α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats
Abstract
:1. Introduction
2. Results
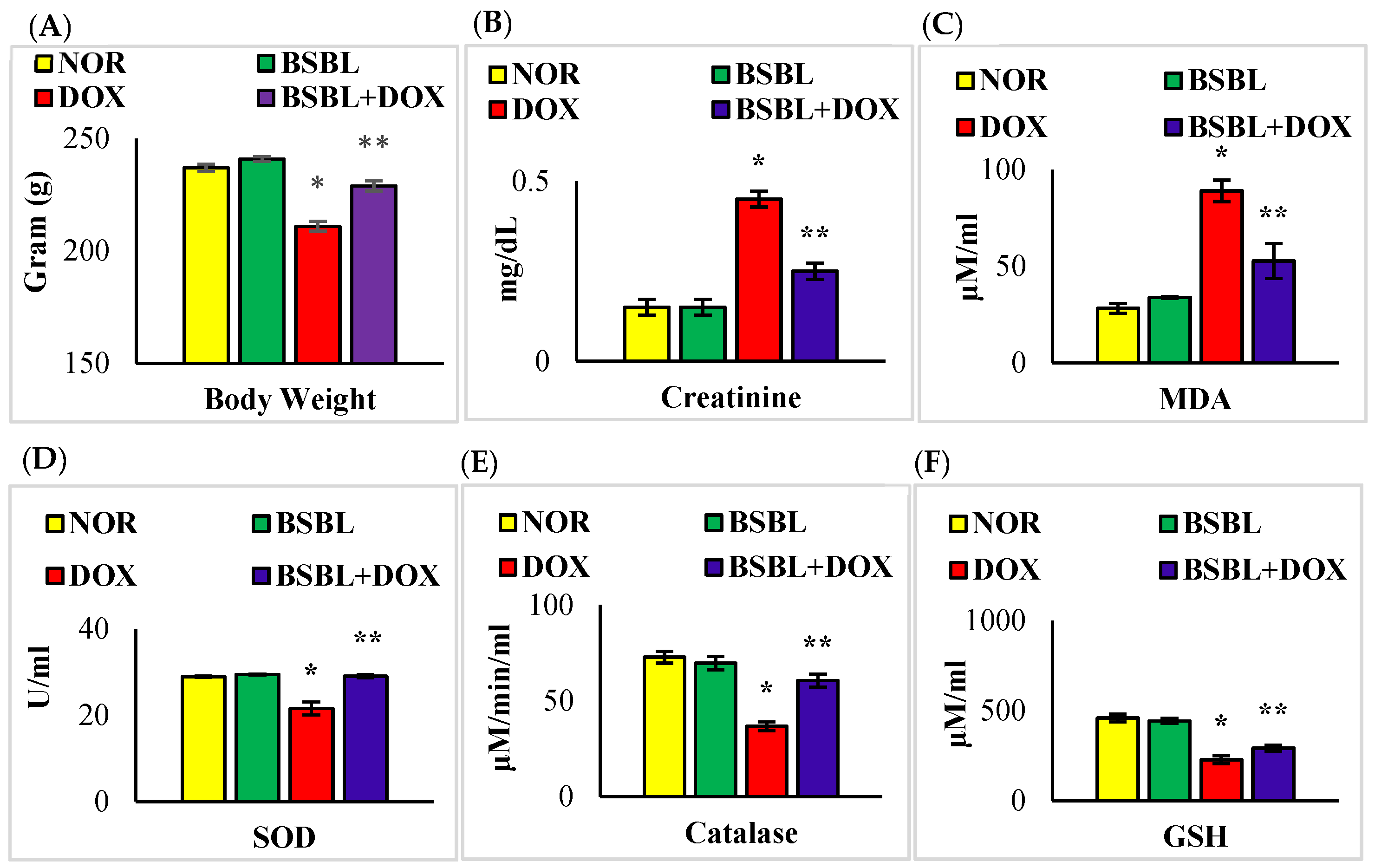
2.1. α-Bisabolol Rescued Weight Loss and Renal Function in DOX-Induced Renal Injury
2.2. α-Bisabolol Inhibited Oxidative Stress in DOX-Induced Renal Injury
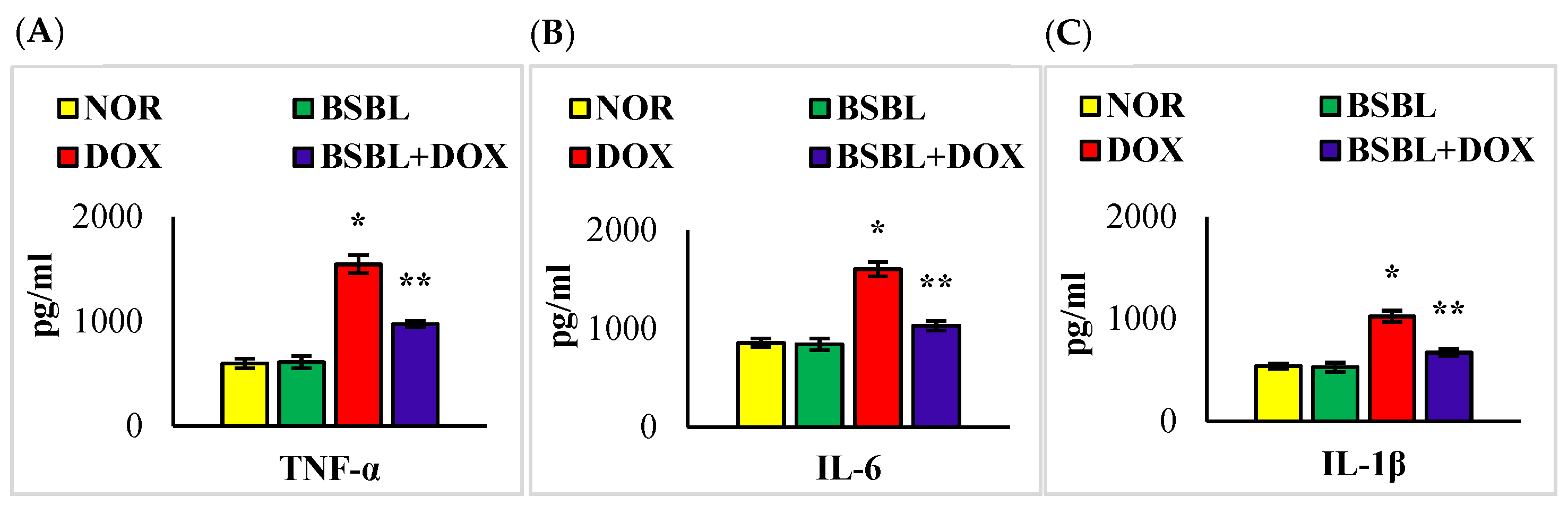
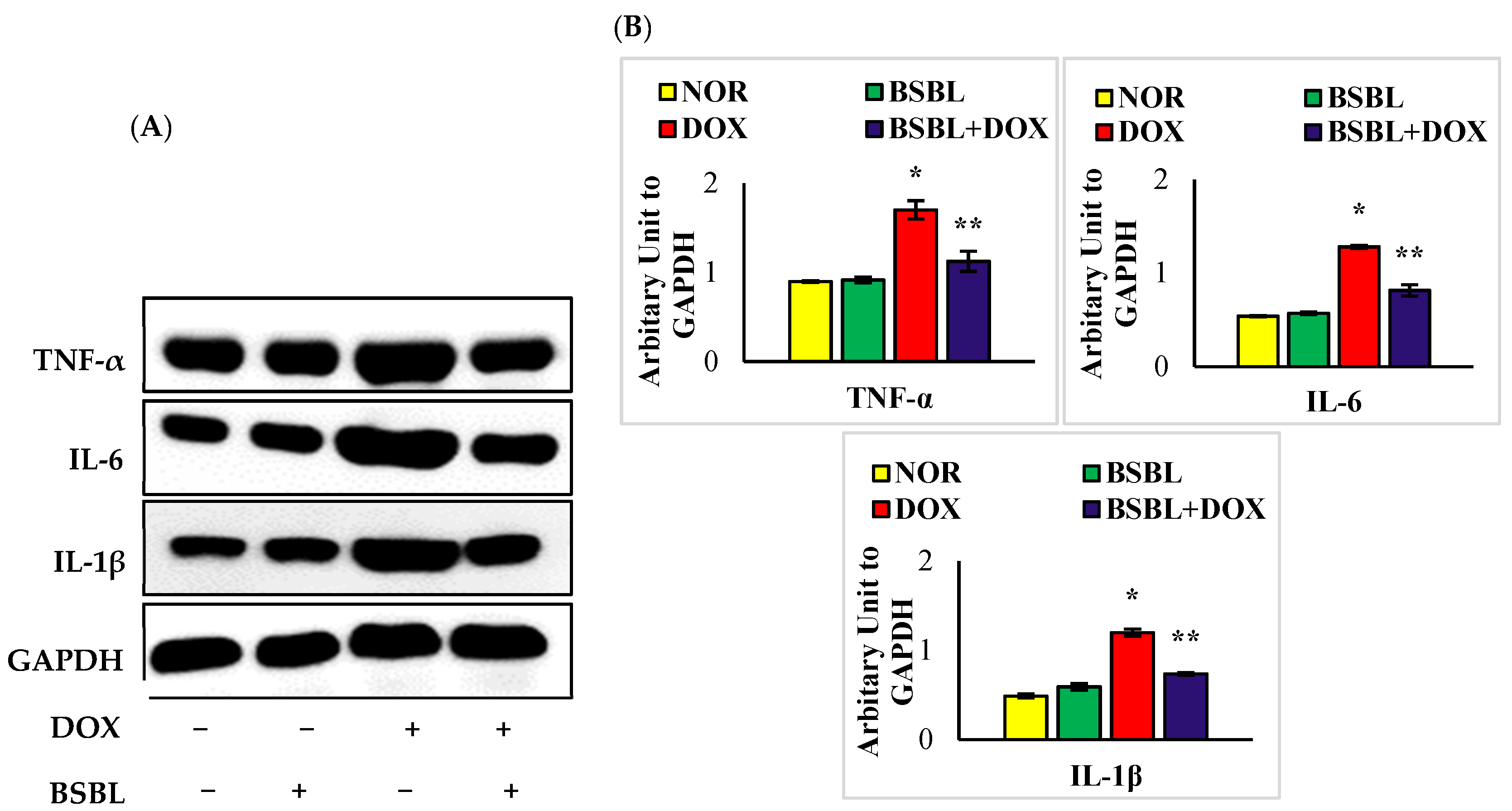
2.3. α-Bisabolol Inhibits the Levels/Expressions of Pro-Inflammatory Cytokines in the Kidneys
2.4. α-Bisabolol Protects the Renal Architecture in DOX-Injected Rats
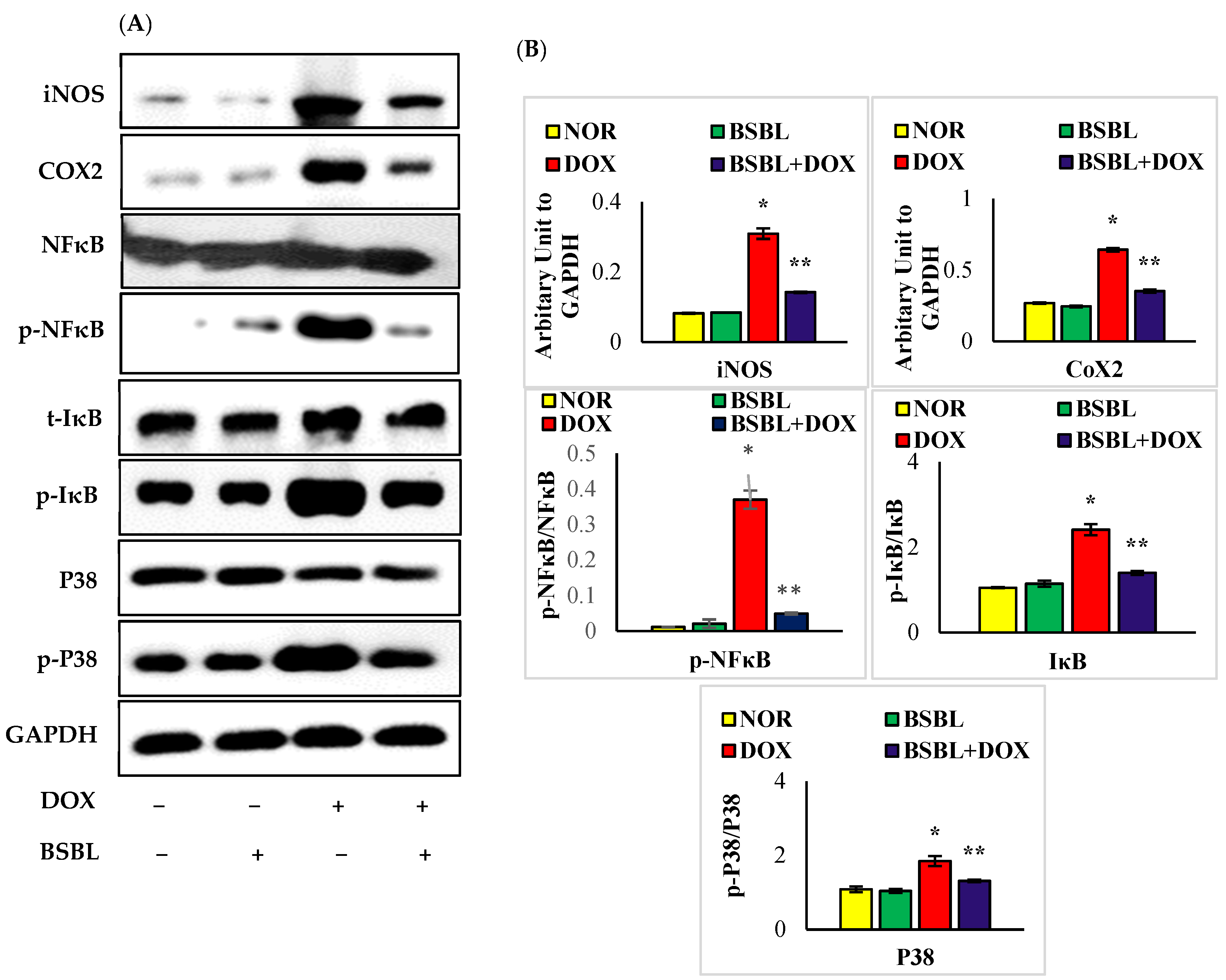
2.5. α-Bisabolol Attenuates the Expressions of Inflammatory Mediators and Downregulates NF-κB/MAPK Signaling Pathway
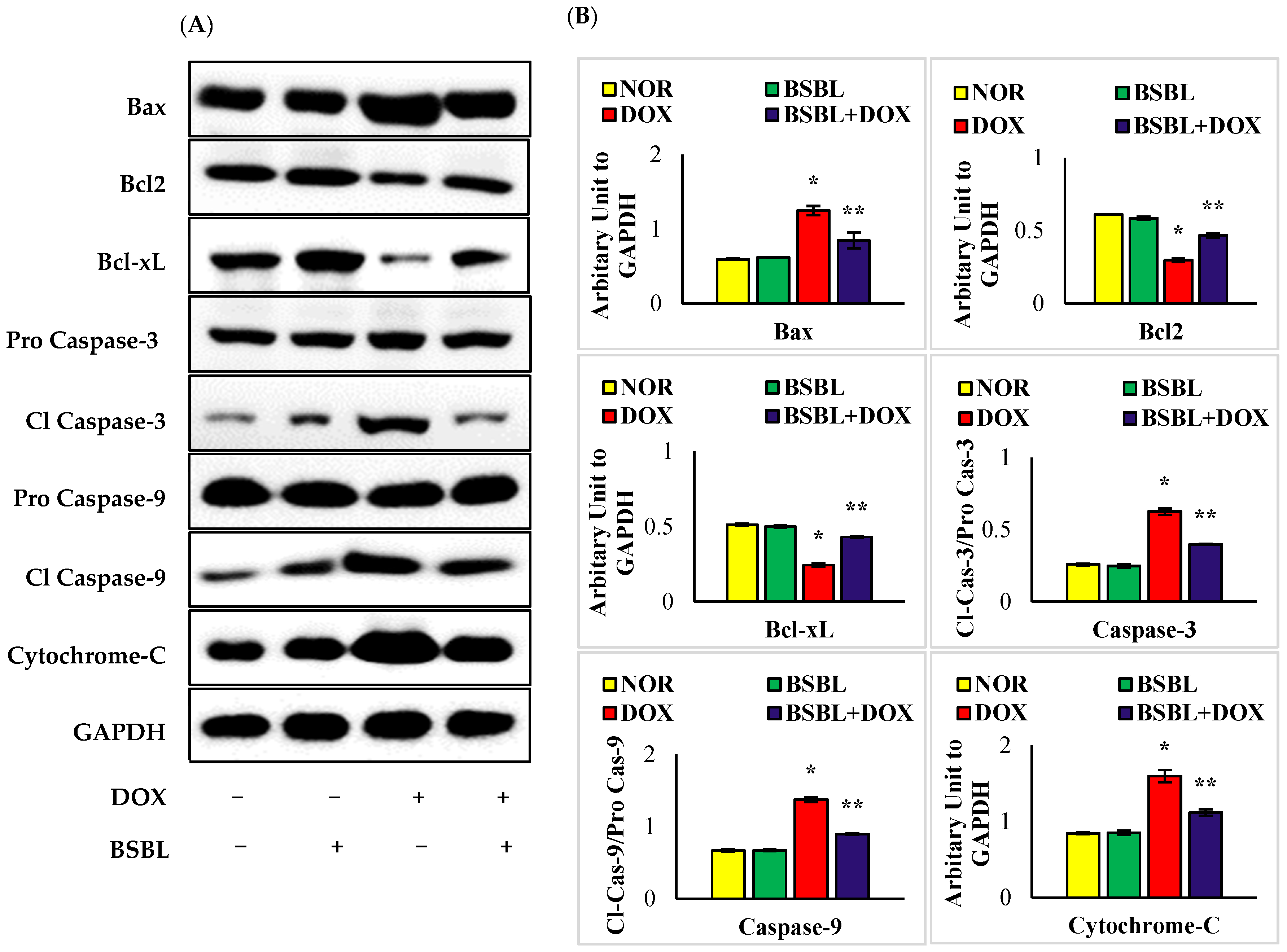
2.6. α-Bisabolol Attenuates Caspase-Dependent Renal Apoptosis
3. Discussion
4. Materials and Methods
4.1. Drugs, Chemicals, and Antibodies
4.2. Experimental Animals
4.3. Experimental Design
4.4. Biochemical Parameters
4.4.1. Estimation of Blood urea Nitrogen and Creatinine
4.4.2. Estimation of Lipid Peroxidation Products and Antioxidants
4.4.3. Estimation of Pro-Inflammatory and Anti-Inflammatory Cytokines
4.5. Western Blot Analysis
4.6. Estimation of Protein Content in the Kidney
4.7. Histopathological Evaluation
