Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol
Abstract
1. Introduction
2. Radiotherapy
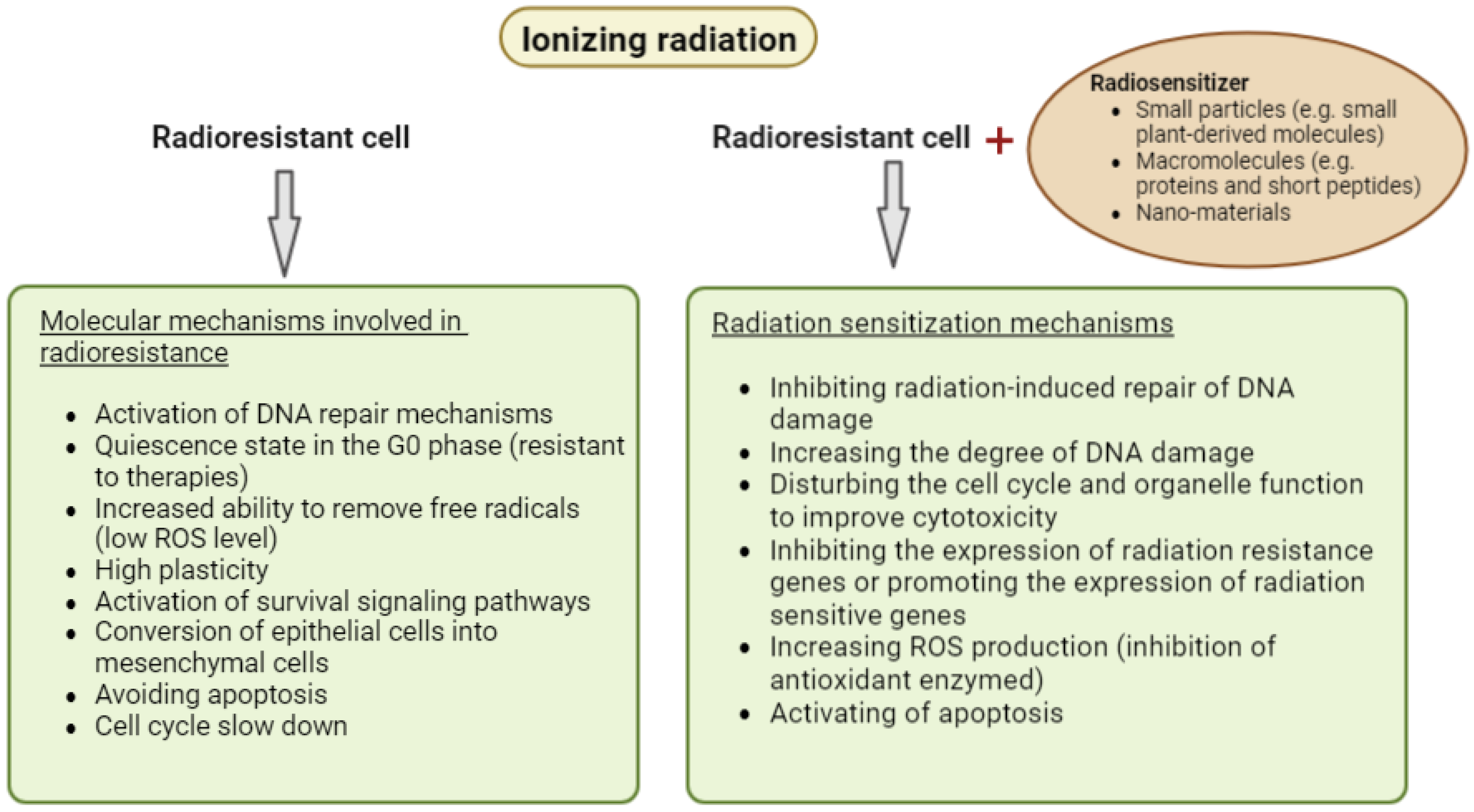
3. Radiosensitizers
4. Resveratrol
4.1. Resveratrol in Longevity and Cancer Therapy in Animal Model Studies
4.2. Clinical Trials of Resveratrol in the Treatment of Cancer and Selected Age-Related Diseases
4.3. Resveratrol as a Radiosensitizer
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, S.C.; Kayamba, V.; Peek, M.R., Jr.; Heimburger, D. Cancer Control in Low- and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Lonati, L.; Barbieri, S.; Guardamagna, I.; Ottolenghi, A.; Baiocco, G. Radiation-induced cell cycle perturbations: A computational tool validated with flow-cytometry data. Sci. Rep. 2021, 11, 925. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 834. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef]
- Harada, H. Hypoxia-inducible factor 1–mediated characteristic features of cancer cells for tumor radioresistance. J. Radiat. Res. 2016, 57, i99–i105. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Vijayappa, S.; Kurimasa, A.; Ogawa, K.; Chen, D.J. Tumor Cell Radiosensitivity Is a Major Determinant of Tumor Response to Radiation. Cancer Res. 2006, 66, 8352–8355. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar]
- Tiwari, P.; Mishra, K.P. Flavonoids sensitize tumor cells to radiation: Molecular mechanisms and relevance to cancer radiotherapy. Int. J. Radiat. Biol. 2020, 96, 360–369. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int. J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef]
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.-T.; Koong, A.C.; Giaccia, A.J.; Yu, B.; et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761. [Google Scholar] [CrossRef]
- Alfonzetti, T.; Yasmin-Karim, S.; Ngwa, W.; Avery, S. Phytoradiotherapy: An Integrative Approach to Cancer Treatment by Combining Radiotherapy With Phytomedicines. Front. Oncol. 2021, 10, 624663. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Zhao, C.; Kang, Q.; Zhang, X.; Suo, K.; Cao, N.; Hao, L.; Lu, J. Potential of natural products as radioprotectors and radiosensitizers: Opportunities and challenges. Food Funct. 2021, 12, 5204–5218. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Rosa, I.M.L. Biodiversity, traditional medicine and public health: Where do they meet? J. Ethnobiol. Ethnomed. 2007, 3, 14. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Priya, S.; Satheeshkumar, P.K. Natural Products from Plants. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–163. [Google Scholar]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciênc. 2019, 91 (Suppl. S3). [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Adams, G.E. CHEMICAL RADIOSENSITIZATION OF HYPOXIC CELLS. Br. Med Bull. 1973, 29, 48–53. [Google Scholar] [CrossRef]
- Fowler, J.F.; Adams, G.E.; Denekamp, J. Radiosensitizers of hypoxic cells in solid tumours. Cancer Treat. Rev. 1976, 3, 227–256. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol. Ther. 2009, 8, 1994–2001. [Google Scholar] [CrossRef]
