The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures
Abstract
1. Introduction
2. Results
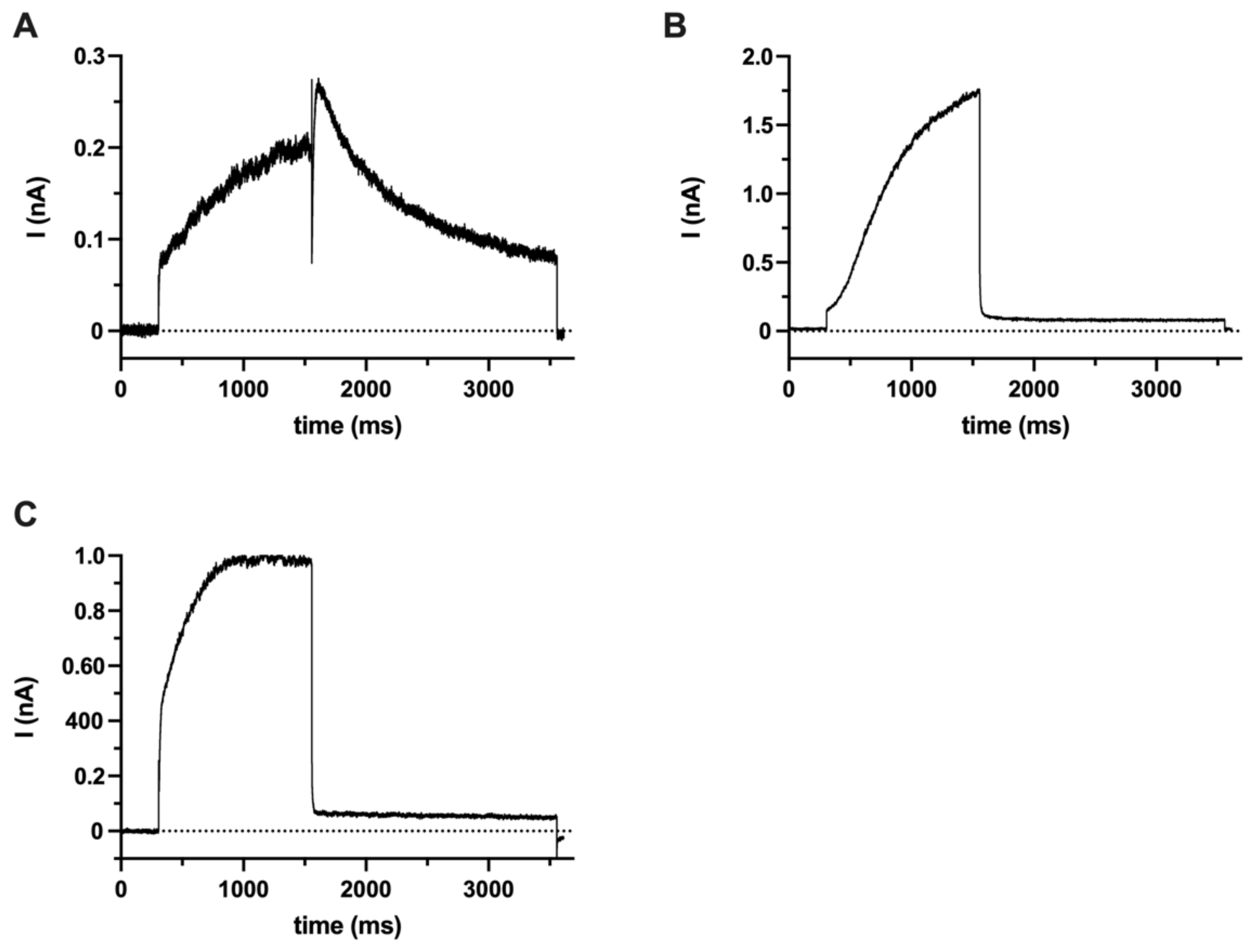
2.1. hEag1 (Kv10.1) Is Expressed in SaOS-2 and MG-63 Cells
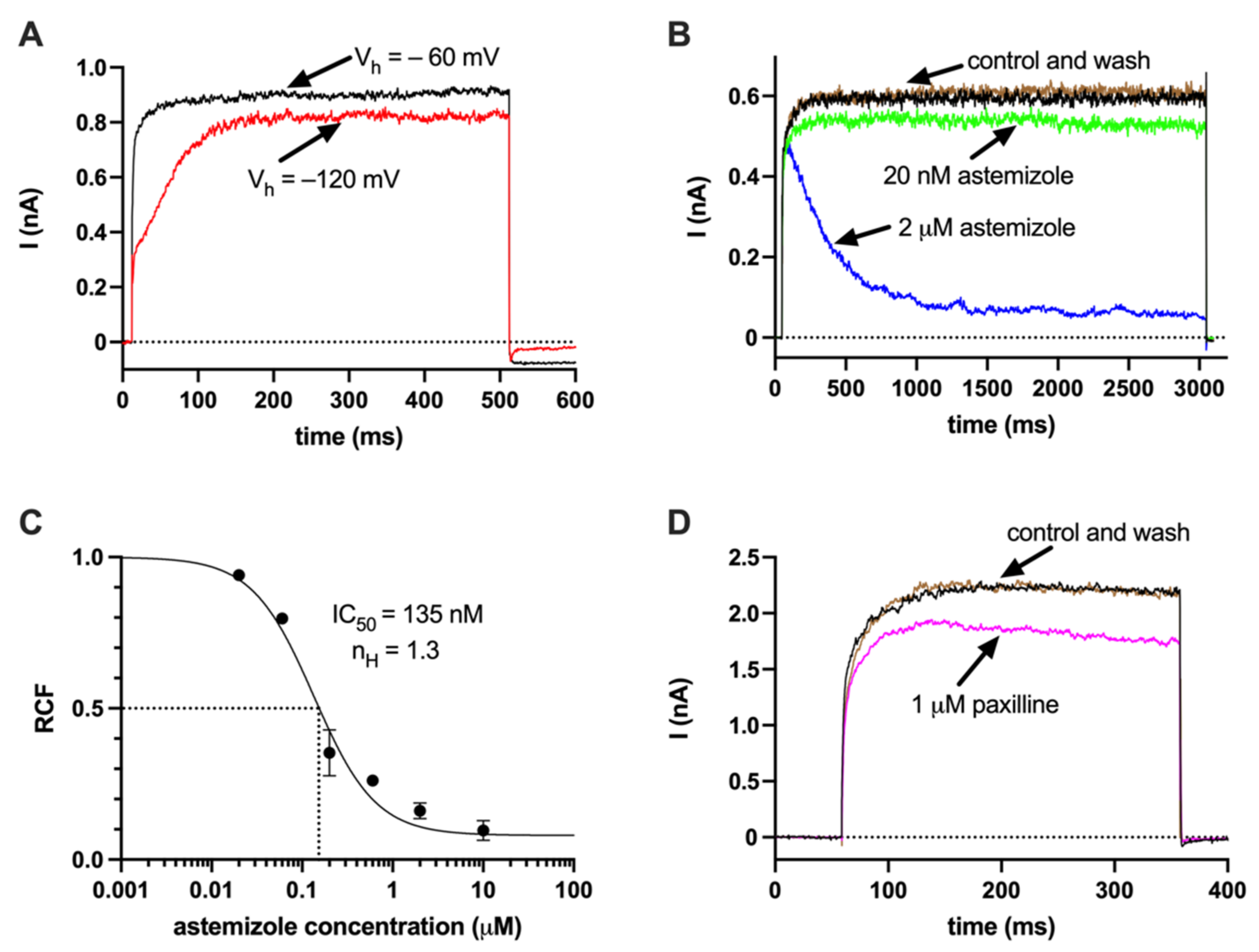
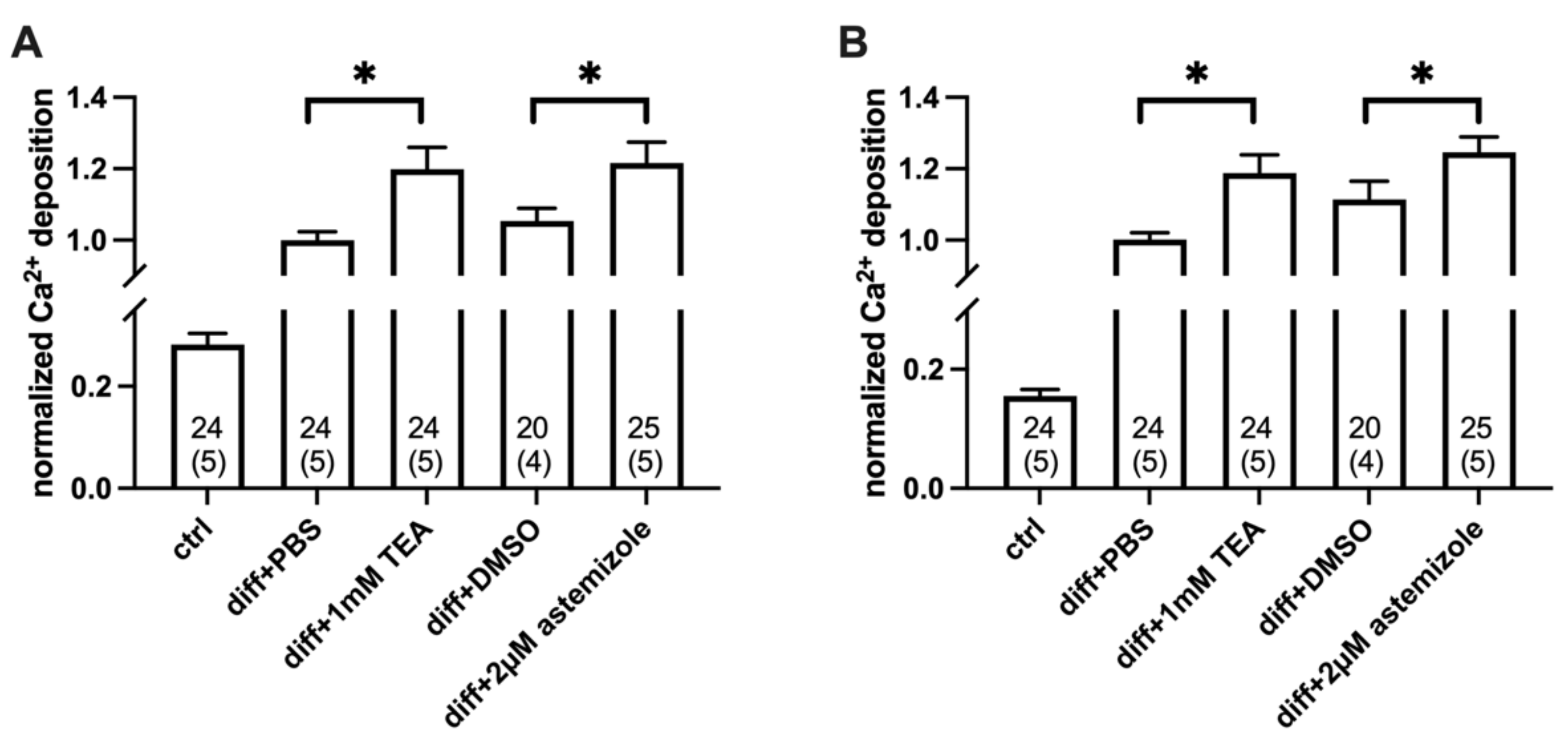
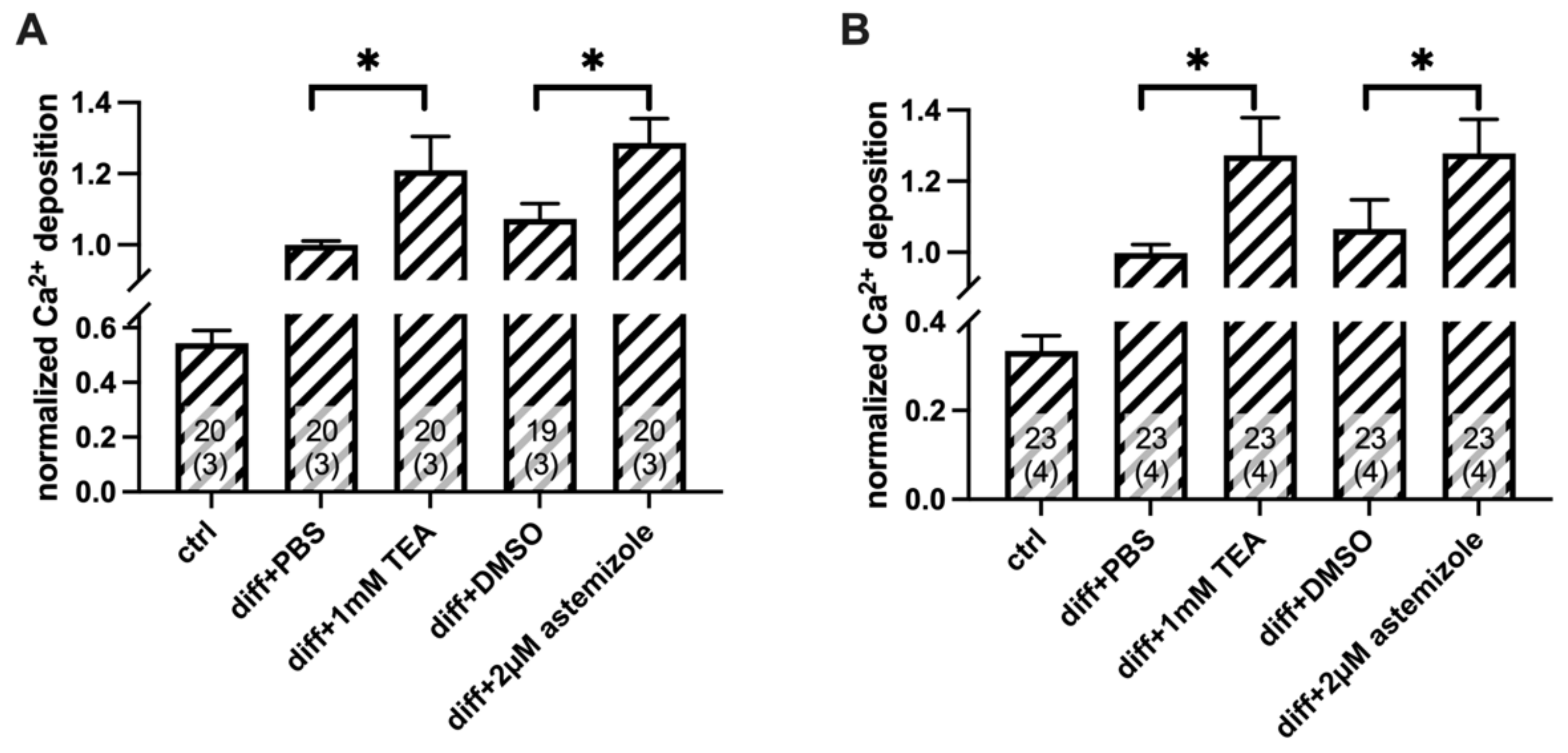
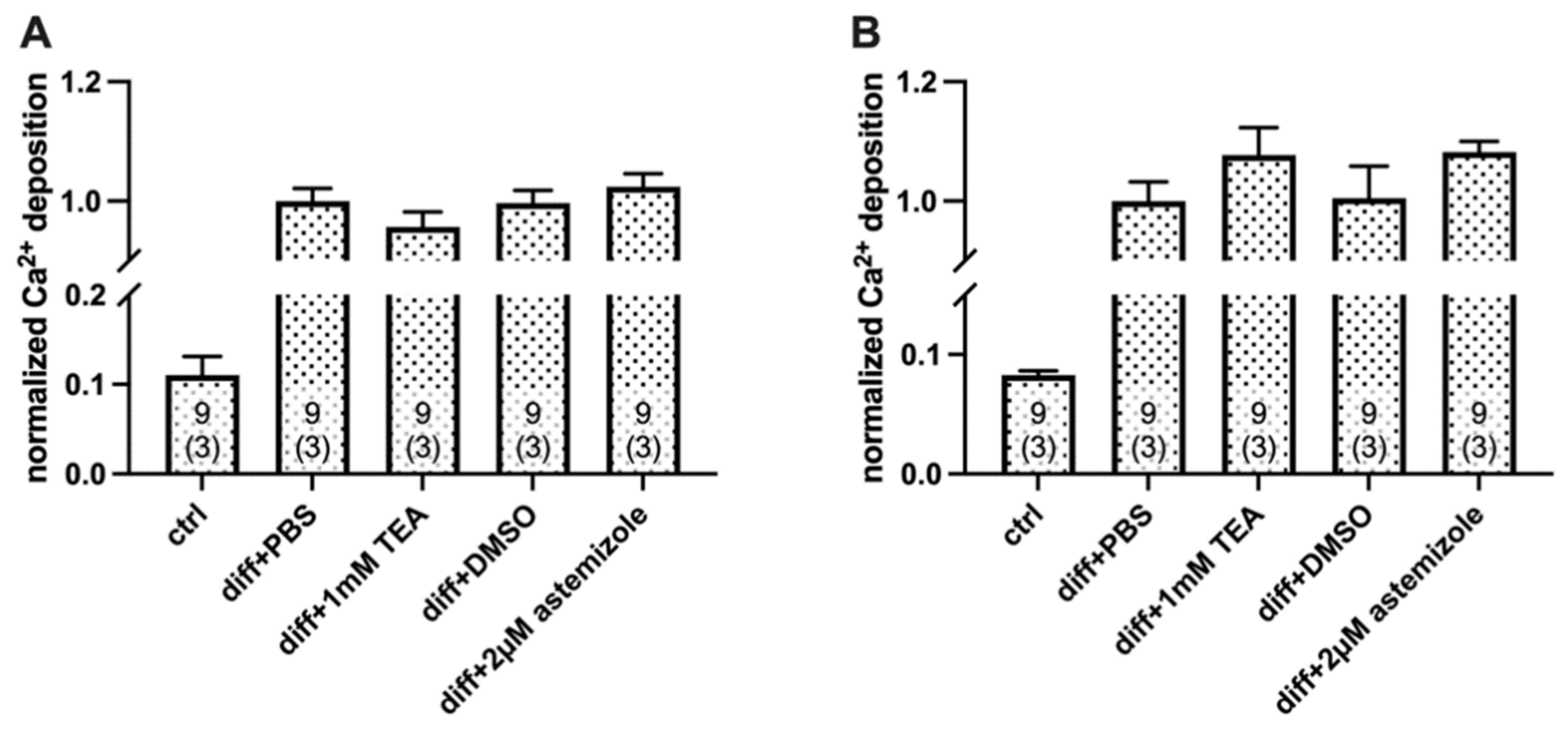
2.2. Ca2+ Deposition Is Enhanced in the Presence of K+ Channel Inhibitors
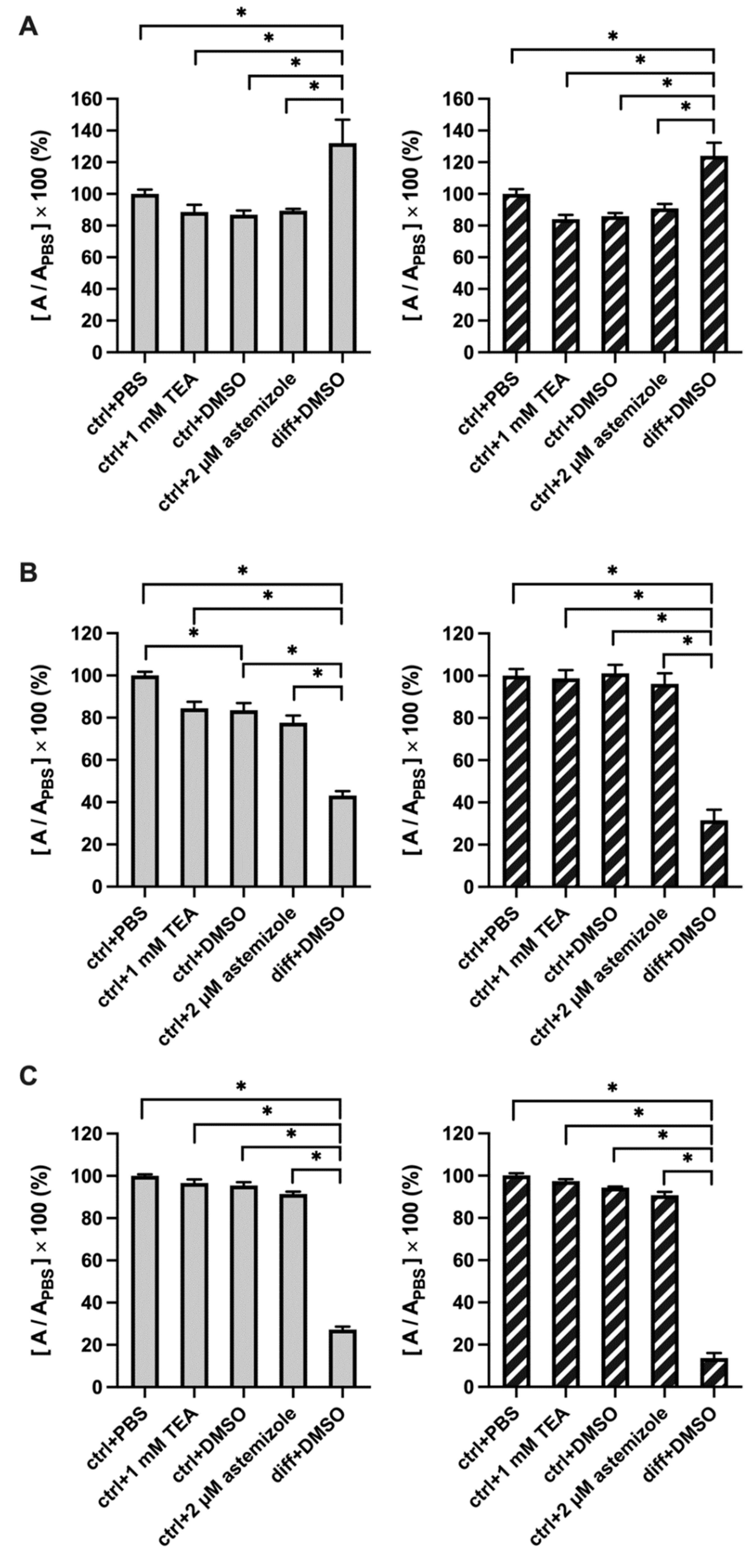
2.3. TEA and Astemizole Do Not Induce Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation and Ion Channel Expression
4.2. RNA Extraction and cDNA Synthesis
4.3. Reverse Transcription PCR (RT-PCR) and Quantitative PCR (QPCR)
4.4. Western Blot
4.5. Electrophysiology and Chemicals
4.6. Calcium Deposition Assay
4.7. Cell Viability/Proliferation
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, X.; Ma, W.; He, X.; Jha, R.K. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Med. Sci. Monit. 2011, 17, RA177–RA190. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; del Camino, D.; Sanchez, A.; Alves, F.; Bruggemann, A.; Beckh, S.; Stuhmer, W. Oncogenic potential of EAG K+ channels. EMBO J. 1999, 18, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Comes, N.; Serrano-Albarras, A.; Capera, J.; Serrano-Novillo, C.; Condom, E.; Ramon, Y.C.S.; Ferreres, J.C.; Felipe, A. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim. Biophys. Acta 2015, 1848, 2477–2492. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stuhmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Weseloh, R.M.; Mello de Queiroz, F.; Knotgen, H.; Sanchez, A.; Rubio, M.E.; Martin, S.; Schliephacke, T.; Jenke, M.; Heinz Joachim, R.; et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol. Cancer 2006, 5, 41. [Google Scholar] [CrossRef]
- Teisseyre, A.; Palko-Labuz, A.; Sroda-Pomianek, K.; Michalak, K. Voltage-Gated Potassium Channel Kv1.3 as a Target in Therapy of Cancer. Front. Oncol. 2019, 9, 933. [Google Scholar] [CrossRef]
- Huang, X.; Jan, L.Y. Targeting potassium channels in cancer. J. Cell Biol. 2014, 206, 151–162. [Google Scholar] [CrossRef]
- Panyi, G.; Beeton, C.; Felipe, A. Ion channels and anti-cancer immunity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130106. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, D.; Wu, X.; Sha, M.; Kang, L.; Ding, Z. Voltage-gated potassium channel Kv1.3 is highly expressed in human osteosarcoma and promotes osteosarcoma growth. Int. J. Mol. Sci. 2013, 14, 19245–19256. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Zeng, W.; Zhong, Y.; Liu, Q.; Wu, J. Silencing of Eag1 Gene Inhibits Osteosarcoma Proliferation and Migration by Targeting STAT3-VEGF Pathway. Biomed Res. Int. 2015, 2015, 617316. [Google Scholar] [CrossRef]
