Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation
Abstract
:1. Introduction
2. Results
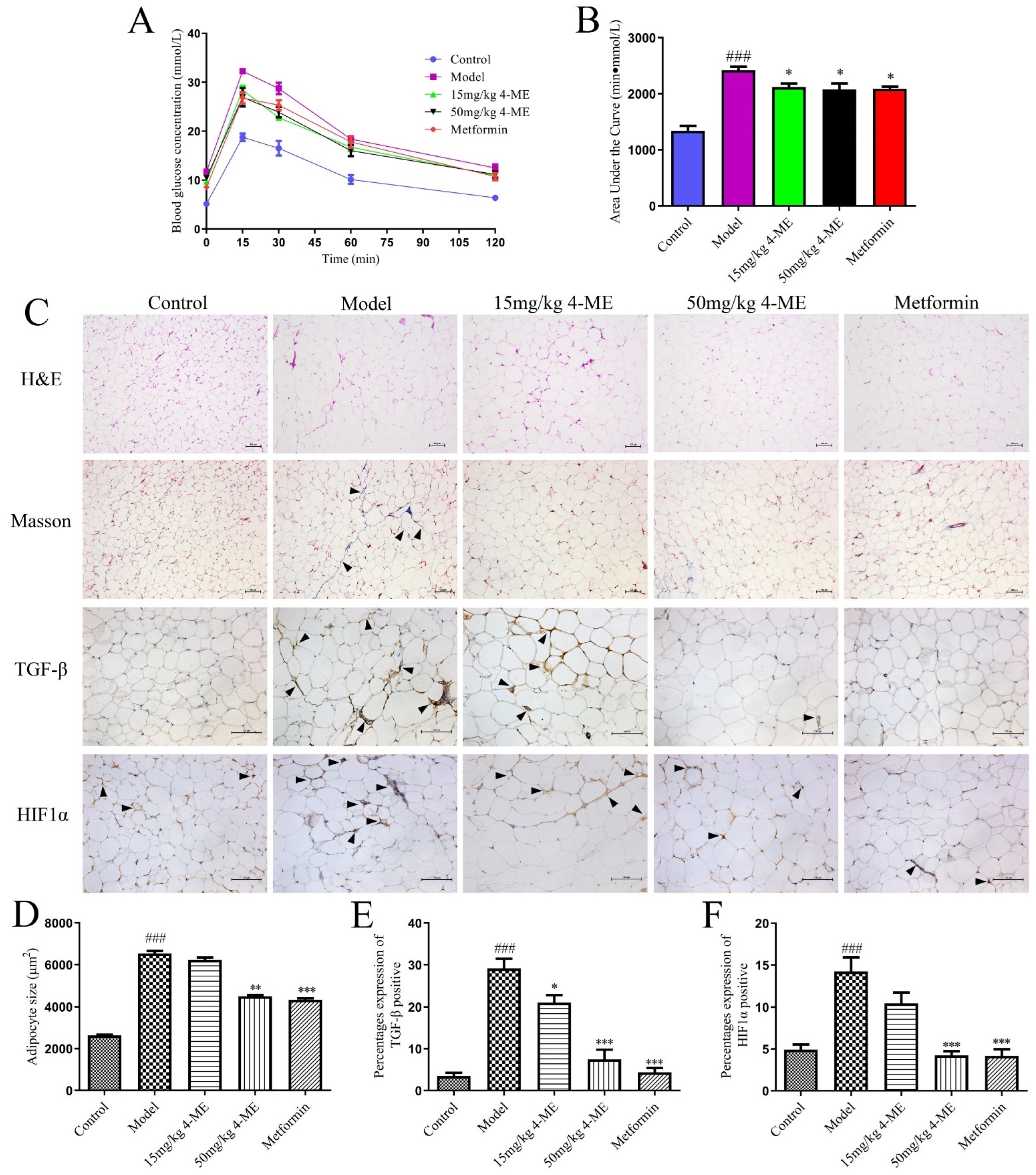
2.1. Effects of 4-ME upon Visceral Obesity and Insulin Resistance in HFD-Fed Mice
2.2. Effects of 4-ME upon Adipose Tissue Microenvironment in HFD-Fed Mice
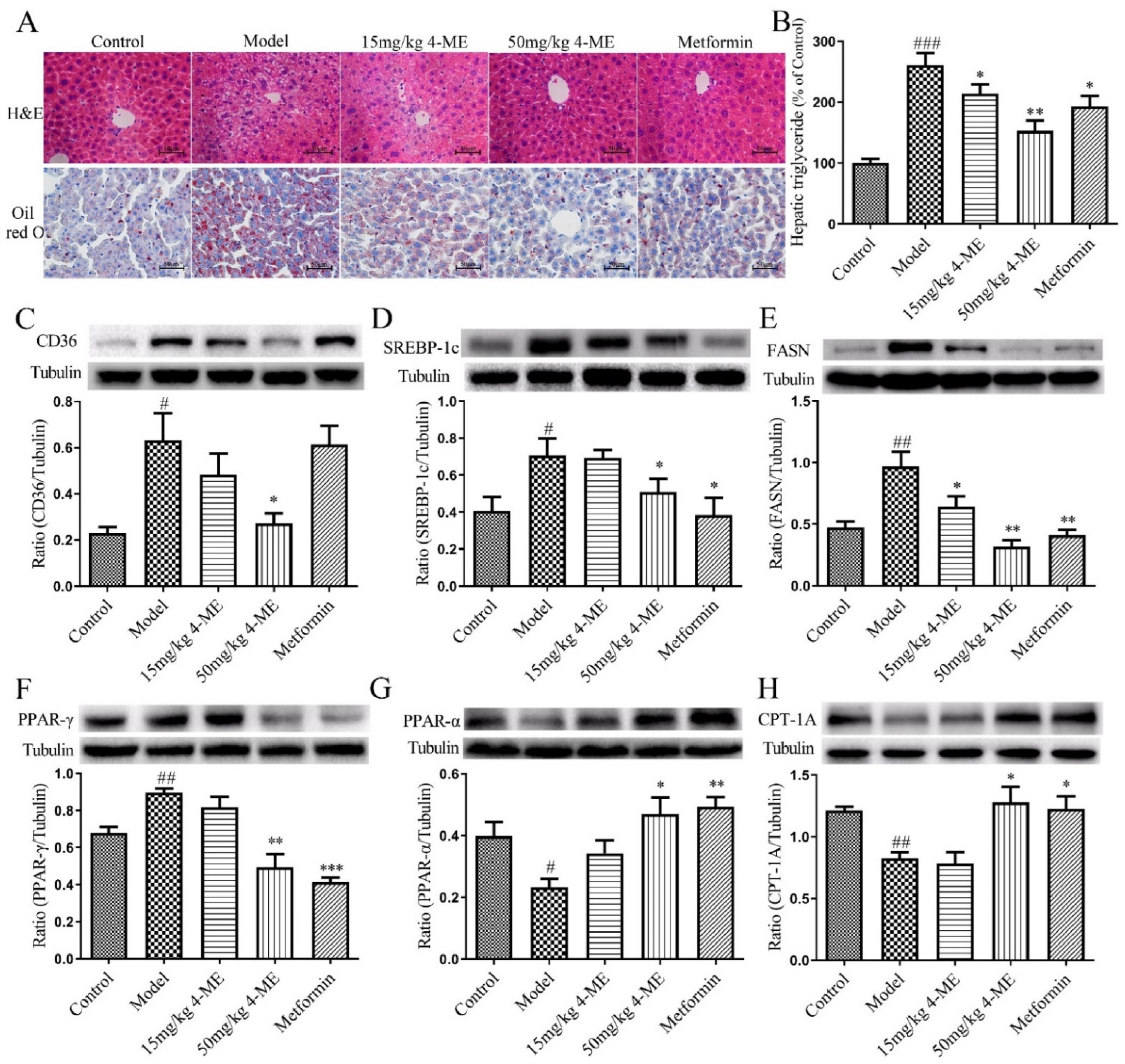
2.3. Effects of 4-ME upon Lipid Metabolism and Hepatic Steatosis in HFD-Fed Mice
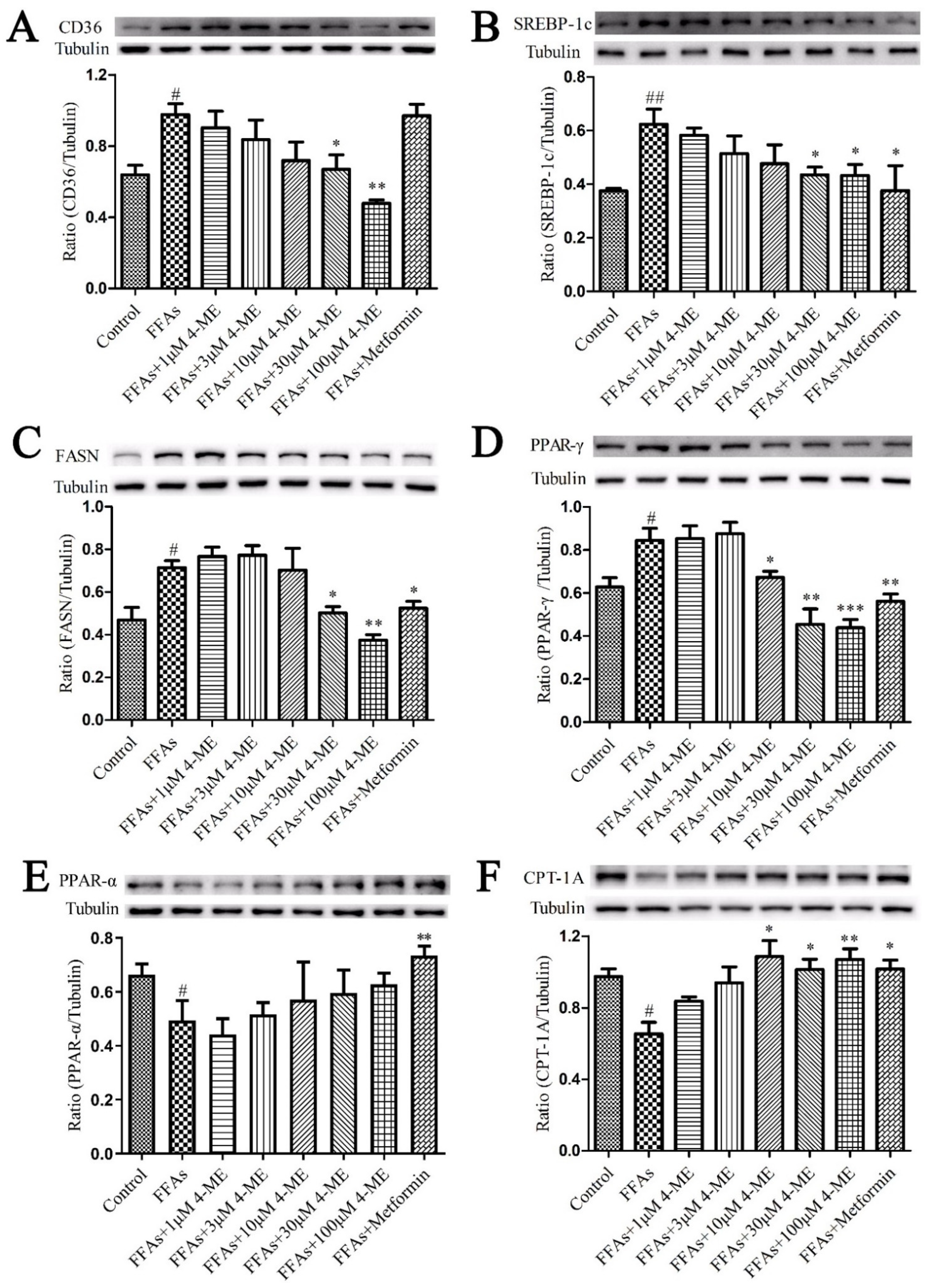
2.4. Effects of 4-ME on the Expression of Proteins Involved in NEFA Uptake, Lipogenesis, and Fatty Acid β-Oxidation in Livers of HFD-Fed Mice
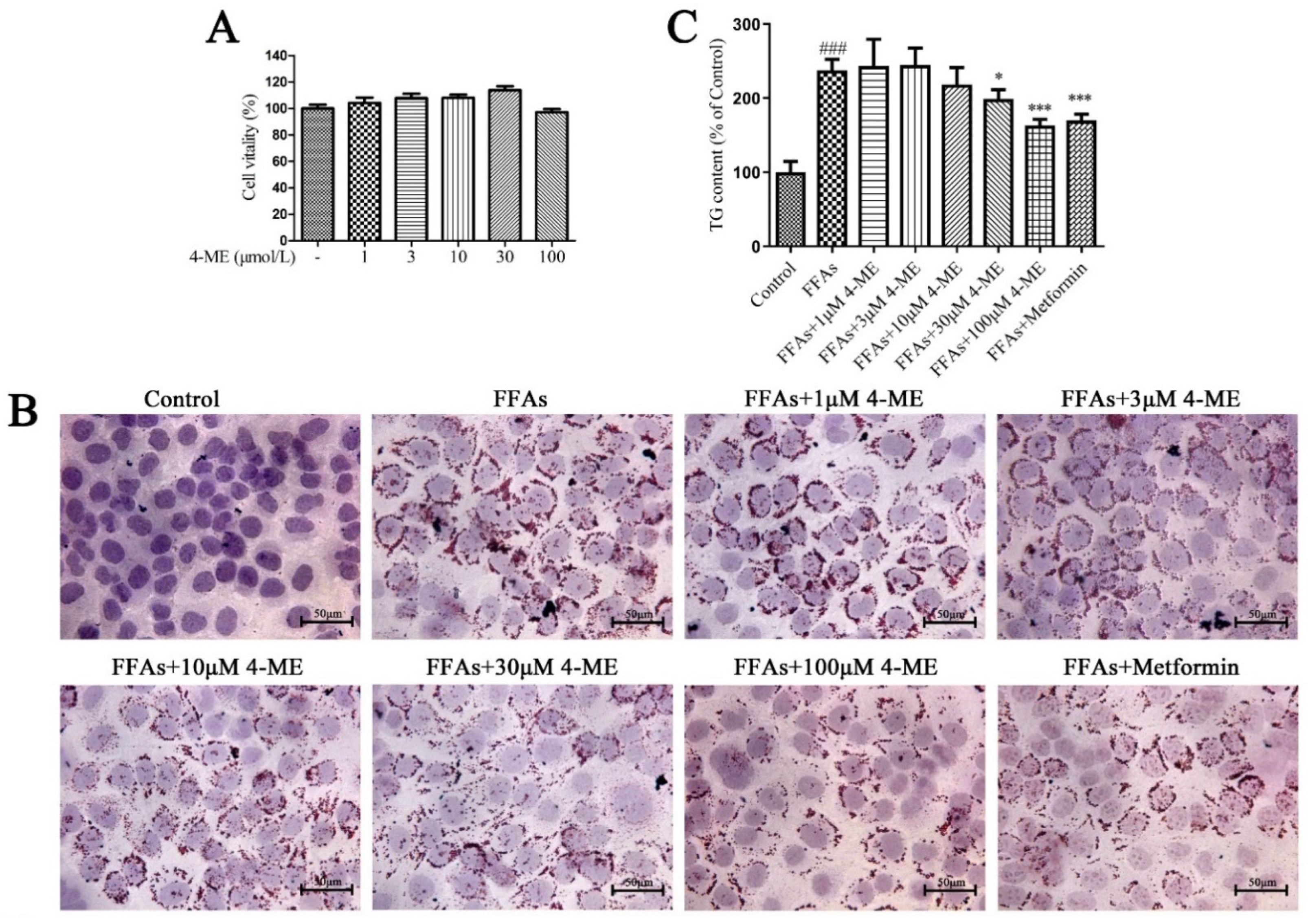
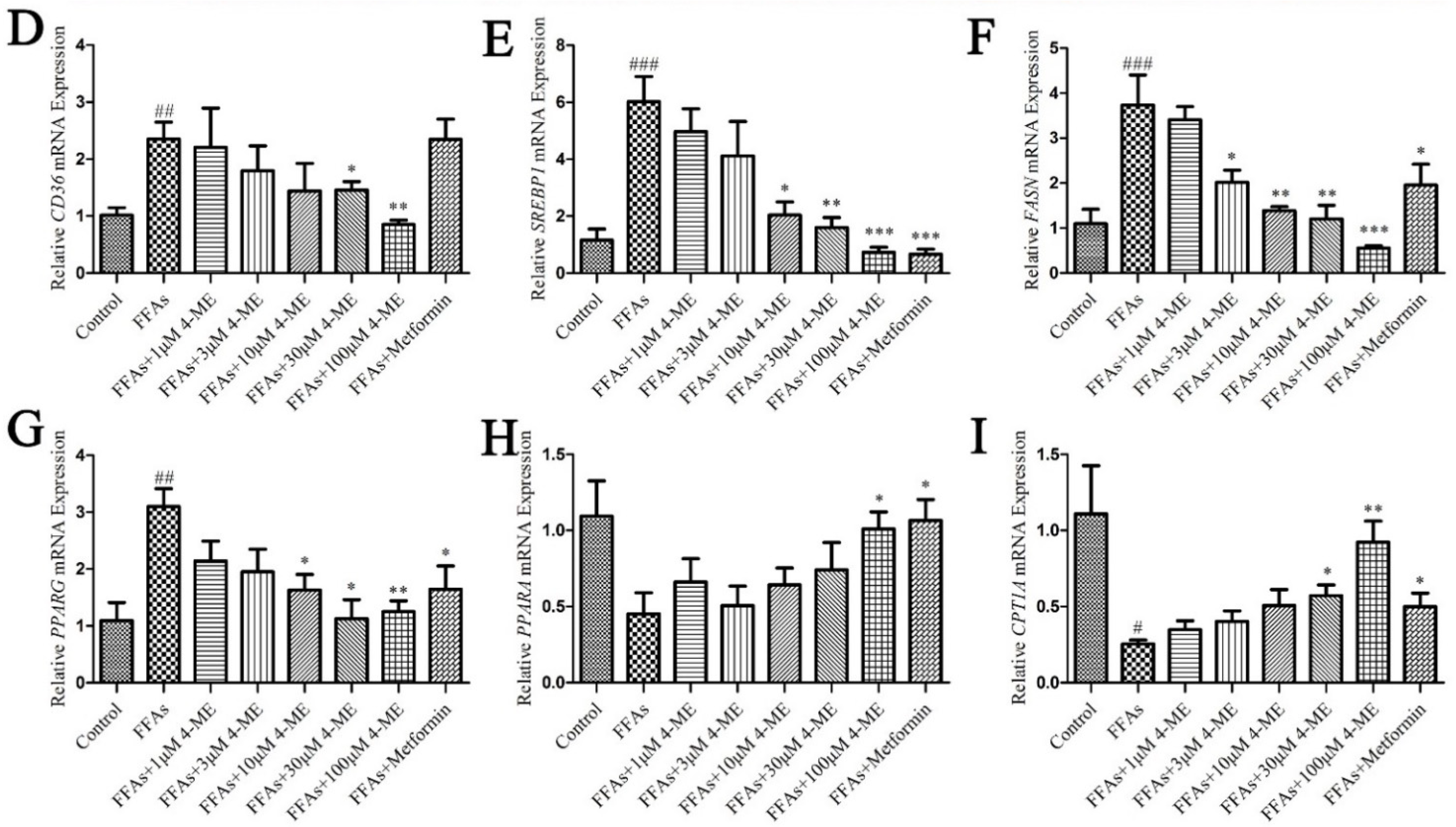
2.5. Effects of 4-ME upon Lipid Deposition in FFA-Incubated LO2 Cells
2.6. Nrf2 Is Necessary for 4-ME Reducing Lipid Deposition
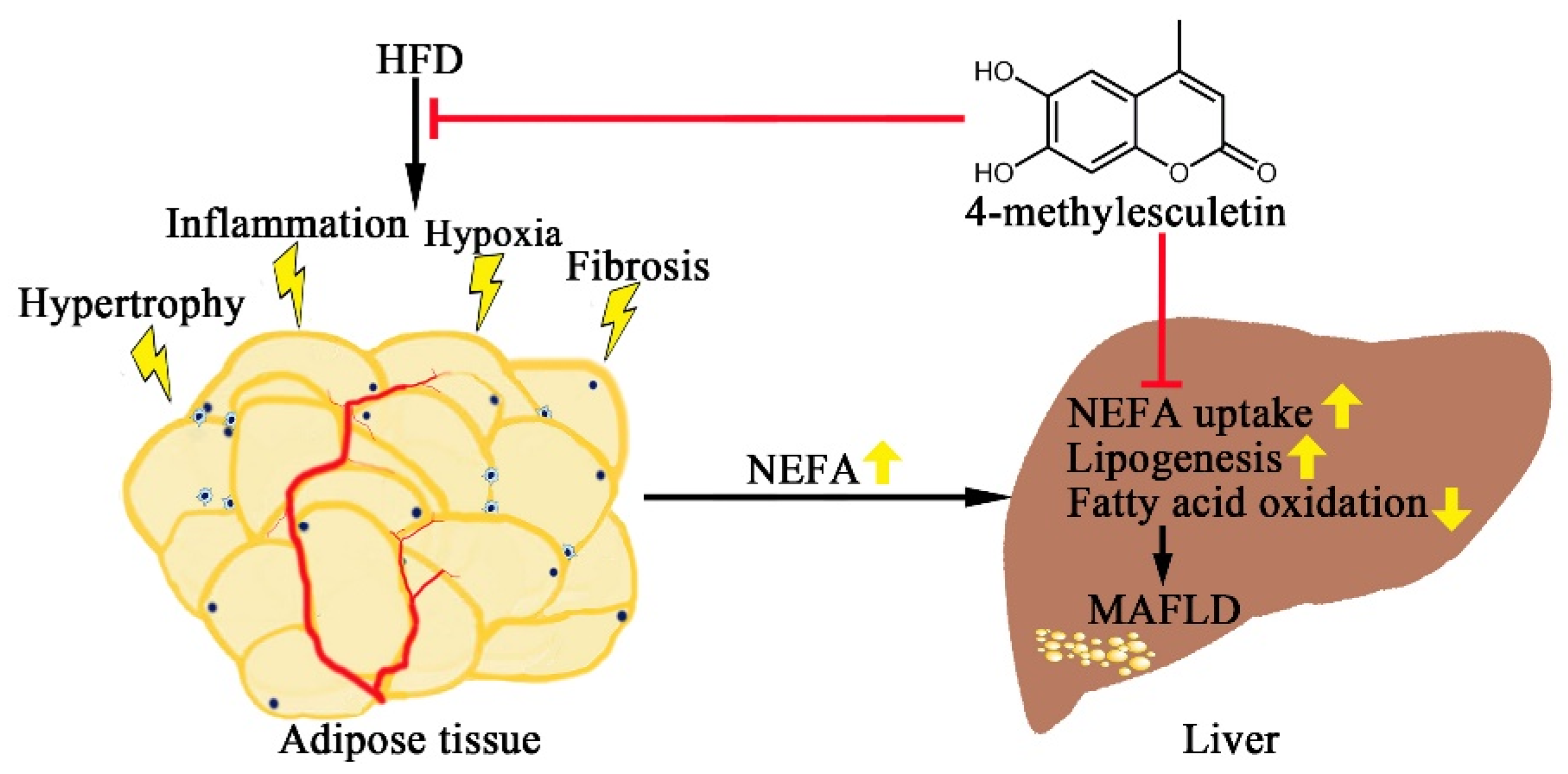
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals Studies
4.3. Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. Hepatic and Intracellular Triglyceride Determination
4.6. Oil Red O Staining
4.7. Intraperitoneal Glucose Tolerance Test
4.8. Serum Analysis
4.9. Histological and Immunohistochemistry Studies
