Abstract
DNA methylation of both viral and host DNA is one of the major mechanisms involved in the development of Epstein–Barr virus-associated gastric carcinoma (EBVaGC); thus, epigenetic treatment using demethylating agents would seem to be promising. We have verified the effect of MC180295, which was discovered by screening for demethylating agents. MC180295 inhibited cell growth of the EBVaGC cell lines YCCEL1 and SNU719 in a dose-dependent manner. In a cell cycle analysis, growth arrest and apoptosis were observed in both YCCEL1 and SNU719 cells treated with MC180295. MKN28 cells infected with EBV were sensitive to MC180295 and showed more significant inhibition of cell growth compared to controls without EBV infection. Serial analysis of gene expression analysis showed the expression of genes belonging to the role of BRCA1 in DNA damage response and cell cycle control chromosomal replication to be significantly reduced after MC180295 treatment. We confirmed with quantitative PCR that the expression levels of BRCA2, FANCM, RAD51, TOP2A, and CDC45 were significantly decreased by MC180295. LMP1 and BZLF1 are EBV genes with expression that is epigenetically regulated, and MC180295 could up-regulate their expression. In conclusion, MC180295 inhibited the growth of EBVaGC cells by suppressing DNA repair and the cell cycle.
1. Introduction
Epstein–Barr virus (EBV) is a ubiquitous human herpes virus closely associated with both lymphoid and epithelial malignancies, and its association with nasopharyngeal carcinoma and gastric carcinoma is well-established [1]. Gastric cancer was responsible for over one million new cases of cancer in 2020 and an estimated 769,000 deaths, ranking fifth for incidence and fourth for mortality globally [2]. About 9% of gastric cancers have been identified as EBV-positive. Thus, EBV-associated gastric carcinoma (EBVaGC) is the most common cancer among EBV-related malignancies [3].
The prognosis for unresectable recurrent gastric cancer is extremely poor. Chemotherapy for gastric cancer is comprised mainly of cytotoxic agents, and molecularly targeted agents are still rare. Only programmed cell death 1 (PD-1) and human epidermal growth factor receptor 2 have recently become new targets in advanced gastric cancer [4]. In addition, the tumor microenvironment has emerged as a potential therapeutic target in several malignancies including gastric cancer [5]. Several agents targeting the tumor microenvironment are currently under assessment in both preclinical and clinical studies. The development of chemotherapy based on the pathogenesis of gastric cancer is clearly desired.
The Cancer Genome Atlas (TCGA) divides gastric adenocarcinomas into four molecular subtypes: (1) EBVaGC, (2) microsatellite instability (MSI), (3) chromosomal instability, and (4) genomically stable tumors. The characteristics of EBVaGC are reported to include the harboring of recurrent PIK3CA mutations, DNA hypermethylation, amplification of JAK2, and overexpression of PD-L1 and PD-L2 [6]. Viral DNA methylation regulates EBV latency type and inhibits the expression of EBV latent genes that are possible targets of cytotoxic T lymphocytes [7,8]. Methylation of host cell DNA inactivates tumor suppressor genes and tumor-associated antigens. Methylation of both viral and host DNA is one of the major mechanisms involved in the development of EBVaGC [9,10].
MC180295 is an aminothiazole compound that was discovered by screening for demethylating agents. Zhang et al. reported that MC180295 acted as a specific inhibitor of cyclin dependent kinase 9 (CDK9) and reactivated epigenetically silenced genes in multiple cancer cells [11]. We verified the effect of this new CDK9 inhibitor, MC180295, on EBVaGC in this study.
2. Results
2.1. Growth Inhibition by MC180295 in EBV-Associated Gastric Cancer Cells
MC180295 inhibited cell growth of the EBVaGC cell lines YCCEL1 and SNU719 in a dose-dependent manner. The inhibition of cell growth of YCCEL1 by MC180295 was significant at 4 days after exposure to 1 μM MC180295 (Figure 1a). The inhibition of cell growth of SNU719 by MC180295 was significant at 2 to 4 days after exposure to 1 μM MC180295 (Figure 1b). MKN28 cells infected with EBV were sensitive to MC180295 and showed more significant inhibition of cell growth compared to EBV-uninfected MKN28 cells and controls treated with DMSO (Figure 1c).
