and chorioretinal atrophies in gyrate atrophy of the choroid and retina with hyperornithinemia. Biochim. Biophys. Acta 1980, 613, 79–84. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Tsikas, D.; Redfors, B. Pilot Study on Acute Effects of Pharmacological Intraperitoneal L-Homoarginine on Homeostasis of Lysine and Other Amino Acids in a Rat Model of Isoprenaline-Induced Takotsubo Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 4734. [Google Scholar] [CrossRef]
- Tsikas, D. A critical review and discussion of analytical methods in the L-arginine/nitric oxide area of basic and clinical research. Anal. Biochem. 2008, 379, 139–163. [Google Scholar] [CrossRef]
- Dreissigacker, U.; Wendt, M.; Wittke, T.; Tsikas, D.; Maassen, N. Positive correlation between plasma nitrite and performance during high-intensive exercise but not oxidative stress in healthy men. Nitric Oxide 2010, 23, 128–135. [Google Scholar] [CrossRef]
- Pyne, D.B. Exercise-induced muscle damage and inflammation: A review. Aust. J. Sci. Med. Sport. 1994, 26, 49–58. [Google Scholar] [PubMed]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed. Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Böger, R.H.; Bode-Böger, S.M.; Gutzki, F.M.; Frölich, J.C. Quantification of nitrite and nitrate in human urine and plasma as pentafluorobenzyl derivatives by gas chromatography-mass spectrometry using their 15N-labelled analogs. J. Chromatogr. B Biomed. Appl. 1994, 661, 185–191. [Google Scholar] [CrossRef]
- Shannon, O.M.; Allen, J.D.; Bescos, R.; Burke, L.; Clifford, T.; Easton, C.; Gonzalez, J.T.; Jones, A.M.; Jonvik, K.L.; Larsen, F.J.; et al. Dietary Inorganic Nitrate as an Ergogenic Aid: An Expert Consensus Derived via the Modified Delphi Technique. Sports Med. 2022. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Blackwell, J.R.; L'Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Burleigh, M.; Liddle, L.; Muggeridge, D.J.; Monaghan, C.; Sculthorpe, N.; Butcher, J.; Henriquez, F.; Easton, C. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxid. 2019, 89, 54–63. [Google Scholar] [CrossRef]
- González-Soltero, R.; Bailén, M.; de Lucas, B.; Ramírez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef]
- Hanusch, B.; Brinkmann, F.; Mayorandan, S.; Chobanyan-Jürgens, K.; Wiemers, A.; Jansen, K.; Ballmann, M.; Schmidt-Choudhury, A.; Bollenbach, A.; Derichs, N.; et al. Local and Systemic Alterations of the L-Arginine/Nitric Oxide Pathway in Sputum, Blood, and Urine of Pediatric Cystic Fibrosis Patients and Effects of Antibiotic Treatment. J. Clin. Med. 2020, 9, 3802. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B. Homoarginine inhibition of Escherichia coli B. J. Biol. Chem. 1955, 212, 617–622. [Google Scholar] [CrossRef]
- Peyru, G.M.; Maas, W.K. Inhibition of Escherichia coli B by homoarginine. J. Bacteriol. 1967, 94, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, P.J.; Timkovich, R.; Cusanovich, M.A. Lysine to 13C-labeled homoarginine conversion in microbial cytochromes c. Electron transport rates and 13C nuclear magnetic resonance spectroscopy. J. Mol. Biol. 1981, 145, 583–602. [Google Scholar] [CrossRef]
- Taghikhani, E.; Maas, R.; Fromm, M.F.; König, J. The renal transport protein OATP4C1 mediates uptake of the uremic toxin asymmetric dimethylarginine (ADMA) and efflux of cardioprotective L-homoarginine. PLoS ONE 2019, 14, e0213747. [Google Scholar] [CrossRef]
- Unkles, S.E.; Rouch, D.A.; Wang, Y.; Siddiqi, M.Y.; Glass, A.D.; Kinghorn, J.R. Two perfectly conserved arginine residues are required for substrate binding in a high-affinity nitrate transporter. Proc. Natl. Acad. Sci. USA 2004, 101, 17549–17554. [Google Scholar] [CrossRef]
- Hanff, E.; Hafner, P.; Bollenbach, A.; Bonati, U.; Kayacelebi, A.A.; Fischer, D.; Tsikas, D. Effects of single and combined metformin and L-citrulline supplementation on L-arginine-related pathways in Becker muscular dystrophy patients: Possible biochemical and clinical implications. Amino Acids 2018, 50, 1391–1406. [Google Scholar] [CrossRef]
- Tsikas, D.; Surdacki, A. Biotransformation of organic nitrates by glutathione S-transferases and other enzymes: An appraisal of the pioneering work by William, B. Jakoby. Anal. Biochem. 2022, 644, 113993. [Google Scholar] [CrossRef]
- Use of Anticoagulants in Diagnostic Laboratory Investigations. WHO/DIL/LAB/99.1 Rev.2. Available online: https://apps.who.int/iris/handle/10665/65957 (accessed on 24 July 2022).