4.10. Western Blotting
4.11. Total RNA Extraction and Quantitative RT-PCR
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, L.; Li, Y.J.; Zhang, D.; Chen, Y.; Kostecká, P.; Kmoníèková, E.; Zídek, Z. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharm. Rep. 2013, 65, 410–420. [Google Scholar] [CrossRef]
- Paya, M.; Halliwell, B.; Hoult, J.R.S. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Ning, C.; Liu, K.; Wang, X.; Liu, Z.; Sun, H.; Ma, X.; Sun, P.; Meng, Q. Hepatoprotection of auraptene from peels of citrus fruits against thioacetamide-induced hepatic fibrosis in mice by activating farnesoid X receptor. Food Funct. 2018, 9, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Sunitha, K.; Thushara, R.M.; Sebastin Santhosh, M.; Shanmuga Sundaram, M.; Kemparaju, K.; Girish, K.S. Antiarthritic and antiinflammatory propensity of 4-methylesculetin, a coumarin derivative. Biochimie 2013, 95, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; de Oliveira, E.C.S.; Tanimoto, A.; Zorzella-Pezavento, S.F.G.; de Oliveira, S.L.; Sartori, A.; Di Stasi, L.C. 4-methylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chem. Biol. Interact. 2018, 280, 59–63. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, Z.; Zhang, L.; Liu, Y.; Ding, H.; Yin, S. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017, 8, 2886–2896. [Google Scholar] [CrossRef]
- Nugara, R.N.; Inafuku, M.; Takara, K.; Iwasaki, H.; Oku, H. Pteryxin: A coumarin in Peucedanum japonicum Thunb leaves exerts antiobesity activity through modulation of adipogenic gene network. Nutrition 2014, 30, 1177–1184. [Google Scholar] [CrossRef]
- Singh, L.R.; Kumar, A.; Upadhyay, A.; Gupta, S.; Palanati, G.R.; Sikka, K.; Siddiqi, M.I.; Yadav, P.N.; Sashidhara, K.V. Discovery of coumarin-dihydroquinazolinone analogs as niacin receptor 1 agonist with in-vivo anti-obesity efficacy. Eur. J. Med. Chem. 2018, 152, 208–222. [Google Scholar] [CrossRef]
- Watanabe, A.; Kato, T.; Ito, Y.; Yoshida, I.; Harada, T.; Mishima, T.; Fujita, K.; Watai, M.; Nakagawa, K.; Miyazawa, T. Aculeatin, a coumarin derived from Toddalia asiatica (L.) Lam., enhances differentiation and lipolysis of 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2014, 453, 787–792. [Google Scholar] [CrossRef]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol. Leg. Content 