- Williams, K.J.; Telfer, B.A.; Shannon, A.M.; Babur, M.; Stratford, I.J.; Wedge, S.R. Combining radiotherapy with AZD2171, a potent inhibitor of vascular endothelial growth factor signaling: Pathophysiologic effects and therapeutic benefit. Mol. Cancer Ther. 2007, 6, 599–606. [Google Scholar] [CrossRef][Green Version]
- Schwartz, D.L.; Powis, G.; Thitai-Kumar, A.; He, Y.; Bankson, J.; Williams, R.; Lemos, R.; Oh, J.; Volgin, A.; Soghomonyan, S.; et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol. Cancer Ther. 2009, 8, 947–958. [Google Scholar] [CrossRef]
- Mu, F.; Liu, T.; Zheng, H.; Xie, X.; Lei, T.; He, X.; Du, S.; Tong, R.; Wang, Y. Mangiferin induces radiosensitization in glioblastoma cells by inhibiting nonhomologous end joining. Oncol. Rep. 2018, 40, 3663–3673. [Google Scholar] [CrossRef]
- Wen, C.; Zhou, Y.; Zhou, C.; Zhang, Y.; Hu, X.; Li, J.; Yin, H. Enhanced Radiosensitization Effect of Curcumin Delivered by PVP-PCL Nanoparticle in Lung Cancer. J. Nanomater. 2017, 2017, 9625909. [Google Scholar] [CrossRef]
- Prabakaran, P.J.; Javaid, A.M.; Swick, A.D.; Werner, L.R.; Nickel, K.P.; Sampene, E.; Hu, R.; Ong, I.M.; Bruce, J.Y.; Hartig, G.K.; et al. Radiosensitization of Adenoid Cystic Carcinoma with MDM2 Inhibition. Clin. Cancer Res. 2017, 23, 6044–6053. [Google Scholar] [CrossRef]
- Wang, W.-H.; Shen, C.-Y.; Chien, Y.-C.; Chang, W.-S.; Tsai, C.-W.; Lin, Y.-H.; Hwang, J.-J. Validation of Enhancing Effects of Curcumin on Radiotherapy with F98/FGT Glioblastoma-Bearing Rat Model. Int. J. Mol. Sci. 2020, 21, 4385. [Google Scholar] [CrossRef]
- Mekkawy, M.H.; Fahmy, H.A.; Nada, A.S.; Ali, O.S. Radiosensitizing Effect of Bromelain Using Tumor Mice Model via Ki-67 and PARP-1 Inhibition. Integr. Cancer Ther. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Jia, L.; Ma, S.; Hou, X.; Wang, X.; Qased, A.B.L.; Sun, X.; Liang, N.; Li, H.; Yi, H.; Kong, D.; et al. The Synergistic Effects of Traditional Chinese Herbs and Radiotherapy for Cancer Treatment (Review). Oncol. Lett. 2013, 5, 1439–1447. [Google Scholar]
- Chappie, T.A.; Humphrey, J.M.; Allen, M.P.; Estep, K.G.; Fox, C.B.; Lebel, L.A.; Liras, S.; Marr, E.S.; Menniti, F.S.; Pandit, J.; et al. Discovery of a Series of 6,7-Dimethoxy-4-pyrrolidylquinazoline PDE10A Inhibitors. J. Med. Chem. 2007, 50, 182–185. [Google Scholar] [CrossRef]
- Gomes, D.A.; Joubert, A.M.; Visagie, M.H. In Vitro Effects of Papaverine on Cell Proliferation, Reactive Oxygen Species, and Cell Cycle Progression in Cancer Cells. Molecules 2021, 26, 6388. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef]
- Ai, D.; Ye, J.; Wei, S.; Li, Y.; Luo, H.; Cao, J.; Zhu, Z.; Zhao, W.; Lin, Q.; Yang, H.; et al. Comparison of 3 Paclitaxel-Based Chemoradiotherapy Regimens for Patients With Locally Advanced Esophageal Squamous Cell Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e220120. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, J.; Zhu, Z.; Zhao, W.; Zhou, J.; Wu, C.; Tang, H.; Fan, M.; Li, L.; Lin, Q.; et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J. Clin. Oncol. 2019, 37, 1695–1703. [Google Scholar] [CrossRef]
- Einstein, M.H.; Frimer, M.; Kuo, D.Y.-S.; Reimers, L.L.; Mehta, K.; Mutyala, S.; Huang, G.S.; Hou, J.Y.; Goldberg, G.L. Phase II trial of adjuvant pelvic radiation “sandwiched” between combination paclitaxel and carboplatin in women with uterine papillary serous carcinoma. Gynecol. Oncol. 2012, 124, 21–25. [Google Scholar] [CrossRef]