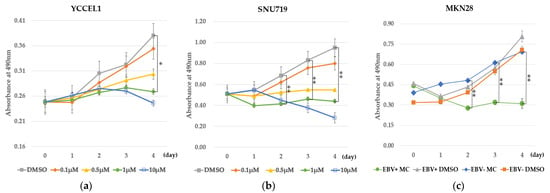
Figure 1.
MC180295 inhibits cell growth in EBV-positive gastric cancer cells. (a) The inhibition of cell growth of YCCEL1 by MC180295 was significant at 4 days after exposure to 1 μM MC180295. (b) The inhibition of cell growth of SNU719 by MC180295 was significant at 2 to 4 days after exposure to 1 μM MC180295. (c) EBV-positive MKN28 cells showed marked growth inhibition by 1 μM MC180295, compared to EBV-uninfected MKN28 cells and controls treated with DMSO. The y-axis represents absorbance at 490 nm in the MTS assay. Data were obtained from three independent experiments. Bars denote SE (n = 3), * p < 0.05. ** p < 0.01.
In a cell cycle analysis, a concomitant decrease in the proportion of cells in the S phase was observed in both SNU719 and YCCEL1 cells treated with MC180295: from 15.6% to 5.4% in SNU719 and from 31.0% to 9.1% in YCCEL1. The sub-G1 fraction increased from 0.9% to 12.6% in SNU719 cells and from 1.5% to 21.0% in YCCEL1 at 48 h after MC180295 treatment. In YCCEL1 cells, the percentage of cells in the sub-G1 fraction increased to 26.4% at 72 h after MC180295 treatment (Figure 2).
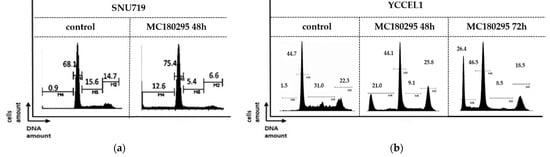
Figure 2.
Cell cycle analysis after MC180295 treatment in SNU719 (a) and YCCEL1 (b). The percentage of cells in S phase decreased, and those in the sub-G1 fraction increased at 48 h after treatment. In YCCEL1 cells, the percentage of cells in the sub-G1 fraction increased to 26.4% at 72 h after MC180295 treatment.
2.2. Patterns of Gene Expression after MC180295 Treatment
The results of serial analysis of gene expression (SAGE) were integrated by Ingenuity Pathways Analysis. We found two pathways in which gene expression varied significantly after MC180295 treatment. The expressions of genes belonging to the pathways “role of BRCA1 in DNA damage response” and “cell cycle control chromosomal replication” were significantly reduced in both YCCEL1 and SNU719 cells. Table 1 shows the change of gene expression of the pathway “role of BRCA1 in DNA damage response”. We selected FANCM, BRCA2, and RAD51 for real-time PCR. Table 2 shows the change of gene expression of the pathway “cell cycle control of chromosomal replication”. The genes down-regulated by MC180295 were common in both YCCEL1 and SNU719 cells. We selected TOP2A and CDC45 for real-time PCR.

Table 1.
Change of gene expression of pathway “role of BRCA1 in DNA damage response”.

Table 2.
Change of gene expression of pathway “cell cycle control of chromosomal replication”.
In quantitative PCR, the expression levels of BRCA2, FANCM, and RAD51 as well as those of TOP2A and CDC45, decreased in both the YCCEL1 and SNU719 cells treated with MC180295 (Figure 3).
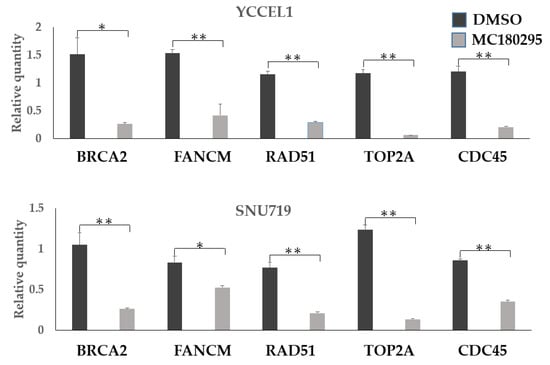
Figure 3.