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Kitteringham, N.R.; Abdullah, A.; Walsh, J.; Randle, L.; Jenkins, R.E.; Sison, R.; Goldring, C.E.; Powell, H.; Sanderson, C.; Williams, S.; et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J. Proteom. 2010, 73, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Investig. 2015, 125, 1790–1792. [Google Scholar] [CrossRef]
- Seebacher, F.; Zeigerer, A.; Kory, N.; Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020, 108, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose Tissue-Liver Cross Talk in the Control of Whole-Body Metabolism: Implications in Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol. Cell. Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinkens, R.; Goossens, G.H.; Jocken, J.W.; Blaak, E.E. Targeting fatty acid metabolism to improve glucose metabolism. Obes. Rev. 2015, 16, 715–757. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.; Jacobs, R.L.; Febbraio, M.; Young, M.E.; Soltys, C.L.; Ong, H.; Vance, D.E.; Dyck, J.R. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007, 56, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Kim, W.D.; Hwang, S.J.; Choi, H.S.; Gilroy, R.K.; Wan, Y.J.; Kim, S.G. Nrf2 inhibits LXRalpha-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid. Redox Signal. 2011, 15, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Annie-Mathew, A.S.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D.V.L. The pivotal role of Nrf2 activators in adipocyte biology. Pharmacol. Res. 2021, 173, 105853. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Park, Y.; Sung, J.; Yang, J.; Ham, H.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of esculetin on free-fatty-acid-induced lipid accumulation in human HepG2 cells through activation of AMP-activated protein kinase. Food Sci. Biotechnol. 2017, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Ham, J.R.; Lee, M.K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem. Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Ou, X.; Luo, G.; Xie, Y.; Sun, R.; Wang, Y.; Qi, X.; Hu, M.; Liu, Z.; et al. Breast Cancer Resistance Protein and Multidrug Resistance Protein 2 Determine the Disposition of Esculetin-7-O-Glucuronide and 4-Methylesculetin-7-O-Glucuronide. Drug Metab. Dispos. 