- Rich, T.; Harris, J.; Abrams, R.; Erickson, B.; Doherty, M.; Paradelo, J.; Small, W.; Safran, H.; Wanebo, H.J. Phase II Study of External Irradiation and Weekly Paclitaxel for Nonmetastatic, Unresectable Pancreatic Cancer: RTOG-98-12. Am. J. Clin. Oncol. Cancer Clin. Trials 2004, 27, 51–56. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Chendil, D.; Ranga, R.S.; Meigooni, D.; Sathishkumar, S.; Ahmed, M.M. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 2004, 23, 1599–1607. [Google Scholar] [CrossRef]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabón, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S. Curcumin Modulates the Radiosensitivity of Colorectal Cancer Cells by Suppressing Constitutive and Inducible NF-κB Activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef]
- Boqiang, C.; Ma, J.; Guo, X.; Sun, J.; Yu, Y.; Cao, B.; Zhang, L.; Ding, X.; Huang, J.; Shao, J. Curcumin Enhances the Radiosensitivity of U87 Cells by Inducing DUSP-2 Up-Regulation. Cell. Physiol. Biochem. 2015, 35, 1381–1393. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef]
- Termini, D.; Hartogh, D.J.D.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytotherapy Res. 2019, 33, 370–378. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, B.; Jin, X.; Li, P.; Zheng, X.; Zhao, T.; Li, F.; Li, Q. Genistein Mediates the Selective Radiosensitizing Effect in NSCLC A549 Cells via Inhibiting Methylation of the Keap1 Gene Promoter Region. Oncotarget 2016, 7, 27267. [Google Scholar]
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Gerszon, J.; Rodacka, A.; Puchała, M. Antioxidant Properties of Resveratrol and its Protective Effects in Neurodegenerative Diseases. Adv. Cell Biol. 2014, 2014, 97–117. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Lu, I.-J.; Fu, Y.-S.; Fang, Y.-P.; Huang, Y.-B.; Wu, P.-C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloid. Surf. B Biointerface. 2016, 148, 650–656. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Magalhães, M.; Pereira-Silva, M.; Caldas, M.; Ferreira, L.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Targeting Cancer Via Resveratrol-Loaded Nanoparticles Administration: Focusing on In Vivo Evidence. AAPS J. 2019, 21, 57. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Wong, Y.T.; Gruber, J.; Jenner, A.M.; Ng, M.P.-E.; Ruan, R.; Tay, F.E.H. Elevation of oxidative-damage biomarkers during aging in F2 hybrid mice: Protection by chronic oral intake of resveratrol. Free Radic. Biol. Med. 2009, 46, 799–809. [Google Scholar] [CrossRef]
- Tung, B.T.; Rodríguez-Bies, E.; Ballesteros-Simarro, M.; Motilva, V.; Navas, P.; López-Lluch, G. Modulation of Endogenous Antioxidant Activity by Resveratrol and Exercise in Mouse Liver is Age Dependent. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 69, 398–409. [Google Scholar] [CrossRef]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Williamson, C.L.; Dabkowski, E.R.; Hollander, J.M.; Alway, S.E. Suppression of Oxidative Stress by Resveratrol After Isometric Contractions in Gastrocnemius Muscles of Aged Mice. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2010, 65, 815–831. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Wang, M.; Lakatta, E.; Sonntag, W.; Ungvari, Z. Age-Associated Proinflammatory Secretory Phenotype in Vascular Smooth Muscle Cells From the Non-human Primate Macaca mulatta: Reversal by Resveratrol Treatment. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2012, 67, 811–820. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.-T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Abraham, J.; Johnson, R. Consuming a Diet Supplemented with Resveratrol Reduced Infection-Related Neuroinflammation and Deficits in Working Memory in Aged Mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef]
- Jeong, S.I.; Shin, J.A.; Cho, S.; Kim, H.W.; Lee, J.Y.; Kang, J.L.; Park, E.-M. Resveratrol attenuates peripheral and brain inflammation and reduces ischemic brain injury in aged female mice. Neurobiol. Aging 2016, 44, 74–84. [Google Scholar] [CrossRef]
- Argüelles, S.; Guerrero-Castilla, A.; Cano, M.; Muñoz, M.F.; Ayala, A. Advantages and disadvantages of apoptosis in the aging process. Ann. New York Acad. Sci. 2019, 1443, 20–33. [Google Scholar] [CrossRef]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, Y.; Hu, X.; Xu, Y. Resveratrol ameliorates neuronal apoptosis and cognitive impairment by activating the SIRT1/RhoA pathway in rats after anesthesia with sevoflurane. Bosn. J. Basic Med Sci. 2022, 22, 110–117. [Google Scholar] [CrossRef]
- Xiong, W.X.; Chai, Z.T.; Wang, B.; Zhou, G.X.; Cang, J.; Xue, Z.G.; Ge, S.J. Resveratrol Alleviates Learning and Memory Impairment in Aged Rats after General Anesthesia with Sevoflurane and Nitrous Oxide via SIRT1-P53 Signaling Pathway. Int. J. Clin. Exp. Med. 2016, 9, 21118–21130. [Google Scholar]
- Ginés, C.; Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Hipólito-Luengo, S.; Alcaide, A.; Ramos, M.; Cercas, E.; Vallejo, S.; Romero, A.; Talero, E.; Sánchez-Ferrer, C.F.; Motilva, V.; Peiró, C. Dual Effects of Resveratrol on Cell Death and Proliferation of Colon Cancer Cells. Nutr. Cancer 2017, 69, 1019–1027. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Koohian, F.; Shanei, A.; Shahbazi-Gahrouei, D.; Hejazi, S.H.; Moradi, M.-T. The Radioprotective Effect of Resveratrol Against Genotoxicity Induced by γ-Irradiation in Mice Blood Lymphocytes. Dose-Response 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol Reduces Radiation-Induced Chromosome Aberration Frequencies in Mouse Bone Marrow Cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Xing, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Fan, S.; Liu, Q.; et al. Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1. Int. J. Mol. Sci. 2014, 15, 5928–5939. [Google Scholar] [CrossRef]
- Said, R.S.; El-Demerdash, E.; Nada, A.S.; Kamal, M. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 2016, 103, 140–150. [Google Scholar] [CrossRef]
- Radwan, R.R.; Karam, H.M. Resveratrol attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR signaling pathway. Environ. Toxicol. 2020, 35, 223–230. [Google Scholar] [CrossRef]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a potential radiation sensitizer for glioma stem cells both in vitro and in vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef]