- Moreau, R.; Hurst, A.M.; Lapointe, J.Y.; Lajeunesse, D. Activation of maxi-K channels by parathyroid hormone and prostaglandin E2 in human osteoblast bone cells. J. Membr. Biol. 1996, 150, 175–184. [Google Scholar] [CrossRef]
- Henney, N.C.; Li, B.; Elford, C.; Reviriego, P.; Campbell, A.K.; Wann, K.T.; Evans, B.A. A large-conductance (BK) potassium channel subtype affects both growth and mineralization of human osteoblasts. Am. J. Physiol. Cell Physiol. 2009, 297, C1397–C1408. [Google Scholar] [CrossRef]
- Yang, J.E.; Song, M.S.; Shen, Y.; Ryu, P.D.; Lee, S.Y. The Role of KV7.3 in Regulating Osteoblast Maturation and Mineralization. Int. J. Mol. Sci. 2016, 17, 407. [Google Scholar] [CrossRef]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; McKinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stuhmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef]
- Hammadi, M.; Chopin, V.; Matifat, F.; Dhennin-Duthille, I.; Chasseraud, M.; Sevestre, H.; Ouadid-Ahidouch, H. Human ether a-gogo K+ channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J. Cell. Physiol. 2012, 227, 3837–3846. [Google Scholar] [CrossRef]
- Menendez, S.T.; Villaronga, M.A.; Rodrigo, J.P.; Alvarez-Teijeiro, S.; Garcia-Carracedo, D.; Urdinguio, R.G.; Fraga, M.F.; Pardo, L.A.; Viloria, C.G.; Suarez, C.; et al. Frequent aberrant expression of the human ether a go-go (hEAG1) potassium channel in head and neck cancer: Pathobiological mechanisms and clinical implications. J. Mol. Med. 2012, 90, 1173–1184. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Erdelyi, K.; Kovacs, K.; Kovacs, I.; Bai, P.; Rajnavolgyi, E.; Virag, L. Hydrogen peroxide-induced poly(ADP-ribosyl)ation regulates osteogenic differentiation-associated cell death. Free Radic. Biol. Med. 2012, 53, 1552–1564. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, D.; Fu, X.; Liu, Q.; Kang, L.; Ding, Z. Silencing of Ether a go-go 1 by shRNA inhibits osteosarcoma growth and cell cycle progression. Int. J. Mol. Sci. 2014, 15, 5570–5581. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stuhmer, W. Eag1: An emerging oncological target. Cancer Res. 2008, 68, 1611–1613. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, D.; Gao, Q.; Zhai, W.; Ding, Z.; Wu, J. MicroRNA-34a inhibits human osteosarcoma proliferation by downregulating ether a go-go 1 expression. Int. J. Med. Sci. 2013, 10, 676–682. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, D.; Lin, B.; Zhai, W.; Ding, Z.; Wu, J. p38 MAPK regulates the expression of ether a go-go potassium channel in human osteosarcoma cells. Radiol. Oncol. 2013, 47, 42–49. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Lian, K.; Lin, B.; Guo, L.; Ding, Z. Overexpression of potassium channel ether a go-go in human osteosarcoma. Neoplasma 2012, 59, 207–215. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.D.; Liu, Q.J.; Zeng, W.R.; Wu, X.Y.; Lin, B.; Wu, J. Expression of Ether a go-go 1 and its molecular mechanism of regulating the malignant phenotype of osteosarcoma. Zhonghua Zhong Liu Za Zhi 2016, 38, 818–825. [Google Scholar] [CrossRef]
- Rodan, S.B.; Imai, Y.; Thiede, M.A.; Wesolowski, G.; Thompson, D.; Bar-Shavit, Z.; Shull, S.; Mann, K.; Rodan, G.A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987, 47, 4961–4966. [Google Scholar]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Plotkin, L.I.; Bruzzaniti, A. Molecular signaling in bone cells: Regulation of cell differentiation and survival. Adv. Protein Chem. Struct Biol. 2019, 116, 237–281. [Google Scholar] [CrossRef]
- Salter, D.M.; Robb, J.E.; Wright, M.O. Electrophysiological responses of human bone cells to mechanical stimulation: Evidence for specific integrin function in mechanotransduction. J. Bone Miner. Res. 1997, 12, 1133–1141. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, Y.; Zhang, X.; Cai, Q.; Deng, X.; Yang, X. Mimicking the electrophysiological microenvironment of bone tissue using electroactive materials to promote its regeneration. J. Mater. Chem. B 2020, 8, 10221–10256. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Comparison of the depolarization response of human mesenchymal stem cells from different donors. Sci. Rep. 2015, 5, 18279. [Google Scholar] [CrossRef]
- Meszaros, B.; Papp, F.; Mocsar, G.; Kokai, E.; Kovacs, K.; Tajti, G.; Panyi, G. The voltage-gated proton channel hHv1 is functionally expressed in human chorion-derived mesenchymal stem cells. Sci. Rep. 2020, 10, 7100. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Aubin, R.; Lapointe, J.Y.; Lajeunesse, D. Pharmacological and biochemical evidence for the regulation of osteocalcin secretion by potassium channels in human osteoblast-like MG-63 cells. J. Bone Miner. Res. 1997, 12, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Ludwig, J.; Steffan, R.; Pongs, O.; Stuhmer, W.; Heinemann, S.H. Extracellular Mg2+ regulates activation of rat eag potassium channel. Pflugers Arch. 1996, 432, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Terlau, H.; Wunder, F.; Bruggemann, A.; Pardo, L.A.; Marquardt, A.; Stuhmer, W.; Pongs, O. Functional expression of a rat homologue of the voltage gated either a go-go potassium channel reveals differences in selectivity and activation kinetics between the Drosophila channel and its mammalian counterpart. EMBO J. 1994, 13, 4451–4458. [Google Scholar] [CrossRef]