2019, 47, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, W.; Wang, C.; Qi, J.; Li, H. Mogroside V Protects against Hepatic Steatosis in Mice on a High-Fat Diet and LO2 Cells Treated with Free Fatty Acids via AMPK Activation. Evid. Based Complement. Alternat. Med. 2020, 2020, 7826874. [Google Scholar] [CrossRef] [PubMed]


| Groups (n = 8) | Body Weight (g) | Epididymal Fat/Body Weight (mg/g) | The Lee Index | ||||
|---|---|---|---|---|---|---|---|
| 0 Week | 2 Week | 4 Week | 6 Week | 8 Week | |||
| Control | 37.1 ± 0.4 | 36.4 ± 0.3 | 35.2 ± 0.8 | 35.5 ± 0.5 | 34.6 ± 0.97 | 15.6 ± 1.2 | 0.301 ± 0.001 |
| Model | 48.8 ± 2.0 ### | 49.3 ± 2.3 ### | 49.8 ± 2.9 ### | 51.8 ± 3.1 ### | 52.6 ± 3.1 ### | 46.0 ± 6.8 ### | 0.338 ± 0.003 ### |
| 15 mg/kg 4-ME | 48.8 ± 2.3 | 48.1 ± 2.0 | 48.9 ± 2.1 | 50.3 ± 2.2 | 49.5 ± 2.5 | 37.3 ± 2.8 | 0.331 ± 0.004 |
| 50 mg/kg 4-ME | 48.6 ± 2.9 | 48.4 ± 2.6 | 48.0 ± 2.8 | 47.0 ± 1.1 | 45.8 ± 2.3 * | 30.7 ± 2.8 * | 0.320 ± 0.002 *** |
| Metformin | 49.0 ± 2.0 | 48.1 ± 1.6 | 47.9 ± 1.4 | 46.7 ± 2.2 | 45.5 ± 1.1 * | 29.3 ± 3.1 * | 0.326 ± 0.003 * |