- Mikami, S.; Ota, I.; Masui, T.; Uchiyama, T.; Okamoto, H.; Kimura, T.; Takasawa, S.; Kitahara, T. Resveratrol-induced REG III expression enhances chemo- and radiosensitivity in head and neck cancer in xenograft mice. Oncol. Rep. 2019, 42, 436–442. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lien, H.-M.; Kao, M.-C.; Lo, U.-G.; Lin, L.-C.; Lin, C.-J.; Chang, S.-J.; Chen, C.-C.; Hsieh, J.-T.; Lin, H.; et al. Sensitization of Radioresistant Prostate Cancer Cells by Resveratrol Isolated from Arachis hypogaea Stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Nguyen, A.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and Colon Cancer. Cancer Manag. Res. 2009, 1, 25. [Google Scholar]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and Its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S. Erratum: Resveratrol Inhibits Proliferation, Induces Apoptosis, and Overcomes Chemoresistance through down-Regulation of STAT3 and Nuclear Factor-ΚB-Regulated Antiapoptotic and Cell Survival Gene Products in Human Multiple Myeloma Cells (Blood (2007) 109. Blood 2013, 122, 1325–1326. [Google Scholar] [CrossRef][Green Version]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jorgensen, J.O.L.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B.; Richelsen, B.; et al. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.-E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer NIH Public Access. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflam. 2017, 14, 1. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A Randomized, Double-Blind, Placebo-Controlled Trial of Resveratrol for Alzheimer Disease. Neurology 2015, 85, 1383–1391. [Google Scholar]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnar, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial 1,2. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef]
- De Ligt, M.; Bergman, M.; Fuentes, R.M.; Essers, H.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; Schrauwen, P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: A randomized trial. Am. J. Clin. Nutr. 2020, 112, 1029–1038. [Google Scholar] [CrossRef]
- de Ligt, M.; Bruls, Y.; Hansen, J.; Habets, M.-F.; Havekes, B.; Nascimento, E.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Lichtenbelt, W.M.; et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Synergism between resveratrol and other phytochemicals: Implications for obesity and osteoporosis. Mol. Nutr. Food Res. 2011, 55, 1177–1185. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Bo, S.; Togliatto, G.; Gambino, R.; Ponzo, V.; Lombardo, G.; Rosato, R.; Cassader, M.; Brizzi, M.F. Impact of sirtuin-1 expression on H3K56 acetylation and oxidative stress: A double-blind randomized controlled trial with resveratrol supplementation. Geol. Rundsch. 2018, 55, 331–340. [Google Scholar] [CrossRef]
- Barazzoni, R.; Palmisano, S.; Cappellari, G.G.; Giuricin, M.; Moretti, E.; Vinci, P.; Semolic, A.; Guarnieri, G.; Zanetti, M.; de Manzini, N. Gastric bypass–induced weight loss alters obesity-associated patterns of plasma pentraxin-3 and systemic inflammatory markers. Surg. Obes. Relat. Dis. 2016, 12, 23–32. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Evangelista, A.; Ciccone, G.; Goitre, I.; Saba, F.; Procopio, M.; Cassader, M.; Gambino, R. Effects of 6 months of resveratrol versus placebo on pentraxin 3 in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Geol. Rundsch. 