The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits
Abstract
1. Introduction
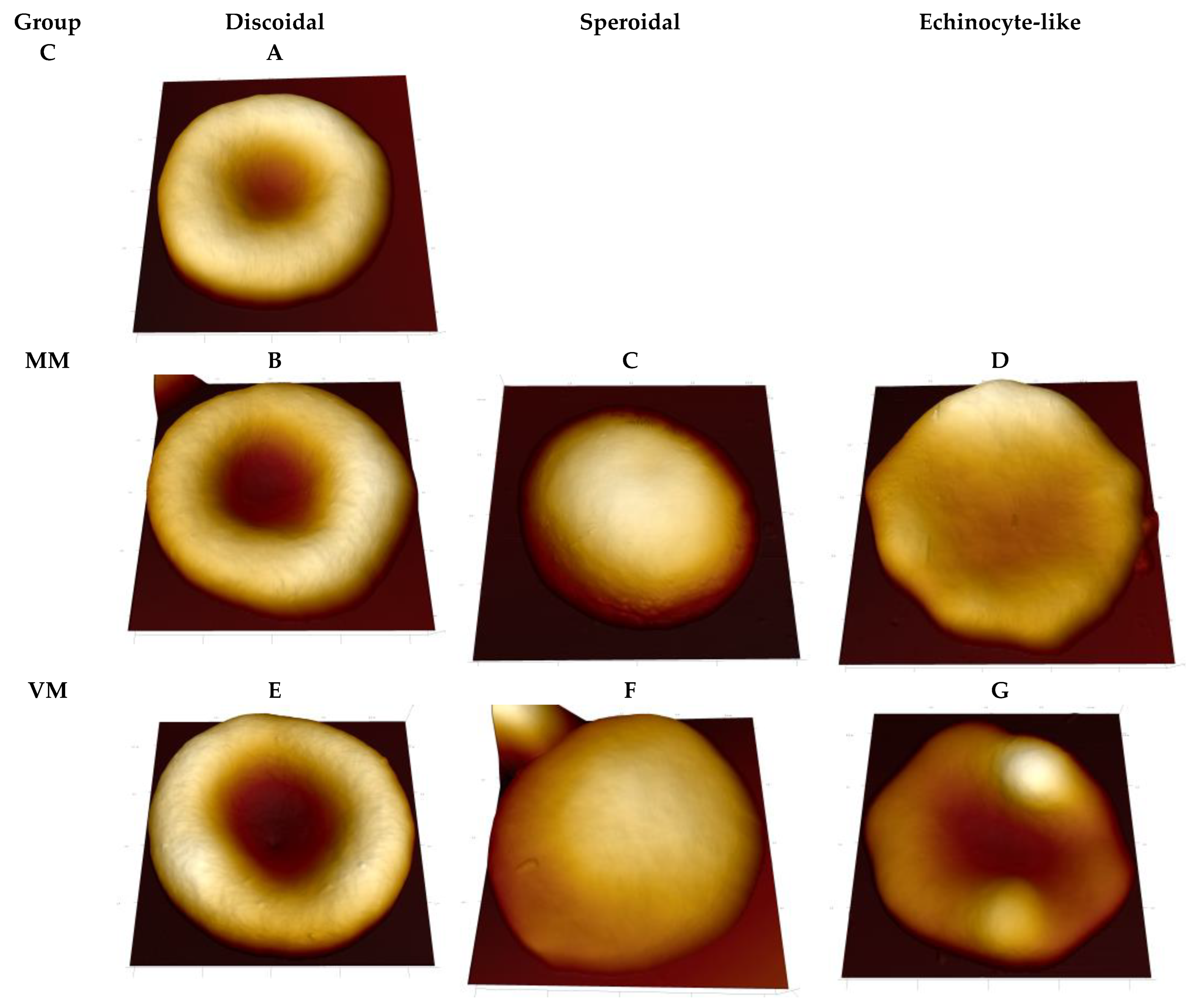
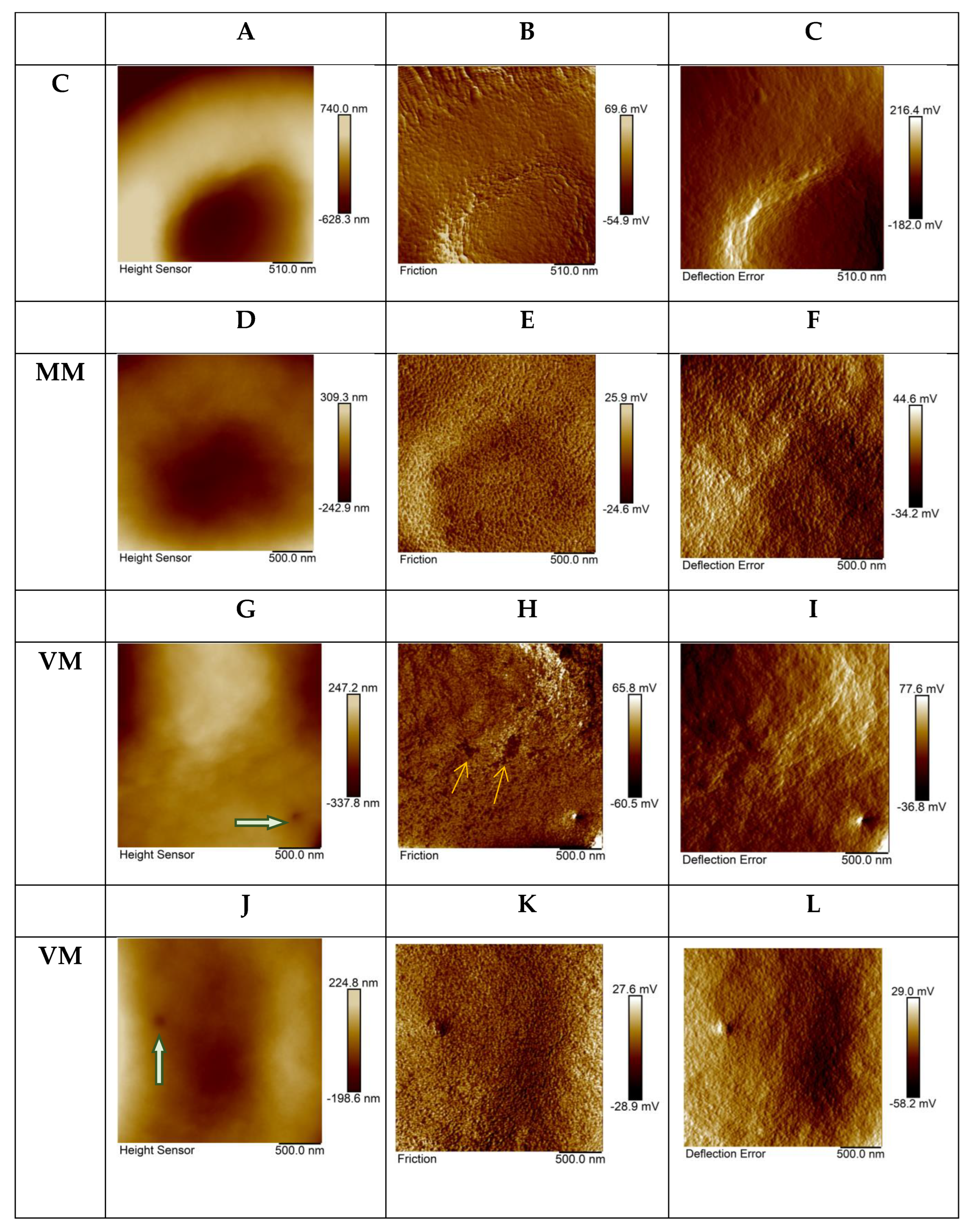
2. Results
3. Discussion
Limitations and Future Studies
4. Materials and Methods
4.1. Animals and Antifungal Drugs Administration Conditions
4.2. Preparation of Erythrocyte Samples for AFM
4.3. Atomic Force Microscopy
4.4. AFM Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.A.; Elhusseiny, A.M.; Siddiqui, M.Z.; Ahmad, K.T.; Sallam, A.B. Fungal Endophthalmitis: A Comprehensive Review. J. Fungi 2021, 7, 996. [Google Scholar] [CrossRef] [PubMed]
- Karachrysafi, S.; Sioga, A.; Komnenou, A.; Karamitsos, A.; Xioteli, M.; Dori, I.; Delis, G.; Kofidou, E.; Anastasiadou, P.; Sotiriou, S.; et al. Histological Effects of Intravitreal Injection of Antifungal Agents in New Zealand White Rabbits: An Electron Microscopic and Immunohistochemical Study. Pharmaceuticals 2020, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.O.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Peter, T.; Bissinger, R.; Liu, G.; Lang, F. Anidulafungin-Induced Suicidal Erythrocyte Death. Cell. Physiol. Biochem. 2016, 38, 2272–2284. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Starodubtseva, M.N. Mechanical Properties of the Cell Surface Layer Measured by Contact Atomic Force Microscopy. In Contact Problems for Soft, Biological and Bioinspired Materials; Borodich, F.M., Jin, X., Eds.; Biologically-Inspired Systems; Springer: Cham, Switzerland, 2022; Volume 15. [Google Scholar] [CrossRef]