| Groups (n = 8) | FBG (mmol/L) | Insulin (mIU/L) | HOMA-IR | Leptin (pg/mL) | HDL-C (mmol/L) | LDL-C (mmol/L) | T-CHO (mmol/L) | TG (mmol/L) | NEFA (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 4.9 ± 0.3 | 4.3 ± 0.3 | 0.91 ± 0.04 | 499.4 ± 31.2 | 1.2 ± 0.1 | 0.9 ± 0.1 | 3.7 ± 0.2 | 0.88 ± 0.03 | 441.1 ± 23.8 |
| Model | 11.7 ± 0.8 ### | 6.1 ± 0.3 ### | 3.17 ± 0.16 ### | 634.5 ± 29.3 ## | 0.9 ± 0.1 | 2.1 ± 0.2 ### | 7.0 ± 0.6 ### | 1.27 ± 0.12 ## | 1023.0 ± 88.6 ### |
| 15 mg/kg 4-ME | 9.5 ± 0.5 * | 5.8 ± 0.3 | 2.82 ± 0.34 | 610.2 ± 29.0 | 1.0 ± 0.1 | 2.1 ± 0.1 | 6.6 ± 0.4 | 1.25 ± 0.07 | 797.3 ± 121.1 * |
| 50 mg/kg 4-ME | 9.9 ± 0.6 * | 5.3 ± 0.2 * | 2.35 ± 0.12 ** | 548.8 ± 25.0 * | 1.1 ± 0.2 | 1.7 ± 0.1 | 6.4 ± 0.5 | 1.13± 0.08 | 521.2 ± 18.9 *** |
| Metformin | 8.7 ± 0.5 ** | 6.8 ± 0.2 | 2.53 ± 0.13 ** | 571.7 ±16.2 | 1.4 ± 0.2 * | 1.2 ± 0.1 ** | 6.4 ± 0.3 | 1.18 ± 0.11 | 655.4 ± 48.8 ** |
| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| CD36 | GAG AAC TGT TAT GGG GCT AT | TTC AAC TGG AGA GGC AAA GG |
| SREBP1 | ACG GGA TGG ACT GAC TT | AGG CTT CTT TGC TGT GAG ATG |
| FASN | AAG GAC CTG TCT AGG TTT GAT GC | TGG CTT CAT AGG TGA CTT CCA |
| PPARG | ACT CCA CAT TAC GAA GAC AT | CTC CAC AGA CAC GAC ATT |