- Tang, C.Y.; Bezanilla, F.; Papazian, D.M. Extracellular Mg2+ modulates slow gating transitions and the opening of Drosophila ether-a-Go-Go potassium channels. J. Gen. Physiol. 2000, 115, 319–338. [Google Scholar] [CrossRef]
- Garcia-Ferreiro, R.E.; Kerschensteiner, D.; Major, F.; Monje, F.; Stuhmer, W.; Pardo, L.A. Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J. Gen. Physiol. 2004, 124, 301–317. [Google Scholar] [CrossRef]
- Roy, J.; Vantol, B.; Cowley, E.A.; Blay, J.; Linsdell, P. Pharmacological separation of hEAG and hERG K+ channel function in the human mammary carcinoma cell line MCF-7. Oncol. Rep. 2008, 19, 1511–1516. [Google Scholar]
- Bachmann, M.; Li, W.; Edwards, M.J.; Ahmad, S.A.; Patel, S.; Szabo, I.; Gulbins, E. Voltage-Gated Potassium Channels as Regulators of Cell Death. Front. Cell Dev. Biol. 2020, 8, 611853. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Ahidouch, A.; Pardo, L.A. Kv10.1 K+ channel: From physiology to cancer. Pflugers Arch. 2016, 468, 751–762. [Google Scholar] [CrossRef]
- Csoti, A.; Del Carmen Najera Meza, R.; Bogar, F.; Tajti, G.; Szanto, T.G.; Varga, Z.; Gurrola, G.B.; Toth, G.K.; Possani, L.D.; Panyi, G. sVmKTx, a transcriptome analysis-based synthetic peptide analogue of Vm24, inhibits Kv1.3 channels of human T cells with improved selectivity. Biochem. Pharmacol. 2022, 199, 115023. [Google Scholar] [CrossRef]
- Naseem, M.U.; Carcamo-Noriega, E.; Beltran-Vidal, J.; Borrego, J.; Szanto, T.G.; Zamudio, F.Z.; Delgado-Prudencio, G.; Possani, L.D.; Panyi, G. Cm28, a scorpion toxin having a unique primary structure, inhibits KV1.2 and KV1.3 with high affinity. J. Gen. Physiol. 2022, 154, e202213146. [Google Scholar] [CrossRef]
- Serguienko, A.; Wang, M.Y.; Myklebost, O. Real-Time Vital Mineralization Detection and Quantification during In Vitro Osteoblast Differentiation. Biol. Proced. Online 2018, 20, 14. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Fujita, H.; Yamamoto, M.; Ogino, T.; Kobuchi, H.; Ohmoto, N.; Aoyama, E.; Oka, T.; Nakanishi, T.; Inoue, K.; Sasaki, J. Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem. Funct. 2014, 32, 77–86. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.rot095505. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095497. [Google Scholar] [CrossRef]