- Starodubtseva, M.N.; Mitsura, E.F.; Starodubtsev, I.E.; Chelnokova, I.A.; Yegorenkov, N.I.; Volkova, L.I.; Kharin, Y.S. Nano- and microscale mechanical properties of erythrocytes in hereditary spherocytosis. J. Biomech. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. Friction Determination by Atomic Force Microscopy in Field of Biochemical Science. Micromachines 2018, 9, 313. [Google Scholar] [CrossRef]
- Ebner, A.; Schillers, H.; Hinterdorfer, P. Normal and Pathological Erythrocytes Studied by Atomic Force Microscopy. In Atomic Force Microscopy in Biomedical Research; Braga, P., Ricci, D., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 736. [Google Scholar] [CrossRef]
- Yeow, N.; Tabor, R.F.; Garnier, G. Atomic force microscopy: From red blood cells to immunohaematology. Adv. Colloid Interface Sci. 2017, 249, 149–162. [Google Scholar] [CrossRef]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyakov, V.; Sherstyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors 2022, 22, 2055. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Niece, K.L.; Boyd, N.K.; Akers, K.S. In vitro study of the variable effects of proton pump inhibitors on voriconazole. Antimicrob. Agents Chemother. 2015, 59, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Peter, T.; Bissinger, R.; Signoretto, E.; Mack, A.F.; Lang, F. Micafungin-Induced Suicidal Erythrocyte Death Cell. Physiol. Biochem. 2016, 39, 584–595. [Google Scholar]
- Bouatou, Y.; Samer, C.F.; Ing Lorenzini, K.R.; Daali, Y.; Daou, S.; Fathi, M.; Rebsamen, M.; Desmeules, J.; Calmy, A.; Escher, M. Therapeutic drug monitoring of voriconazole: A case report of multiple drug interactions in a patient with an increased CYP2C19 activity. AIDS Res. Ther. 2014, 11, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, W.; Lin, Y.C.; Luo, Y.L. Mechanical properties of anionic asymmetric bilayers from atomistic simulations. J. Chem. Phys. 2021, 154, 224701. [Google Scholar] [CrossRef]