| PPARA | GCG AAC GAT TCG ACT CAA GC | TAC CGT TGT GTG ACA TCC CG |
| CPT1A | TGT CCA GCC AGA CGA AGA AC | ATC TTG CCG TGC TCA GTG AA |
| GAPDH | GTC TCC TCT GAC TTC AAC AGC G | ACC ACC CTG TTG CTG TAG CCA A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhu, G.; Fu, G.; Zha, W.; Li, H. Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation. Int. J. Mol. Sci. 2022, 23, 10465. https://doi.org/10.3390/ijms231810465
Li L, Zhu G, Fu G, Zha W, Li H. Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation. International Journal of Molecular Sciences. 2022; 23(18):10465. https://doi.org/10.3390/ijms231810465
Chicago/Turabian StyleLi, Linghuan, Guangyao Zhu, Gaohang Fu, Weiwei Zha, and Hanbing Li. 2022. "Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation" International Journal of Molecular Sciences 23, no. 18: 10465. https://doi.org/10.3390/ijms231810465
APA StyleLi, L., Zhu, G., Fu, G., Zha, W., & Li, H. (2022). Metabolic Syndrome Ameliorated by 4-Methylesculetin by Reducing Hepatic Lipid Accumulation. International Journal of Molecular Sciences, 23(18), 10465. https://doi.org/10.3390/ijms231810465