- Voigt, W. Sulforhodamine B assay and chemosensitivity. Methods Mol. Med. 2005, 110, 39–48. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Gomez-Varela, D.; Contreras-Jurado, C.; Furini, S.; Garcia-Ferreiro, R.; Stuhmer, W.; Pardo, L.A. Different relevance of inactivation and F468 residue in the mechanisms of hEag1 channel blockage by astemizole, imipramine and dofetilide. FEBS Lett. 2006, 580, 5059–5066. [Google Scholar] [CrossRef]
- Elmedyb, P.; Calloe, K.; Schmitt, N.; Hansen, R.S.; Grunnet, M.; Olesen, S.P. Modulation of ERG channels by XE991. Basic Clin. Pharmacol. Toxicol. 2007, 100, 316–322. [Google Scholar] [CrossRef]
- Saxena, P.; Zangerl-Plessl, E.M.; Linder, T.; Windisch, A.; Hohaus, A.; Timin, E.; Hering, S.; Stary-Weinzinger, A. New potential binding determinant for hERG channel inhibitors. Sci. Rep. 2016, 6, 24182. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Q.; Chen, Z.; Wu, X.; Zhong, Y.; Wu, J. Silencing of hERG1 Gene Inhibits Proliferation and Invasion, and Induces Apoptosis in Human Osteosarcoma Cells by Targeting the NF-kappaB Pathway. J. Cancer 2016, 7, 746–757. [Google Scholar] [CrossRef]
- Kirsch, T. Determinants of pathological mineralization. Curr. Opin. Rheumatol. 2006, 18, 174–180. [Google Scholar] [CrossRef]
- Grzesiak, J.; Smieszek, A.; Marycz, K. Ultrastructural changes during osteogenic differentiation in mesenchymal stromal cells cultured in alginate hydrogel. Cell Biosci. 2017, 7, 2. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Qin, X.; Wang, L.; Xiao, J.; Song, Y.; Sheng, X.; Guo, M.; Ji, X. Sp1 Plays an Important Role in Vascular Calcification Both In Vivo and In Vitro. J. Am. Heart Assoc. 2018, 7, e007555. [Google Scholar] [CrossRef]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef]
- Agarwal, J.R.; Griesinger, F.; Stuhmer, W.; Pardo, L.A. The potassium channel Ether a go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol. Cancer 2010, 9, 18. [Google Scholar] [CrossRef]
- Bernal-Ramos, G.; Hernandez-Gallegos, E.; Vera, E.; Chavez-Lopez, M.G.; Zuniga-Garcia, V.; Sanchez-Perez, Y.; Garrido, E.; Camacho, J. Astemizole inhibits cell proliferation in human prostate tumorigenic cells expressing ether a-go-go-1 potassium channels. Cell Mol. Biol. 2017, 63, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Lopez, M.G.; Zuniga-Garcia, V.; Hernandez-Gallegos, E.; Vera, E.; Chasiquiza-Anchatuna, C.A.; Viteri-Yanez, M.; Sanchez-Ramos, J.; Garrido, E.; Camacho, J. The combination astemizole-gefitinib as a potential therapy for human lung cancer. OncoTargets Ther. 2017, 10, 5795–5803. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quiroz, J.; Camacho, J. Astemizole: An old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med. Chem. 2011, 11, 307–314. [Google Scholar] [CrossRef] [PubMed]