- Monneret, D.; Mestari, F.; Atlan, G.; Corlouer, C.; Ramani, Z.; Jaffre, J.; Dever, S.; Fressart, V.; Alkouri, R.; Lamari, F.; et al. Hemolysis indexes for biochemical tests and immunoassays on Roche analyzers: Determination of allowable interference limits according to different calculation methods. Scand. J. Clin. Lab. Invest 2015, 75, 162–169. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Isobe, K.; Naito, A.; Ishijima, M.; Nanmoku, T.; Yamamoto, T.; Suzuki, E.; Kawakami, Y. Influence of In Vitro Hemolysis on 80 Different Laboratory Tests. Clin. Lab. 2017, 63, 219–226. [Google Scholar] [CrossRef]
- Mays, J.A.; Greene, D.N.; Merrill, A.E.; Mathias, P.C. Evidence-Based Validation of Hemolysis Index Thresholds by Use of Retrospective Clinical Data. J. Appl. Lab. Med. 2018, 3, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.B.; Cumberbatch, M.; Cohn, S.; Scott, D.; Gunasuntharam, T.; Davidson, C.; Chapman, C. The erythrocyte sodium and potassium in patients treated with digoxin. Br. J. Clin. Pharmacol. 1980, 10, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dreissigacker, U.; Suchy, M.T.; Maassen, N.; Tsikas, D. Human plasma concentrations of malondialdehyde (MDA) and the F2-isoprostane 15(S)-8-iso-PGF(2alpha) may be markedly compromised by hemolysis: Evidence by GC-MS/MS and potential analytical and biological ramifications. Clin. Biochem. 2010, 43, 159–167. [Google Scholar] [CrossRef]
- Steinberg, J.; Gainnier, M.; Michel, F.; Faucher, M.; Arnaud, C.; Jammes, Y. The post-exercise oxidative stress is depressed by acetylsalicylic acid. Respir. Physiol. Neurobiol. 2002, 130, 189–199. [Google Scholar] [CrossRef]
- Shushakov, V.; Stubbe, C.; Peuckert, A.; Endeward, V.; Maassen, N. The relationships between plasma potassium, muscle excitability and fatigue during voluntary exercise in humans. Exp. Physiol. 2007, 92, 705–715. [Google Scholar] [CrossRef]
- Malfatti, C.R.; Burgos, L.T.; Rieger, A.; Rüdger, C.L.; Túrmina, J.A.; Pereira, R.A.; Pavlak, J.L.; Silva, L.A.; Osiecki, R. Decreased erythrocyte NA+,K+-ATPase activity and increased plasma TBARS in prehypertensive patients. ScientificWorldJournal. 2012, 2012, 348246. [Google Scholar] [CrossRef]
- al-Mehdi, A.B.; Shuman, H.; Fisher, A.B. Oxidant generation with K(+)-induced depolarization in the isolated perfused lung. Free Radic. Biol. Med. 1997, 23, 47–56. [Google Scholar] [CrossRef]
- Clifton, P. From sodium intake restriction to nitrate supplementation: Different measures with converging mechanistic pathways. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1079–1086. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Shannon, O.M.; Easton, C.; Shepherd, A.I.; Siervo, M.; Bailey, S.J.; Clifford, T. Dietary nitrate and population health: A narrative review of the translational potential of existing laboratory studies. BMC Sports Sci. Med. Rehabil. 2021, 13, 65. [Google Scholar] [CrossRef]