Gene expression levels after MC180295 treatment. The expression levels of BRCA2, FANCM, and RAD51 decreased in both YCCEL1 and SNU719 cells treated with MC180295, as well as those of TOP2A and CDC45. Data were obtained from three independent experiments. Bars denote SE (n = 3), * p < 0.05. ** p < 0.01.
2.3. Protein Expression of γH2A Histone Family Member X(γH2AX) and Caspase-3 after MC180295 Treatment
Expression of proteins involved in DNA repair and cell cycle regulation were investigated. In SNU719 cells treated with MC180295, expression of γH2A histone family member X (γH2AX) showed an increase up to 24 h but showed a slight decrease at 48 h. In YCCEL1 cells, MC180295 treatment increased γH2AX expression up to 48 h. In SNU719 cells treated with MC180295, expression of cleaved caspase-3 showed an increase up to 24 h but showed a slight decrease at 48 h. In YCCEL1 cells, MC180295 treatment increased cleaved caspase-3 expression at 48 h (Figure 4).
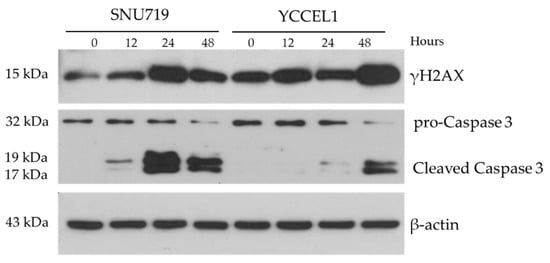
Figure 4.
Protein Expression of γH2A histone family member X (γH2AX) and caspase-3 after MC180295 treatment. The expression levels of γH2AX and cleaved caspase-3 were up-regulated in both YCCEL1 and SNU719 cells treated with MC180295.
2.4. Expression of EBV Genes LMP1 and BZLF1 after MC180295 Treatment
The mRNA expression levels of LMP1 and BZLF1 were increased by 1 μM MC180295 in both the YCCEL1 and SNU719 cells (Figure 5).
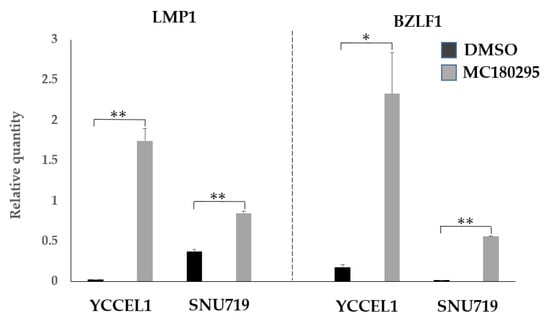
Figure 5.
EBV gene expression after MC180295 treatment. The expression levels of LMP1 and BZLF1 were up-regulated in both YCCEL1 and SNU719 cells treated with MC180295. Data were obtained from three independent experiments. Bars denote SE (n = 3), * p < 0.05. ** p < 0.01.
3. Discussion
Helicobacter pylori and EBV are recognized as risk factors for gastric cancers. Infection by both induces epigenetic alterations in gastric mucosal cells and promotes the development of gastric cancer [12]. Thus, epigenetic treatment using demethylating agents would seem to be promising. There are few studies on clinical trials of demethylating agents for gastric cancers. A Phase I trial using 5-azacitidine prior to neoadjuvant chemotherapy was reported in 2017 and showed the overall response rate to be 67%, with 25% of patients achieving complete response [13]. A Phase II trial is scheduled, which uses a modified regimen. Another phase II clinical trial combining histone deacetylase (HDAC) inhibitor SAHA could not show improvement in the clinical outcome of the patients with metastatic or unresectable gastric cancer [14]. Thus, epigenetic strategies for gastric cancer treatment have not been fully achieved.