- Nguyen, L.; Man, V.H.; Li, M.S.; Derreumaux, P.; Wang, J.; Nguyen, P.H. Elastic moduli of normal and cancer cell membranes revealed by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2022, 24, 6225–6237. [Google Scholar] [CrossRef]
- Mochizuki, K.; Sawada, A.; Suemori, S.; Kawakami, H.; Niwa, Y.; Kondo, Y.; Ohkusu, K.; Yamada, N.; Ogura, S.; Yaguchi, T.; et al. Intraocular penetration of intravenous micafungin in inflamed human eyes. Antimicrob. Agents Chemother. 2013, 57, 4027–4030. [Google Scholar] [CrossRef]
- Shen, Y.C.; Wang, M.Y.; Wang, C.Y.; Tsai, T.C.; Tsai, H.Y.; Lee, Y.F.; Wei, L.C. Clearance of intravitreal voriconazole. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2238–2241. [Google Scholar] [CrossRef]
- Gao, H.; Pennesi, M.E.; Shah, K.; Qiao, X.; Hariprasad, S.M.; Mieler, W.F.; Holz, E.R. Intravitreal voriconazole: An electroretinographic and histopathologic study. Arch. Ophthalmol. 2004, 122, 1687–1692. [Google Scholar] [CrossRef]
- Harrison, J.M.; Glickman, R.D.; Ballentine, C.S.; Trigo, Y.; Pena, M.A.; Kurian, P.; Graybill, J.R. Retinal function assessed by ERG before and after induction of ocular aspergillosis and treatment by the anti-fungal, micafungin, in rabbits. Doc. Ophthalmol. 2005, 110, 37–55. [Google Scholar] [CrossRef]
- Missel, P.J. Simulating Intravitreal Injections in Anatomically Accurate Models for Rabbit, Monkey, and Human Eyes. Pharm. Res. 2012, 29, 3251–3272. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
| Sample | Height | Deflection Error | Friction |
|---|---|---|---|
| Control (n = 20) | 79.5(74.3;95.5) nm | 12.9(10.5;15.5) mV | 7.0(6.0;10.9) mV |
| ΜM (n = 38) | * 29.0(20.5;39.5) nm | ** 7.0(5.0;9.6) mV | *** 11.0(8.3;15.5) mV |