2017, 54, 499–507. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Fraiz, G.M.; da Conceição, A.R.; Vilela, D.L.D.S.; Rocha, D.M.U.P.; Bressan, J.; Hermsdorff, H.H.M. Can resveratrol modulate sirtuins in obesity and related diseases? A systematic review of randomized controlled trials. Eur. J. Nutr. 2021, 60, 2961–2977. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar] [CrossRef]
- Zordoky, B.N.M.; Robertson, I.M.; Dyck, J.R.B. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2014, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Christopher Glass, A.K.; Witztum, J.L. Atherosclerosis: The Road Ahead Review. Cell 2001, 104, 503–516. [Google Scholar]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-Inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; Conesa, M.T.G.; Tomas-Barberan, F.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.-I.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol Supplementation Does Not Improve Metabolic Function in Nonobese Women with Normal Glucose Tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-Dose Resveratrol Supplementation in Obese Men: An Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R.C. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Shabeeb, D.; Musa, A.E. Resveratrol as an Adjuvant for Normal Tissues Protection and Tumor Sensitization. Curr. Cancer Drug Targets 2019, 20, 130–145. [Google Scholar] [CrossRef]
- Yu, H.; Jove, R. The STATs of cancer—New molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.-H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.-W.; Lee, J.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Chang, Y.-L.; Huang, P.-I.; Chiou, G.-Y.; Tseng, L.-M.; Chiou, S.-H.; Chen, M.-H.; Shih, Y.-H.; Chang, C.-H.; Hsu, C.-C.; et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J. Cell. Physiol. 2012, 227, 976–993. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Schulte, B.A.; Yang, A.; Tang, S.; Wang, G.Y. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int. J. Oncol. 2013, 43, 1999–2006. [Google Scholar] [CrossRef]
- Fang, Y.; Herrick, E.J.; Nicholl, M.B. A Possible Role for Perforin and Granzyme B in Resveratrol-Enhanced Radiosensitivity of Prostate Cancer. J. Androl. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Fang, Y.; DeMarco, V.; Nicholl, M.B. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci. 2012, 103, 1090–1098. [Google Scholar] [CrossRef]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol enhances prostate cancer cell response to ionizing radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat. Oncol. 2011, 6, 144. [Google Scholar] [CrossRef]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef]
- Hannun, Y.A. Functions of Ceramide in Coordinating Cellular Responses to Stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef]
- Johnson, G.E.; Ivanov, V.N.; Hei, T.K. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 2008, 13, 790–802. [Google Scholar] [CrossRef]
- Tak, J.K.; Lee, J.H.; Park, J.-W. Resveratrol and piperine enhance radiosensitivity of tumor cells. BMB Rep. 2012, 45, 242–246. [Google Scholar] [CrossRef]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol Induces Premature Senescence in Lung Cancer Cells via ROS-Mediated DNA Damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef]