MC180295 is an aminothiazole compound found by screening for demethylating agents [11]. MC180295 can reactivate epigenetically silenced genes by remodeling chromatin but without affecting DNA methylation, as with HDAC inhibitors. The expression pattern of the genes induced by MC180295 was broadly similar to what was seen with DNA methyltransferase (DNMT) inhibition [11]. Through TCGA molecular classification, both EBVaGC and the MSI subtype were identified as CpG island methylator phenotype (CIMP) [6]. Thus, MC180295 could be a novel candidate for treatment of gastric cancer with CIMP. We showed growth inhibition of EBVaGC cell lines by MC180295 treatment. Some cells treated with MC180295 were arrested, and apoptosis was induced. To our knowledge, this study is the first report to reveal the effectiveness of MC180295 in inhibiting tumor cell proliferation in EBVaGC. A clinical study is underway to evaluate its safety. If MC180295 can be administered to patients with EBVaGC, it may restore expression of tumor suppressor genes with expression that is suppressed by DNA methylation and histone modification [9]. Tumor-associated antigens are also down-regulated in EBVaGC through epigenetic mechanisms [10], and restoration of their expression would be an attractive approach for possible anti-tumor immunity or for combination therapy with immune checkpoint inhibitors. We believe that this would be a major advance in the chemotherapy of unresectable EBVaGC.
We analyzed patterns of gene expression after MC180295 treatment in EBVaGC. Common pathways were significantly suppressed in both the SNU719 and YCCEL1 cell lines. BRCA2 and RAD51, which cooperate with BRCA1 for homologous recombination [15], were significantly down-regulated by MC180295. As BRCA1 recruitment to damaged DNA sites is dependent on CDK9 [16], MC180295 might interfere with the repair of double-strand DNA breaks by acting as a CDK9 inhibitor.
BRCA1- and BRCA2-deficient cells are sensitive to poly (ADP-ribose) polymerase (PARP) inhibitors [17]. PARP is a key regulator in the base excision repair process and participates in the repair of single-strand DNA breaks. Loss of PARP activity is likely to cause the accumulation of single-strand breaks, which are converted to double-strand DNA breaks during replication or homologous recombination repair [18,19]. The increase in damaged DNA results in the lethality of BRCA1- or BRCA2-deficient cells. Since MC180295 could suppress the expression of BRCA1 and BRCA2, DNA damage response for double-stranded DNA breaks was disrupted in the MC180295-treated EBVaGC cells, indicating that the combination of MC190285 and a PARP inhibitor might lead to cancer-cell-specific death.
The genes associated with DNA replication, including TOP2A and CDC45, were down-regulated after MC180295. TOP2A loosens double-stranded DNA by changing the helical structure in processes of DNA replication and transcription [20]. CDC45 plays a central role in the regulation of the initiation and elongation stages of chromosomal DNA replication [21]. Knockdown of TOP2A and CDC45 has been shown to suppress the growth of various cancer cells [22,23]. Cell cycle analysis showed a decrease in the S phase and G1 arrest after MC180295 treatment. These results are thought to be due to the inhibition of DNA replication.
Expression of proteins involved in DNA repair and cell cycle regulation were investigated. γH2AX is a sensitive marker of DNA damage response [24,25]. Caspase-3 plays important role in the process of apoptosis, and detection of cleaved caspase-3 indicates the occurrence of apoptosis [25]. The expression of cleaved caspase-3 and γH2AX increased in a similar fashion after MC180295 treatment. The result showed that the data from the cell cycle analysis were convincing.
Induction of the lytic cycle is one of the therapeutic targets for EBV-related malignancies. BZLF1 is a transcriptional activator that binds to AP-1-like motifs in the promoters of early lytic genes and induces lytic infection in latently EBV-infected cells [26]. BZLF1 is epigenetically regulated, and demethylating agents and HDAC inhibitors are known to induce BZLF1 expression in EBV-carrying cells [27]. LMP1 is also a viral gene with expression that is epigenetically regulated [28]. BZLF1 has been shown to be a binding partner of several DNA damage response proteins, such as 53BP1, Ku80, p53, and RNF8 [29,30,31,32,33]. Moreover, screening of the EBV proteins that inhibit DNA damage response identified EBV BKRF4 as a DNA damage response inhibitor. Since BKRF4 was expressed in both latent and lytic EBV infections, several EBV genes expressed during both lytic and latent infection might be involved with DNA damage response [34].
The limitation of this study is that we only examined the growth-inhibitory effect of MC180295 in EBVaGC lines in vitro. In the future, we are currently planning to investigate the effect of MC180295 on cancer cell lines transplanted into immunodeficient mice.