4.8. Statistical Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khames, A.; Khalaf, M.M.; Gad, A.M.; Abd El-Raouf, O.M.; Kandeil, M.A. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact. 2019, 311, 108777. [Google Scholar] [CrossRef]
- Lahoti, T.S.; Patel, D.; Thekkemadom, V.; Beckett, R.; Ray, S.D. Doxorubicin-induced in vivo nephrotoxicity involves oxidative stress-mediated multiple pro- and anti-apoptotic signaling pathways. Curr. Neurovasc. Res. 2012, 9, 282–295. [Google Scholar] [CrossRef]
- Sanajou, D.; Nazari Soltan Ahmad, S.; Hosseini, V.; Kalantary-Charvadeh, A.; Marandi, Y.; Roshangar, L.; Bahrambeigi, S.; Mesgari-Abbasi, M. β-Lapachone protects against doxorubicin-induced nephrotoxicity via NAD(+)/AMPK/NF-kB in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 633–640. [Google Scholar] [CrossRef]
- Ghibu, S.; Delemasure, S.; Richard, C.; Guilland, J.C.; Martin, L.; Gambert, S.; Rochette, L.; Vergely, C. General oxidative stress during doxorubicin-induced cardiotoxicity in rats: Absence of cardioprotection and low antioxidant efficiency of alpha-lipoic acid. Biochimie 2012, 94, 932–939. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Almajwal, A.; Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci. 2020, 27, 2251–2260. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Qi, Y.; Xu, L.; Song, S.; Yin, L.; Tao, X.; Zhen, Y.; Han, X.; Ma, X.; et al. Protective effects of dioscin against doxorubicin-induced nephrotoxicity via adjusting FXR-mediated oxidative stress and inflammation. Toxicology 2017, 378, 53–64. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Al-Asmari, A.F.; Ansari, M.N.; Al-Anazi, W.A.; Bahashwan, S.; Almutairi, M.M.; Alshammari, M.; et al. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep. 2018, 70, 993–1000. [Google Scholar] [CrossRef]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.-U.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef]
- Shati, A.A. Doxorubicin-induces NFAT/Fas/FasL cardiac apoptosis in rats through activation of calcineurin and P38 MAPK and inhibition of mTOR signalling pathways. Clin. Exp. Pharmacol. Physiol. 2020, 47, 660–676. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef]
- Lee, V.W.; Harris, D.C. Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology 2011, 16, 30–38. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Content and composition of the essential oil of Chamomilla recutita (L.) Rauschert from some European countries. Nat. Prod. Res. 2010, 24, 48–55. [Google Scholar] [CrossRef]
- De Souza, A.T.; Benazzi, T.L.; Grings, M.B.; Cabral, V.; da Silva, E.A.; Cardozo-Filho, L.; Antunes, O.A.C. Supercritical extraction process and phase equilibrium of Candeia (Eremanthus erythropappus) oil using supercritical carbon dioxide. J. Supercrit. Fluids 2008, 47, 182–187. [Google Scholar] [CrossRef]
- Vila, R.; Santana, A.I.; Pérez-Rosés, R.; Valderrama, A.; Castelli, M.V.; Mendonca, S.; Zacchino, S.; Gupta, M.P.; Cañigueral, S. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of α-bisabolol. Bioresour. Technol. 2010, 101, 2510–2514. [Google Scholar] [CrossRef]
- Seki, T.; Kokuryo, T.; Yokoyama, Y.; Suzuki, H.; Itatsu, K.; Nakagawa, A.; Mizutani, T.; Miyake, T.; Uno, M.; Yamauchi, K. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci. 2011, 102, 2199–2205. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Laham, F.; Al-Taee, H.; Azimullah, S.; Ojha, S. Protective effects of α-bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: In vivo and in vitro evidences. J. Biochem. Mol. Toxicol. 2018, 32, e22200. [Google Scholar] [CrossRef]
- Zaaba, N.E.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, B.M.; Ali, B.H.; Adeghate, E.; Nemmar, A. The Nephroprotective Effects of α-Bisabolol in Cisplatin-Induced Acute Kidney Injury in Mice. Biomedicines 2022, 10, 842. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Injac, R.; Boskovic, M.; Perse, M.; Koprivec-Furlan, E.; Cerar, A.; Djordjevic, A.; Strukelj, B. Acute doxorubicin nephrotoxicity in rats with malignant neoplasm can be successfully treated with fullerenol C60(OH)24 via suppression of oxidative stress. Pharmacol. Rep. 2008, 60, 742. [Google