- Lele, W.; Lei, L.; Liting, Q. Resveratrol sensitizes A549 cells to irradiation damage via suppression of store-operated calcium entry with Orai1 and STIM1 downregulation. Exp. Ther. Med. 2021, 21, 587. [Google Scholar] [CrossRef]
- Collins, H.E.; Zhu-Mauldin, X.; Marchase, R.B.; Chatham, J.C. STIM1/Orai1-mediated SOCE: Current perspectives and potential roles in cardiac function and pathology. Am. J. Physiol. Circ. Physiol. 2013, 305, H446–H458. [Google Scholar] [CrossRef]
- Lopez, E.; Frischauf, I.; Jardin, I.; Derler, I.; Muik, M.; Cantonero, C.; Salido, G.M.; Smani, T.; Rosado, J.; Redondo, P.C. STIM1 phosphorylation at Y316 modulates its interaction with SARAF and the activation of SOCE and ICRAC. J. Cell Sci. 2019, 132, jcs.226019. [Google Scholar] [CrossRef]
- Bosch-Presegué, L.; Vaquero, A. The Dual Role of Sirtuins in Cancer. Genes Cancer 2011, 2, 648–662. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Zang, W.; Wang, X.; Liu, Z.; Li, W.; Jia, J. Resveratrol Inhibits the Growth of Gastric Cancer by Inducing G1 Phase Arrest and Senescence in a Sirt1-Dependent Manner. PLoS ONE 2013, 8, e70627. [Google Scholar] [CrossRef]
- Ji, K.; Sun, X.; Liu, Y.; Du, L.; Wang, Y.; He, N.; Wang, J.; Xu, C.; Liu, Q. Regulation of Apoptosis and Radiation Sensitization in Lung Cancer Cells via the Sirt1/NF-κB/Smac Pathway. Cell. Physiol. Biochem. 2018, 48, 304–316. [Google Scholar] [CrossRef]
- Voellger, B.; Waldt, N.; Rupa, R.; Kirches, E.; Melhem, O.; Ochel, H.-J.; Mawrin, C.; Firsching, R. Combined effects of resveratrol and radiation in GH3 and TtT/GF pituitary adenoma cells. J. Neuro-Oncology 2018, 139, 573–582. [Google Scholar] [CrossRef]
- Beucken, T.V.D.; Koch, E.; Chu, K.; Rupaimoole, R.; Prickaerts, P.; Adriaens, M.; Voncken, J.W.; Harris, A.; Buffa, F.; Haider, S.; et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat. Commun. 2014, 5, 5203. [Google Scholar] [CrossRef]
- Carnero, A.; Lleonart, M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. BioEssays 2016, 38 (Suppl. S1), S65–S74. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Y.; Lu, Y.; Liu, M.; Li, M.; Li, J.; Wu, L. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci. Rep. 2017, 7, 10592. [Google Scholar] [CrossRef]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef]
- Eckert, F.; Zwirner, K.; Boeke, S.; Thorwarth, D.; Zips, D.; Huber, S.M. Rationale for Combining Radiotherapy and Immune Checkpoint Inhibition for Patients With Hypoxic Tumors. Front. Immunol. 2019, 10, 407. [Google Scholar] [CrossRef]
- Khoei, S.; Shoja, M.; Mostaar, A.; Faeghi, F. Effects of resveratrol and methoxyamine on the radiosensitivity of iododeoxyuridine in U87MG glioblastoma cell line. Exp. Biol. Med. 2016, 241, 1229–1236. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, W.; Wang, X.; Wang, K.; Du, B.; Xiao, J. Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol. Rep. 2017, 37, 1833–1841. [Google Scholar] [CrossRef]
- da Costa Araldi, I.C.; Bordin, F.P.R.; Cadoná, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.M.M.F.; Bauermann, L.D.F. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem. Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef]
