Microrna-486-5P Regulates Human Pulmonary Artery Smooth Muscle Cell Migration via Endothelin-1
Abstract
:1. Introduction
2. Results
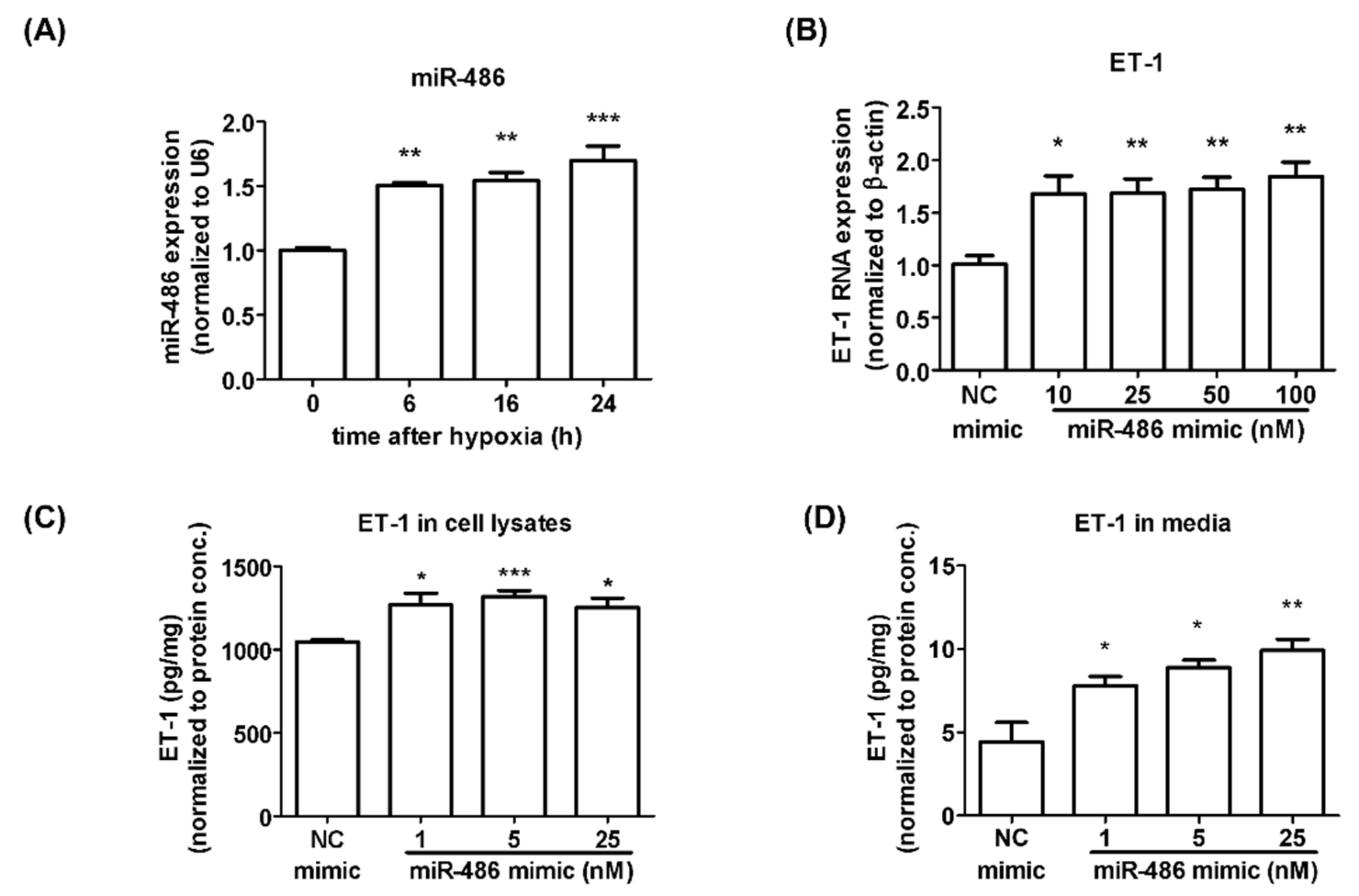
2.1. Alteration of miRNA Expression under Hypoxia Treatment in Isolated HPASMCs
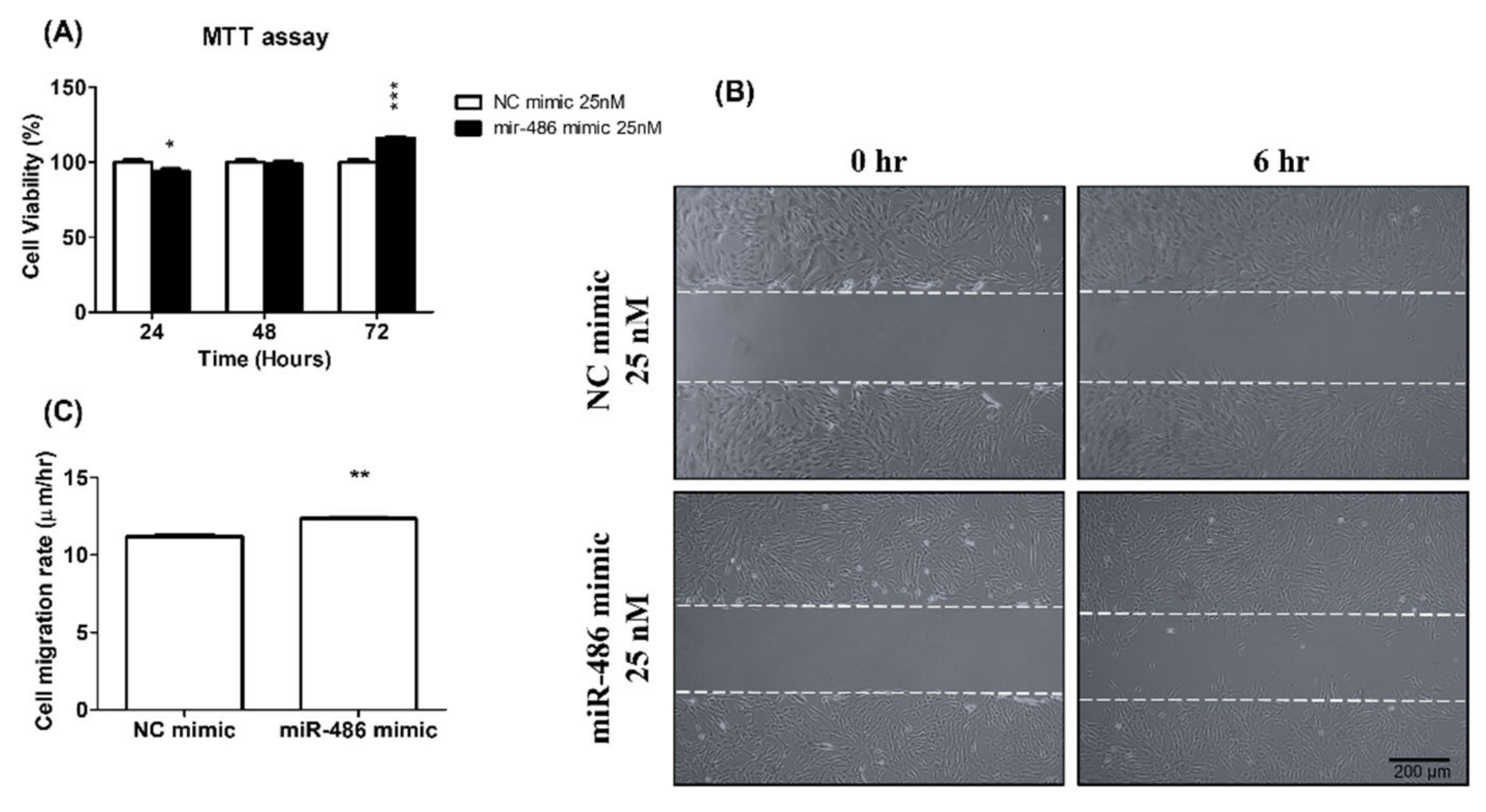
2.2. miR-486-5p Induced HPSMC Proliferation and Migration Ability
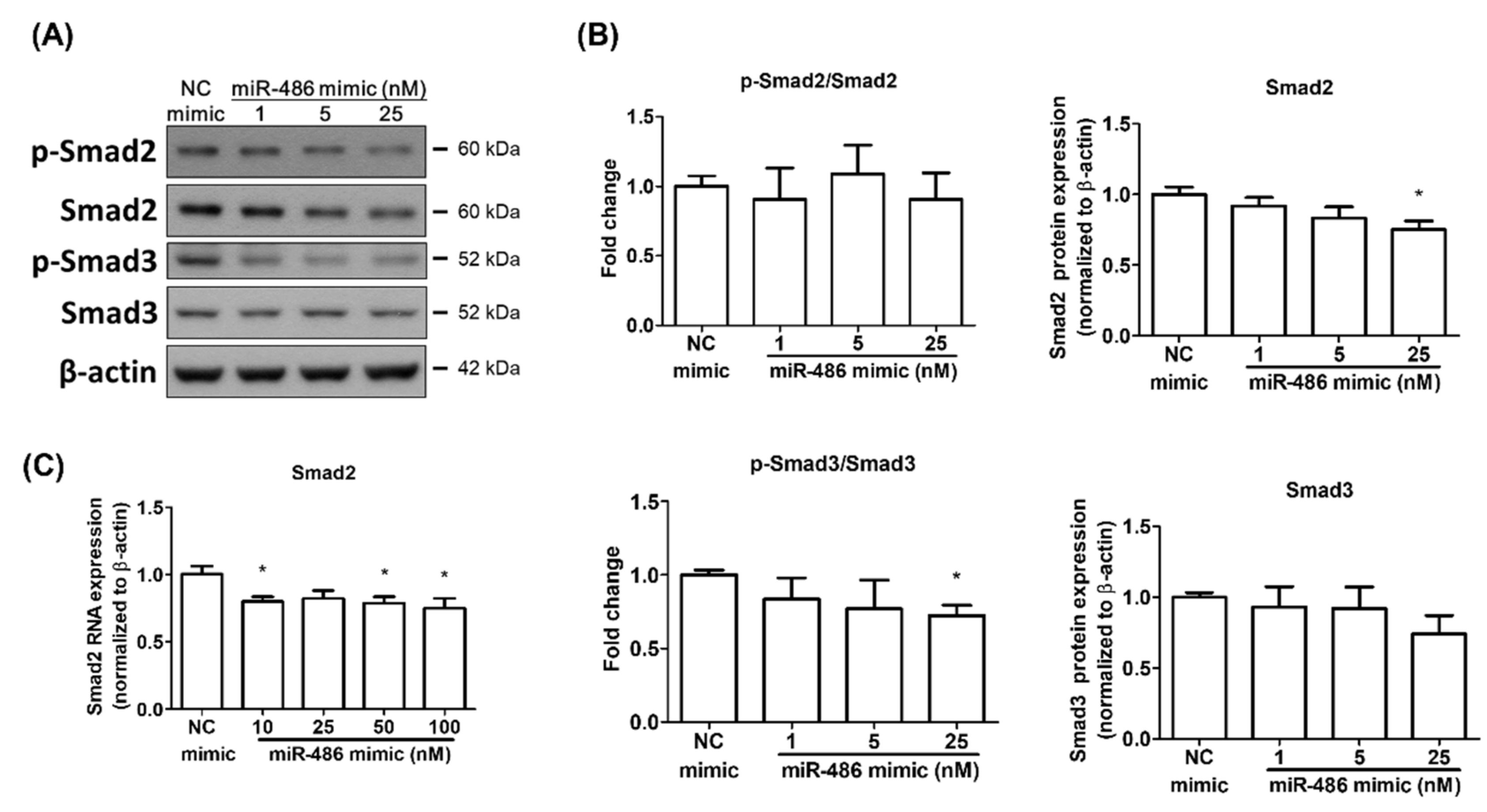
2.3. miR-486-5p Regulated Smad2 and Smad3 in HPSMCs Isolated from a CHD Patient
3. Discussion
4. Materials and Methods
4.1. HPASMC Isolation and Cell Culture
4.2. Induction of Hypoxia in HPASMCs
4.3. Levels of miRNAs in HPASMCs Analyzed by Next-Generation Sequencing (NGS)
4.4. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.5. miRNA Mimic Transfection
4.6. Western Blot Analysis
4.7. Cell Proliferation Assays
4.8. Wound-Healing Assay
4.9. ET-1 ELISA
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galiè, N.; Ghofrani, A.H. New horizons in pulmonary arterial hypertension therapies. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2013, 22, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.K.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43 (Suppl. S12), 13S–24S. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Marecki, J.C.; Richter, A.; Fijalkowska, I.; Flores, S. Pathology of pulmonary hypertension. Clin. Chest Med. 2007, 28, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Adnot, S.; Archer, S.L.; Dupuis, J.; Jones, P.L.; MacLean, M.R.; McMurtry, I.F.; Stenmark, K.R.; Thistlethwaite, P.A.; Weissmann, N.; et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. S1), S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.P. Hypoxia on the pulmonary circulation. How and where it acts. Circ. Res. 1976, 38, 221–231. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Whitman, E.M.; Pisarcik, S.; Luke, T.; Fallon, M.; Wang, J.; Sylvester, J.T.; Semenza, G.L.; Shimoda, L.A. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L309–L318. [Google Scholar] [CrossRef]
- He, J.; Yi, B.; Chen, Y.; Huang, Q.; Wang, H.; Lu, K.; Fu, W. The ET-1-mediated carbonylation and degradation of ANXA1 induce inflammatory phenotype and proliferation of pulmonary artery smooth muscle cells in HPS. PLoS ONE 2017, 12, e0175443. [Google Scholar] [CrossRef]
- Humbert, M.; Lau, E.M.; Montani, D.; Jaïs, X.; Sitbon, O.; Simonneau, G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014, 130, 2189–2208. [Google Scholar] [CrossRef]
- Bouallegue, A.; Daou, G.B.; Srivastava, A.K. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr. Vasc. Pharmacol. 2007, 5, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Stenmark, K.R.; Rabinovitch, M. Emerging therapies for the treatment of pulmonary hypertension. Pediatric Crit. Care Med. A J. Soc. Crit. Care Med. World Fed. Pediatric Intensive Crit. Care Soc. 2010, 11 (Suppl. S2), S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Paulin, R.; Provencher, S.; Bonnet, S. Therapeutic Potential of microRNA Modulation in Pulmonary Arterial Hypertension. Curr. Vasc. Pharmacol. 2015, 13, 331–340. [Google Scholar] [CrossRef]