- De Guadalupe Chavez-Lopez, M.; Hernandez-Gallegos, E.; Vazquez-Sanchez, A.Y.; Gariglio, P.; Camacho, J. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int. J. Gynecol. Cancer 2014, 24, 824–828. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L.; Jiao, L.; Jiang, X.; Lian, F.; Lu, J.; Zhu, K.; Du, D.; Liu, J.; Ding, H.; et al. Astemizole arrests the proliferation of cancer cells by disrupting the EZH2-EED interaction of polycomb repressive complex 2. J. Med. Chem. 2014, 57, 9512–9521. [Google Scholar] [CrossRef]
- Gradl, G.; Faust, D.; Oesch, F.; Wieser, R.J. Density-dependent regulation of cell growth by contactinhibin and the contactinhibin receptor. Curr. Biol. 1995, 5, 526–535. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Huang, T.; Yu, Z.; Ma, K.; Yang, M.; Liu, Q.; Pan, H.; Wang, H.; Wang, J.; et al. Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation. Adv. Sci. 2018, 5, 1700755. [Google Scholar] [CrossRef]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef]
- Dieudonne, F.X.; Severe, N.; Biosse-Duplan, M.; Weng, J.J.; Su, Y.; Marie, P.J. Promotion of osteoblast differentiation in mesenchymal cells through Cbl-mediated control of STAT5 activity. Stem Cells 2013, 31, 1340–1349. [Google Scholar] [CrossRef]
- Hayashi, M.; Novak, I. Molecular basis of potassium channels in pancreatic duct epithelial cells. Channels 2013, 7, 432–441. [Google Scholar] [CrossRef]
- Vergara, C.; Moczydlowski, E.; Latorre, R. Conduction, Blockade and Gating in a Ca2+-activated K+ Channel Incorporated into Planar Lipid Bilayers. Biophys. J. 1984, 45, 73–76. [Google Scholar] [CrossRef]
- Yellen, G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J. Gen. Physiol. 1984, 84, 157–186. [Google Scholar] [CrossRef]
- Schonherr, R.; Gessner, G.; Lober, K.; Heinemann, S.H. Functional distinction of human EAG1 and EAG2 potassium channels. FEBS Lett. 2002, 514, 204–208. [Google Scholar] [CrossRef]
- Hadley, J.K.; Noda, M.; Selyanko, A.A.; Wood, I.C.; Abogadie, F.C.; Brown, D.A. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br. J. Pharmacol. 2000, 129, 413–415. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yue, J.; Che, H.; Sun, H.Y.; Tse, H.F.; Li, G.R. BKCa and hEag1 channels regulate cell proliferation and differentiation in human bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2014, 229, 202–212. [Google Scholar] [CrossRef]