4. Materials and Methods
4.1. Cell Culture
The EBV-associated gastric cancer cell lines YCCEL1 and SNU719 were cultured in medium with 10% FBS. YCCEL1 cells were seeded at a density of 5 × 103 cells/mL onto 96-well dishes, and SNU719 cells were seeded at a density of 1 × 104 cells/mL onto 96-well dishes. Cells were treated with MC180295 (Selleck Chemicals, Houston, TX, USA) or DMSO as a control. MC180295 was diluted to medium at concentrations of 10 μM, 1 μM, 0.5 μM, and 0.1 μM. Cell viability was analyzed by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. MTS was added to the well, and the absorbance was measured at 490 nm. Data were obtained from three independent experiments.
We previously infected EBV recombinants that carried NeoR to the EBV-negative gastric carcinoma cell line MKN28 [35]. MKN28 cells infected with EBV and uninfected control cells were treated with 1 μM MC180295 for 48 h. The harvested cells were then seeded into 96-well plates and cultured in medium containing 1 μM MC180295, and cell proliferation was assessed by MTS.
4.2. Cell Cycle Analysis
Cell cycle analysis was performed for cells treated in the presence or absence of 1 μM MC180295 for 48 h or 72 h. For measurement of DNA content, treated cells were collected and fixed with 70% ethanol. After resuspension, fixed cells were washed and incubated with propidium iodide and RNase. After staining, flow cytometry was used to analyze the cell cycle phase.
4.3. RNA Extraction and SAGE Analysis
The total RNA of cells treated in the presence or absence of 1 μM MC180295 for 48 h was isolated using an All Prep DNA/RNA Mini Kit (Qiagen, Hilden, Germany), in accordance with the protocol of the manufacturer. For SAGE, we cultured YCCEL1 and SNU719 cells and treated them with 1 μM MC180295. After incubation for 48 h, mRNA was extracted from the cells. We synthesized cDNA from mRNA using reverse transcriptase. Gene expression was then comprehensively investigated from the obtained cDNA by SAGE. Based on the results of Ingenuity Pathways Analysis by SAGE, the expression levels of the genes that changed significantly were individually quantified by real-time PCR.
4.4. Real-Time PCR
Real-time RT-PCR was performed with Applied Biosystems SYBR Green I using a LightCycler (Roche Diagnostics, Indianapolis, IN, USA) to quantify the mRNA. The primer sequences of FANCM, BRCA2, RAD51, TOP2A, CDC45, LMP1, BZLF1, and β-actin are listed in Table 3. Data were calculated as a ratio in reference to the mRNA of β-actin. Data were obtained from three independent experiments.

Table 3.
Primers for quantitative real-time PCR.
4.5. Western Blot for γH2AX and Caspase-3
SNU719 or YCCEL1 cells were treated with 1 μM of MC180295 and lysed by RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with protease inhibitor (cOmplete mini, Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitor (PhosStop, Sigma, St. Louis, MO, USA). Five μg of protein samples were electrophoresed on 15% SDS-page gel and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked by BlockAce or Tris buffered saline with 0.01% tween 20 and 5% bovine serum albumin. Then, the membranes were incubated with antibodies specific to anti-phospho-histone H2A.X (Ser139) (γH2AX) (#2577; Cell Signaling Technology, Danvers, MA, USA), anti-caspase-3 (W20054B; Biolegend, San Diego, CA, USA), anti-cleaved caspase-3 (Asp175) (5A1E; Cell Signaling, Danvers, MA, USA), and anti-β-actin (AC-15, Sigma, St. Louis, MO, USA). After washing, the membranes were incubated with horseradish peroxidase (HRP)-linked anti-rabbit IgG (Cell Signaling, Danvers, MA, USA), HRP-linked anti-rat IgG (Beckman Coulter, Brea, CA, USA), and HRP-linked anti-mouse IgG (Cell Signaling, Danvers, MA, USA). Specific bands were visualized using an Immobilon (Millipore, Billerica, MA, USA) and were detected by X-ray film. Anti-β-actin antibody was used as an internal control.
4.6. Statistical Analysis
Results were analyzed statistically by the Student’s t-test, and a value of p < 0.05 was considered to indicate statistical significance. Analysis was performed using StatFlex version 6.0 statistical software (Artech Co., Ltd., Osaka, Japan).
5. Conclusions
MC180295 inhibited the growth of EBVaGC cells by suppressing DNA repair and the cell cycle.