- Farh, K.K.; Grimson, A.; Jan, C.; Lewis, B.P.; Johnston, W.K.; Lim, L.P.; Burge, C.B.; Bartel, D.P. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 2005, 310, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in pulmonary arterial hypertension: Pathogenesis, diagnosis and treatment. J. Am. Soc. Hypertens. JASH 2015, 9, 221–234. [Google Scholar] [CrossRef]
- Courboulin, A.; Paulin, R.; Giguère, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Côté, J.; et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011, 208, 535–548. [Google Scholar] [CrossRef]
- Kang, K.; Peng, X.; Zhang, X.; Wang, Y.; Zhang, L.; Gao, L.; Weng, T.; Zhang, H.; Ramchandran, R.; Raj, J.U.; et al. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 2013, 288, 25414–25427. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Ku, T.; Yun, Y.; Li, G.; Sang, N. MicroRNA-338-5p modulates pulmonary hypertension-like injuries caused by SO(2), NO(2) and PM(2.5) co-exposure through targeting the HIF-1α/Fhl-1 pathway. Toxicol. Res. 2016, 5, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Zhao, S.; Fa, X. Upregulated miR-17 Regulates Hypoxia-Mediated Human Pulmonary Artery Smooth Muscle Cell Proliferation and Apoptosis by Targeting Mitofusin 2. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 3301–3308. [Google Scholar] [CrossRef]
- Shi, X.F.; Wang, H.; Xiao, F.J.; Yin, Y.; Xu, Q.Q.; Ge, R.L.; Wang, L.S. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem. Biophys. Res. Commun. 2016, 470, 670–677. [Google Scholar] [CrossRef]