Scholar] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Soltani Hekmat, A.; Chenari, A.; Alipanah, H.; Javanmardi, K. Protective effect of alamandine on doxorubicin-induced nephrotoxicity in rats. BMC Pharmacol. Toxicol. 2021, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol. Med. Rep. 2016, 13, 3953–3960. [Google Scholar] [CrossRef]
- Refaie, M.M.M.; Amin, E.F.; El-Tahawy, N.F.; Abdelrahman, A.M. Possible protective effect of diacerein on doxorubicin-induced nephrotoxicity in rats. J. Toxicol. 2016, 2016, 9507563. [Google Scholar] [CrossRef]
- El-Sayed, E.M.; Mansour, A.M.; El-Sawy, W.S. Alpha lipoic acid prevents doxorubicin-induced nephrotoxicity by mitigation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2017, 31, e21940. [Google Scholar] [CrossRef]
- Calabrese, V.; Lodi, R.; Tonon, C.; D’Agata, V.; Sapienza, M.; Scapagnini, G.; Mangiameli, A.; Pennisi, G.; Stella, A.M.; Butterfield, D.A. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J. Neurol. Sci. 2005, 233, 145–162. [Google Scholar] [CrossRef]
- Gille, L.; Nohl, H. Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radic. Biol. Med. 1997, 23, 775–782. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Cortez, M.; Carmo, L.S.; Rogero, M.M.; Borelli, P.; Fock, R.A. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation 2013, 36, 379–386. [Google Scholar] [CrossRef]