- Komorowska, D.; Gajewska, A.; Hikisz, P.; Bartosz, G.; Rodacka, A. Comparison of the Effects of Resveratrol and Its Derivatives on the Radiation Response of MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9511. [Google Scholar] [CrossRef]
- Amini, P.; Nodooshan, S.J.; Ashrafizadeh, M.; Eftekhari, S.-M.; Aryafar, T.; Khalafi, L.; Musa, A.E.; Mahdavi, S.R.; Najafi, M.; Farhood, B. Resveratrol Induces Apoptosis and Attenuates Proliferation of MCF-7 Cells in Combination with Radiation and Hyperthermia. Curr. Mol. Med. 2020, 21, 142–150. [Google Scholar] [CrossRef]


| Identifier | Natural Compound | Tumor Type | No. of Patients | Phase of Clinical Trials | Drug Used in Combination with Radiotherapy |
|---|---|---|---|---|---|
| NCT03824327 | Papaverine | Non-Small Cell Lung Cancer or Lung Metastases | 24 | Phase I | Papaverine |
| NCT05136846 | Papaverine and Paclitaxel | Locally Advanced Non-Small Cell Lung Cancer | 28 | Phase I | Carboplatin + Paclitaxel + Durvalumab and Papaverine |
| NCT02459457 | Paclitaxel | Local Advanced Esophageal Cancer | 321 | Phase III | Group 1: Paclitaxel + Cisplatin Group 2: Paclitaxel + Fluorouracil Group 3: Paclitaxel + Carboplatin |
| NCT01591135 | Paclitaxel | Locally Advanced Esophageal Carcinoma | 436 | Phase III | Group 1: Paclitaxel + 5-fluorouracil Group 2: Cisplatin + 5-fluorouracil |
| NCT02280252 | Paclitaxel | Breast Cancer | 69 | Phase II | Paclitaxel |
| NCT00238420 | Paclitaxel | Bladder Cancer | 70 | Phase I/II | Paclitaxel + Trastuzumab |
| NCT00231868 | Paclitaxel | Uterine Cancer | 81 | Phase II | Carboplatin + Paclitaxel |
| NCT00003591 | Paclitaxel | Pancreatic Cancer | 122 | Phase II | Paclitaxel |
| NCT02724618 | Curcumin | Prostate Cancer | 64 | Phase II | Nanocurcumin |
| NCT01917890 | Curcumin | Prostate Cancer | 40 | Completed | Curcumin |
| NCT00745134 | Curcumin | Rectal Cancer | 45 | Phase II | Capecitabine + Curcumin |
| NCT04294836 | Curcumin | Cervical Cancer | 240 | Phase II | Cisplatin + Curcumin |
| NCT02075112 | Genistein | Advanced Squamous Cell Carcinoma of the Head and Neck | 24 | Phase I | Soy isoflavone + Cisplatin |
| NCT00769990 | Genistein | Bone Metastases | 0 | Phase I/II | Genistein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorowska, D.; Radzik, T.; Kalenik, S.; Rodacka, A. Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol. Int. J. Mol. Sci. 2022, 23, 10627. https://doi.org/10.3390/ijms231810627
Komorowska D, Radzik T, Kalenik S, Rodacka A. Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol. International Journal of Molecular Sciences. 2022; 23(18):10627. https://doi.org/10.3390/ijms231810627
Chicago/Turabian StyleKomorowska, Dominika, Tomasz Radzik, Sebastian Kalenik, and Aleksandra Rodacka. 2022. "Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol" International Journal of Molecular Sciences 23, no. 18: 10627. https://doi.org/10.3390/ijms231810627
APA StyleKomorowska, D., Radzik, T., Kalenik, S., & Rodacka, A. (2022). Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol. International Journal of Molecular Sciences, 23(18), 10627. https://doi.org/10.3390/ijms231810627