| VM (n = 42) | * 26.5(22.0;40.25) nm | ** 7.0(4.3;8.9) mV | **** 10.0(8.0;13.2) mV |
| Sample | Peak 1 | Peak 2 | Peak 3 | R2 |
|---|---|---|---|---|
| Control | 43 nm (59%) | 82 nm (30%) | 161 nm (11%) | 0.996 |
| ΜM | 48 nm (59%) | 94 nm (32%) | 206 nm (8%) | 0.995 |
| VM | 46 nm (64%) | 91 nm (28%) | 199 nm (8%) | 0.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtseva, M.N.; Karachrysafi, S.; Shkliarava, N.M.; Chelnokova, I.A.; Kavvadas, D.; Papadopoulou, K.; Samara, P.; Papaliagkas, V.; Sioga, A.; Komnenou, A.; et al. The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits. Int. J. Mol. Sci. 2022, 23, 10464. https://doi.org/10.3390/ijms231810464
Starodubtseva MN, Karachrysafi S, Shkliarava NM, Chelnokova IA, Kavvadas D, Papadopoulou K, Samara P, Papaliagkas V, Sioga A, Komnenou A, et al. The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits. International Journal of Molecular Sciences. 2022; 23(18):10464. https://doi.org/10.3390/ijms231810464
Chicago/Turabian StyleStarodubtseva, Maria N., Sofia Karachrysafi, Nastassia M. Shkliarava, Irina A. Chelnokova, Dimitrios Kavvadas, Kyriaki Papadopoulou, Paraskevi Samara, Vasileios Papaliagkas, Antonia Sioga, Anastasia Komnenou, and et al. 2022. "The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits" International Journal of Molecular Sciences 23, no. 18: 10464. https://doi.org/10.3390/ijms231810464
APA StyleStarodubtseva, M. N., Karachrysafi, S., Shkliarava, N. M., Chelnokova, I. A., Kavvadas, D., Papadopoulou, K., Samara, P., Papaliagkas, V., Sioga, A., Komnenou, A., Karampatakis, V., & Papamitsou, T. (2022). The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits. International Journal of Molecular Sciences, 23(18), 10464. https://doi.org/10.3390/ijms231810464