- He, J.; Xiao, B.; Li, X.; He, Y.; Li, L.; Sun, Z. MiR-486-5p Suppresses Proliferation and Migration of Hepatocellular Carcinoma Cells through Downregulation of the E3 Ubiquitin Ligase CBL. BioMed Res. Int. 2019, 2019, 2732057. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Xu, K.; Zhu, L.; Mao, M.; Zhang, F.; Cui, L. MiR-486-5p Act as a Biomarker in Endometrial Carcinoma: Promotes Cell Proliferation, Migration, Invasion by Targeting MARK1. OncoTargets Ther. 2020, 13, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, K.; Su, S.; Li, J.; Li, C. miR-486-5p is upregulated in osteoarthritis and inhibits chondrocyte proliferation and migration by suppressing SMAD2. Mol. Med. Rep. 2018, 18, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.H.; Chao, C.F.; Tsai, S.H.; Chen, J.Y.; Chang, L.T. Autocrine effects of endothelin on in vitro proliferation of vascular smooth muscle cells from spontaneously hypertensive and normotensive rats. Clin. Exp. Hypertens. 2006, 28, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, L.; Yu, P.; Liu, Y.; Chen, W. MicroRNA-486-5p inhibits the growth of human hypertrophic scar fibroblasts by regulating Smad2 expression. Mol. Med. Rep. 2019, 19, 5203–5210. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.; Lei, X.; Zhu, Z.; Pei, C.; Qin, L. MicroRNA-486-5p suppresses TGF-β2-induced proliferation, invasion and epithelial-mesenchymal transition of lens epithelial cells by targeting Smad2. J. Biosci. 2017, 42, 575–584. [Google Scholar] [CrossRef]