- Hegle, A.P.; Marble, D.D.; Wilson, G.F. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc. Natl. Acad. Sci. USA 2006, 103, 2886–2891. [Google Scholar] [CrossRef]
- Pardo, L.A. Voltage-gated potassium channels in cell proliferation. Physiology 2004, 19, 285–292. [Google Scholar] [CrossRef]
- Kaczmarek, L.K. Non-conducting functions of voltage-gated ion channels. Nat. Rev. Neurosci 2006, 7, 761–771. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Moody, A.T.; Levin, M.; Kaplan, D.L. Membrane Potential Depolarization Alters Calcium Flux and Phosphate Signaling During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Bioelectricity 2019, 1, 56–66. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Jono, S.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H. Vascular calcification and inorganic phosphate. Am. J. Kidney Dis. 2001, 38, S34–S37. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.Y.; Giachelli, C.M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 2006, 98, 905–912. [Google Scholar] [CrossRef]
- Voelkl, J.; Lang, F.; Eckardt, K.U.; Amann, K.; Kuro, O.M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell. Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular calcification: The killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef]
- Zavaczki, E.; Jeney, V.; Agarwal, A.; Zarjou, A.; Oros, M.; Katko, M.; Varga, Z.; Balla, G.; Balla, J. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011, 80, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimo, D.A.; Wyler, S.C.; Romani, A.M.; O’Neill, W.C.; Dubyak, G.R. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: Synergistic modulation by cyclic AMP and hyperphosphatemia. Am. J. Physiol. Cell Physiol. 2010, 298, C702–C713. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.M.; Ocana, D.B.; Diaz, L.; Larrea, F.; Avila-Chavez, E.; Cadena, A.; Hinojosa, L.M.; Lara, G.; Villanueva, L.A.; Vargas, C.; et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004, 64, 6996–7001. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; Contreras-Jurado, C.; Zientkowska, M.; Alves, F.; Stuhmer, W. Role of voltage-gated potassium channels in cancer. J. Membr. Biol. 2005, 205, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Quiroz, J.; Gonzalez-Gonzalez, M.E.; Diaz, L.; Ordaz-Rosado, D.; Segovia-Mendoza, M.; Prado-Garcia, H.; Larrea, F.; Garcia-Becerra, R. Astemizole, an Inhibitor of Ether-a-Go-Go-1 Potassium Channel, Increases the Activity of the Tyrosine Kinase Inhibitor Gefitinib in Breast Cancer Cells. Rev. Investig. Clin. 2019, 71, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Lopez, M.G.; Zuniga-Garcia, V.; Castro-Magdonel, B.E.; Vera, E.; Garrido, E.; Sanchez-Ramos, J.; Ponce-Castaneda, M.V.; Cabrera-Munoz, M.L.; Escobar, Y.; Ortiz, C.S.; et al. Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and its Regulation by pRb in HeLa Cells. Genes 2020, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, E.J.; Head, S.A.; Ai, N.; Zhang, B.; Wu, C.; Li, R.J.; Liu, Y.; Chakravarty, H.; Zhang, S.; et al. Astemizole Inhibits mTOR Signaling and Angiogenesis by Blocking Cholesterol Trafficking. Int. J. Biol. Sci. 2018, 14, 1175–1185. [Google Scholar] [CrossRef]
- De Guadalupe Chavez-Lopez, M.; Perez-Carreon, J.I.; Zuniga-Garcia, V.; Diaz-Chavez, J.; Herrera, L.A.; Caro-Sanchez, C.H.; Acuna-Macias, I.; Gariglio, P.; Hernandez-Gallegos, E.; Chiliquinga, A.J.; et al. Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumour Biol. 2015, 36, 6149–6158. [Google Scholar] [CrossRef]
- Richards, K.L.; Milligan, C.J.; Richardson, R.J.; Jancovski, N.; Grunnet, M.; Jacobson, L.H.; Undheim, E.A.B.; Mobli, M.; Chow, C.Y.; Herzig, V.; et al. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc. Natl. Acad. Sci. USA 2018, 115, E8077–E8085. [Google Scholar] [CrossRef]
- Li, L.; Buchet, R.; Wu, Y. Dimethyl sulfoxide-induced hydroxyapatite formation: A biological model of matrix vesicle nucleation to screen inhibitors of mineralization. Anal. Biochem. 2008, 381, 123–128. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steventon, G.; Hylands, P.J.; Lee, C.C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészáros, B.; Csoti, A.; Szanto, T.G.; Telek, A.; Kovács, K.; Toth, A.; Volkó, J.; Panyi, G. The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures. Int. J. Mol. Sci. 2022, 23, 10533. https://doi.org/10.3390/ijms231810533
Mészáros B, Csoti A, Szanto TG, Telek A, Kovács K, Toth A, Volkó J, Panyi G. The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures. International Journal of Molecular Sciences. 2022; 23(18):10533. https://doi.org/10.3390/ijms231810533
Chicago/Turabian StyleMészáros, Beáta, Agota Csoti, Tibor G. Szanto, Andrea Telek, Katalin Kovács, Agnes Toth, Julianna Volkó, and Gyorgy Panyi. 2022. "The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures" International Journal of Molecular Sciences 23, no. 18: 10533. https://doi.org/10.3390/ijms231810533
APA StyleMészáros, B., Csoti, A., Szanto, T. G., Telek, A., Kovács, K., Toth, A., Volkó, J., & Panyi, G. (2022). The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures. International Journal of Molecular Sciences, 23(18), 10533. https://doi.org/10.3390/ijms231810533