Author Contributions
Conceptualization, T.F. and J.N. (Jun Nishikawa); methodology, S.F., N.K., K.S. and K.F.; investigation, J.N. (Junzo Nojima), R.O., A.G., K.H., S.H., A.P.W. and H.I.; supervision, H.Y., Y.S., T.Y. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by JSPS KAKENHI Grant No. 18K07974.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are very grateful to Tomomi Hoshida of Yamaguchi University Hospital for their invaluable help in analyzing the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zur Hausen, H.; Schulte-Holthausen, H.; Klein, G.; Henle, W.; Henle, G.; Clifford, P.; Santesson, L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970, 228, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009, 137, 824–833. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Rojas Llimpe, F.L.; Di Fabio, F.; De Biase, D.; Rihawi, K. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER paradigm shift? Cancers 2021, 13, 1664. [Google Scholar] [CrossRef]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: Novel translational implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Masucci, M.G.; Contreras-Salazar, B.; Ragnar, E.; Falk, K.; Minarovits, J.; Ernberg, I.; Klein, G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt’s lymphoma line rael. J. Virol. 1989, 63, 3135–3141. [Google Scholar] [CrossRef]
- Imai, S.; Koizumi, S.; Sugiura, M.; Tokunaga, M.; Uemura, Y.; Yamamoto, N.; Tanaka, S.; Sato, E.; Osato, T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9131–9135. [Google Scholar] [CrossRef]
- Kaneda, A.; Matsusaka, K.; Aburatani, H.; Fukayama, M. Epstein-Barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 2012, 72, 3445–3450. [Google Scholar] [CrossRef]
- Nishikawa, J.; Iizasa, H.; Yoshiyama, H.; Nakamura, M.; Saito, M.; Sasaki, S.; Shimokuri, K.; Yanagihara, M.; Sakai, K.; Suehiro, Y.; et al. The role of epigenetic regulation in Epstein-Barr virus-associated gastric cancer. Int. J. Mol. Sci. 2017, 18, 1606. [Google Scholar] [CrossRef]
- Zhang, H.; Pandey, S.; Travers, M.; Sun, H.; Morton, G.; Madzo, J.; Chung, W.; Khowsathit, J.; Perez-Leal, O.; Barrero, C.A.; et al. Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell 2018, 175, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.; Ushijima, T.; Tan, P. How to stomach an epigenetic insult: The gastric cancer epigenome. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Shah, M.A.; Klute, K.; Ocean, A.; Popa, E.; Altorki, N.; Lieberman, M.; Schreiner, A.; Yantiss, R.; Christos, P.J.; et al. Phase I study of epigenetic priming with azacitidine prior to standard neoadjuvant chemotherapy for patients with resectable gastric and esophageal adenocarcinoma: Evidence of tumor hypomethylation as an indicator of major histopathologic response. Clin. Cancer Res. 2017, 23, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Ryu, M.H.; Na, Y.S.; Ryoo, B.Y.; Lee, C.W.; Kang, Y.K. Vorinostat in combination with capecitabine plus cisplatin as a first-line chemotherapy for patients with metastatic or unresectable gastric cancer: Phase II study and biomarker analysis. Br. J. Cancer 2016, 114, 1185–1190. [Google Scholar] [CrossRef]
- Zhao, W.; Steinfeld, J.B.; Liang, F.; Chen, X.; Maranon, D.G.; Jian Ma, C.; Kwon, Y.; Rao, T.; Wang, W.; Sheng, C.; et al. BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature 2017, 550, 360–365. [Google Scholar] [CrossRef]
- Nepomuceno, T.C.; Fernandes, V.C.; Gomes, T.T.; Carvalho, R.S.; Suarez-Kurtz, G.; Monteiro, A.N.; Carvalho, M.A. BRCA1 recruitment to damaged DNA sites is dependent on CDK9. Cell Cycle 2017, 16, 665–672. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Godon, C.; Cordelières, F.P.; Biard, D.; Giocanti, N.; Mégnin-Chanet, F.; Hall, J.; Favaudon, V. PARP inhibition versus PARP-1 silencing: Different outcomes in terms of single-strand break repair and radiation susceptibility. Nucleic Acids Res. 2008, 36, 4454–4464. [Google Scholar] [CrossRef]