- Macuh, M.; Knap, B. Effects of Nitrate Supplementation on Exercise Performance in Humans: A Narrative Review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Rushing, B.R.; Sumner, S.J.; Hackney, A.C. Dietary Supplements for Athletic Performance in Women: Beta-Alanine, Caffeine, and Nitrate. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Smeets, E.T.H.C.; Mensink, R.P.; Kleinloog, J.P.D.; Joris, P.J. Acute Effects of Inorganic Nitrate Intake on Brachial and Femoral Flow-Mediated Vasodilation, and on Carotid Artery Reactivity Responses: Results of a Randomized, Double-Blinded, Placebo-Controlled Cross-Over Study in Abdominally Obese Men. Nutrients 2022, 14, 3560. [Google Scholar] [CrossRef] [PubMed]
- Hanff, E.; Eisenga, M.F.; Beckmann, B.; Bakker, S.J.; Tsikas, D. Simultaneous pentafluorobenzyl derivatization and GC-ECNICI-MS measurement of nitrite and malondialdehyde in human urine: Close positive correlation between these disparate oxidative stress biomarkers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1043, 167–175. [Google Scholar] [CrossRef]
- Tsikas, D.; Mikuteit, M. N-Acetyl-L-cysteine in human rheumatoid arthritis and its effects on nitric oxide (NO) and malondialdehyde (MDA): Analytical and clinical considerations. Amino Acids 2022, 54, 1251–1260. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef]
- Hanff, E.; Lützow, M.; Kayacelebi, A.A.; Finkel, A.; Maassen, M.; Yanchev, G.R.; Haghikia, A.; Bavendiek, U.; Buck, A.; Lücke, T.; et al. Simultaneous GC-ECNICI-MS measurement of nitrite, nitrate and creatinine in human urine and plasma in clinical settings. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1047, 207–214. [Google Scholar] [CrossRef]
- Hanff, E.; Ruben, S.; Kreuzer, M.; Bollenbach, A.; Kayacelebi, A.A.; Das, A.M.; von Versen-Höynck, F.; von Kaisenberg, C.; Haffner, D.; Ückert, S.; et al. Development and validation of GC-MS methods for the comprehensive analysis of amino acids in plasma and urine and applications to the HELLP syndrome and pediatric kidney transplantation: Evidence of altered methylation, transamidination, and arginase activity. Amino Acids 2019, 51, 529–547. [Google Scholar] [CrossRef]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Suchy, M.T.; Trettin, A.; Modun, D.; Stuke, N.; Maassen, N.; Frölich, J.C. Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2α and nitric oxide (NO). Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 95–111. [Google Scholar] [CrossRef]


| Analyte | NaCl Group (PL) | NaNO3 Group (N) | D (%) (N − PL) | p b Value | AUC-ROC |
|---|---|---|---|---|---|
| Alanine | 477 [414–544] | 420 [502–568] | −12 | 0.3352 | 0.55 |
| Threonine | 143 [114–161] | 129 [110–148] | −10 | 0.0626 | 0.60 |
| Glycine | 250 [234–279] | 258 [214–299] | +3 | 0.4175 | 0.54 |
| Valine | 280 [261–319] | 277 [238–326] | −1 | 0.5982 | 0.53 |
| Serine | 149 [128–175] | 144 [128–176] | −3 | 0.8966 | 0.51 |
| Sarcosine | 1.87 [1.40–2.34] | 1.35 [1.11–1.79] | −28 | <0.0001 | 0.73 |
| Leucine/isoleucine | 217 [195–252] | 215 [197–246] | −1 | 0.7005 | 0.52 |
| Guanidinoacetate | 3.85 [2.77–4.52] | 3.56 [3.09–4.18] | −8 | 0.7751 | 0.52 |
| Asparagine/aspartate | 69.8 [62.2–76.1] | 67.7 [59.4–77.2] | −3 | 0.4795 | 0.54 |
| Hydroxy-proline | 10.8 [7.26–20.1] | 11.3 [9.51–14.0] | +4 | 0.4870 | 0.54 |
| Proline | 247 [203–290] | 253 [236–304] | +2 | 0.1761 | 0.57 |