- Sutariya, B.; Saraf, M. α-asarone reduce proteinuria by restoring antioxidant enzymes activities and regulating necrosis factor κB signaling pathway in doxorubicin-induced nephrotic syndrome. Biomed. Pharmacother. 2018, 98, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Abraham, P.; Kota, R.; Isaac, B. NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem. Toxicol. 2018, 118, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jawień, J.; Gajda, M.; Olszanecki, R.; Korbut, R. BAY x 1005 attenuates atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 2007, 58, 583–588. [Google Scholar]
- Reiterer, G.; Toborek, M.; Hennig, B. Quercetin protects against linoleic acid-induced porcine endothelial cell dysfunction. J. Nutr. 2004, 134, 771–775. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; El-Harbi, K.M.; Khoshhal, S.; Ahmed, N.; Elkablawy, M.A.; Shaaban, A.A.; Abo-Haded, H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 2019, 11, 47. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem. Pharmacol. 2011, 81, 891–909. [Google Scholar] [CrossRef]
- Park, E.J.; Kwon, H.K.; Choi, Y.M.; Shin, H.J.; Choi, S. Doxorubicin induces cytotoxicity through upregulation of pERK-dependent ATF3. PLoS ONE 2012, 7, e44990. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, Y.; Chi, Y. Role of p38 MAPK activation and mitochondrial cytochrome-c release in allicin-induced apoptosis in SK-N-SH cells. Anticancer Drugs 2016, 27, 312–317. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Jha, N.K.; Saraswathiamma, D.; Subramanya, S.; Albawardi, A.; Ojha, S. α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats. Int. J. Mol. Sci. 2022, 23, 10528. https://doi.org/10.3390/ijms231810528
Arunachalam S, Nagoor Meeran MF, Azimullah S, Jha NK, Saraswathiamma D, Subramanya S, Albawardi A, Ojha S. α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats. International Journal of Molecular Sciences. 2022; 23(18):10528. https://doi.org/10.3390/ijms231810528
Chicago/Turabian StyleArunachalam, Seenipandi, M. F. Nagoor Meeran, Sheikh Azimullah, Niraj Kumar Jha, Dhanya Saraswathiamma, Sandeep Subramanya, Alia Albawardi, and Shreesh Ojha. 2022. "α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats" International Journal of Molecular Sciences 23, no. 18: 10528. https://doi.org/10.3390/ijms231810528
APA StyleArunachalam, S., Nagoor Meeran, M. F., Azimullah, S., Jha, N. K., Saraswathiamma, D., Subramanya, S., Albawardi, A., & Ojha, S. (2022). α-Bisabolol Attenuates Doxorubicin Induced Renal Toxicity by Modulating NF-κB/MAPK Signaling and Caspase-Dependent Apoptosis in Rats. International Journal of Molecular Sciences, 23(18), 10528. https://doi.org/10.3390/ijms231810528