- Schultz, N.; Lopez, E.; Saleh-Gohari, N.; Helleday, T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003, 31, 4959–4964. [Google Scholar] [CrossRef]
- Keszthelyi, A.; Minchell, N.E.; Baxter, J. The causes and consequences of topological stress during DNA replication. Genes 2016, 7, 134. [Google Scholar] [CrossRef]
- Broderick, R.; Nasheuer, H.P. Regulation of Cdc45 in the cell cycle and after DNA damage. Biochem. Soc. Trans. 2009, 37, 926–930. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Zhao, J.; Bai, J.H. Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A. J. Cell. Biochem. 2018, 119, 7256–7263. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Yang, Y.; He, J.; Bai, J.; Zheng, Y.; Luo, Z. Knockdown of circular RNA hsa_circ_0062270 suppresses the progression of melanoma via downregulation of CDC45. Histol. Histopathol. 2022, 37, 373–383. [Google Scholar] [PubMed]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Plesca, D.; Mazumder, S.; Almasan, A. DNA damage response and apoptosis. Methods Enzymol. 2008, 446, 107–122. [Google Scholar]
- Kalla, M.; Schmeinck, A.; Bergbauer, M.; Pich, D.; Hammerschmidt, W. AP-1 homolog BZLF1 of Epstein-Barr virus has two essential functions dependent on the epigenetic state of the viral genome. Proc. Natl. Acad. Sci. USA 2010, 107, 850–855. [Google Scholar] [CrossRef]
- Hagemeier, S.R.; Barlow, E.A.; Meng, Q.; Kenney, S.C. The cellular ataxia telangiectasia-mutated kinase promotes Epstein-Barr virus lytic reactivation in response to multiple different types of lytic reactivation-inducing stimuli. J. Virol. 2012, 86, 13360–13370. [Google Scholar] [CrossRef]
- Hu, L.F.; Minarovits, J.; Cao, S.L.; Contreras-Salazar, B.; Rymo, L.; Falk, K.; Klein, G.; Ernberg, I. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5′-flanking region. J. Virol. 1991, 65, 1558–1567. [Google Scholar] [CrossRef]
- Bailey, S.G.; Verrall, E.; Schelcher, C.; Rhie, A.; Doherty, A.J.; Sinclair, A.J. Functional interaction between Epstein-Barr virus replication protein Zta and host DNA damage response protein 53BP1. J. Virol. 2009, 83, 11116–11122. [Google Scholar] [CrossRef]
- Chen, C.C.; Yang, Y.C.; Wang, W.H.; Chen, C.S.; Chang, L.K. Enhancement of Zta-activated lytic transcription of Epstein-Barr virus by Ku80. J. Gen. Virol. 2011, 92, 661–668. [Google Scholar] [CrossRef]
- Dreyfus, D.H.; Liu, Y.; Ghoda, L.Y.; Chang, J.T. Analysis of an ankyrin-like region in Epstein Barr Virus encoded (EBV) BZLF-1 (ZEBRA) protein: Implications for interactions with NF-κB and p53. Virol. J. 2011, 8, 422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, Y.; Shirata, N.; Kudoh, A.; Iwahori, S.; Nakayama, S.; Murata, T.; Isomura, H.; Nishiyama, Y.; Tsurumi, T. Expression of Epstein-Barr virus BZLF1 immediate-early protein induces p53 degradation independent of MDM2, leading to repression of p53-mediated transcription. Virology 2009, 388, 204–211. [Google Scholar] [CrossRef]
- Yang, J.; Deng, W.; Hau, P.M.; Liu, J.; Lau, V.M.; Cheung, A.L.; Huen, M.S.; Tsao, S.W. Epstein-Barr virus BZLF1 protein impairs accumulation of host DNA damage proteins at damage sites in response to DNA damage. Lab. Investig. 2015, 95, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Sitz, J.; Shen, Q.; Leblanc-Lacroix, A.; Campos, E.I.; Borozan, I.; Marcon, E.; Greenblatt, J.; Fradet-Turcotte, A.; Jin, D.Y.; et al. A Screen for Epstein-Barr Virus Proteins That Inhibit the DNA Damage Response Reveals a Novel Histone Binding Protein. J. Virol. 2018, 92, e00262-18. [Google Scholar] [CrossRef]
- Yoshiyama, H.; Imai, S.; Shimizu, N.; Takada, K. Epstein-Barr virus infection of human gastric carcinoma cells: Implication of the existence of a new virus receptor different from CD21. J. Virol. 1997, 71, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).