| Methionine | 52.2 [44.0–75.1] | 51.7 [45.4–84.1] | −1 | 0.7153 | 0.52 |
| Glutamine/glutamate | 787 [732–855] | 836 [800–905] | +6 | 0.0003 | 0.69 |
| Ornithine/citrulline | 49.8 [46.2–58.0] | 59.6 [49.4–67.5] | +16 | 0.0152 | 0.63 |
| Phenylalanine | 68.9 [62.3–72.2] | 63.6 [56.3–69.6] | −8 | 0.0026 | 0.66 |
| Tyrosine | 69.1 [56.4–78.1] | 59.7 [52.7–63.7] | −14 | 0.0051 | 0.65 |
| Lysine | 201 [147–217] | 179 [162–214] | −11 | 0.2967 | 0.56 |
| Arginine | 98.6 [86.6–112.4] | 103 [85.5–114] | +14 | 0.9796 | 0.50 |
| Homoarginine | 1.27 [1.06–1.68] | 1.68 [1.16–1.97] | +24 | 0.0001 | 0.70 |
| Tryptophan | 44.3 [37.8–51.0] | 40.8 [35.4–45.2] | −8 | 0.0047 | 0.65 |
| ADMA | 0.42 [0.38–0.50] | 0.44 [0.38–0.49] | +5 | 0.8088 | 0.51 |
| GABR | 1.89 [1.63–2.09] | 1.81 [1.47–2.10] | −4 | 0.0921 | 0.59 |
| Creatinine | 118 [97–127] | 106 [91–141] | −10 | 0.4533 | 0.58 |
| Malondialdehyde | 0.319 [0.294–0.415] | 0.284 [0.236–0.351] | −10 | 0.0002 | 0.693 |
| Potassium | 4.63 [4.37–4.86] | 4.50 [4.29–4.71] | −3 | 0.023 | 0.605 |
| Nitrite | 0.88 [0.14–1.06] | 1.47 [1.26–1.80] | +40 | <0.0001 | 0.951 |
| Nitrate | 35.5 [26.7–67.7] | 263 [170–306] | +640 | <0.0001 | 0.866 |
| Nitrate/nitrite ratio (PNOx) | 56.4 [309–203] | 130 [50–227] | +57 | 0.0478 | 0.605 |
| Kgaa (×1000) | 6.9 [6.1–8.6] | 7.7 [6.7–9.4] | +11 | 0.0334 | 0.614 |
| Kharg (×1000) | 3.5 [2.8–5.8] | 4.9 [4.2–6.6] | +29 | <0.0001 | 0.733 |
| Kgaa/Kharg | 1.96 [1.30–3.17] | 1.57 [1.31–2.01] | −20 | 0.0066 | 0.645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikas, D.; Maassen, N.; Thorns, A.; Finkel, A.; Lützow, M.; Röhrig, M.A.; Blau, L.S.; Dimina, L.; Mariotti, F.; Beckmann, B.; et al. Short-Term Supplementation of Sodium Nitrate vs. Sodium Chloride Increases Homoarginine Synthesis in Young Men Independent of Exercise. Int. J. Mol. Sci. 2022, 23, 10649. https://doi.org/10.3390/ijms231810649
Tsikas D, Maassen N, Thorns A, Finkel A, Lützow M, Röhrig MA, Blau LS, Dimina L, Mariotti F, Beckmann B, et al. Short-Term Supplementation of Sodium Nitrate vs. Sodium Chloride Increases Homoarginine Synthesis in Young Men Independent of Exercise. International Journal of Molecular Sciences. 2022; 23(18):10649. https://doi.org/10.3390/ijms231810649
Chicago/Turabian StyleTsikas, Dimitrios, Norbert Maassen, Antonie Thorns, Armin Finkel, Moritz Lützow, Magdalena Aleksandra Röhrig, Larissa Sarah Blau, Laurianne Dimina, François Mariotti, Bibiana Beckmann, and et al. 2022. "Short-Term Supplementation of Sodium Nitrate vs. Sodium Chloride Increases Homoarginine Synthesis in Young Men Independent of Exercise" International Journal of Molecular Sciences 23, no. 18: 10649. https://doi.org/10.3390/ijms231810649
APA StyleTsikas, D., Maassen, N., Thorns, A., Finkel, A., Lützow, M., Röhrig, M. A., Blau, L. S., Dimina, L., Mariotti, F., Beckmann, B., Shushakov, V., & Jantz, M. (2022). Short-Term Supplementation of Sodium Nitrate vs. Sodium Chloride Increases Homoarginine Synthesis in Young Men Independent of Exercise. International Journal of Molecular Sciences, 23(18), 10649. https://doi.org/10.3390/ijms231810649