- Ji, X.; Wu, B.; Fan, J.; Han, R.; Luo, C.; Wang, T.; Yang, J.; Han, L.; Zhu, B.; Wei, D.; et al. The Anti-fibrotic Effects and Mechanisms of MicroRNA-486-5p in Pulmonary Fibrosis. Sci. Rep. 2015, 5, 14131. [Google Scholar] [CrossRef]
- Ogo, T.; Chowdhury, H.M.; Yang, J.; Long, L.; Li, X.; Torres Cleuren, Y.N.; Morrell, N.W.; Schermuly, R.T.; Trembath, R.C.; Nasim, M.T. Inhibition of overactive transforming growth factor-β signaling by prostacyclin analogs in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 48, 733–741. [Google Scholar] [CrossRef]
- Rol, N.; Kurakula, K.B.; Happé, C.; Bogaard, H.J.; Goumans, M.J. TGF-β and BMPR2 Signaling in PAH: Two Black Sheep in One Family. Int. J. Mol. Sci. 2018, 19, 2585. [Google Scholar] [CrossRef]
- Zabini, D.; Granton, E.; Hu, Y.; Miranda, M.Z.; Weichelt, U.; Breuils Bonnet, S.; Bonnet, S.; Morrell, N.W.; Connelly, K.A.; Provencher, S.; et al. Loss of SMAD3 Promotes Vascular Remodeling in Pulmonary Arterial Hypertension via MRTF Disinhibition. Am. J. Respir. Crit. Care Med. 2018, 197, 244–260. [Google Scholar] [CrossRef]
- Han, X.M.; Tian, P.Y.; Zhang, J.L. MicroRNA-486-5p inhibits ovarian granulosa cell proliferation and participates in the development of PCOS via targeting MST4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7217–7223. [Google Scholar]
- Hu, H.; Xu, H.; Lu, F.; Zhang, J.; Xu, L.; Xu, S.; Jiang, H.; Zeng, Q.; Chen, E.; He, Z. Exosome-Derived miR-486-5p Regulates Cell Cycle, Proliferation and Metastasis in Lung Adenocarcinoma via Targeting NEK2. Front. Bioeng. Biotechnol. 2020, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.H.; Liu, Z.F.; Yang, B.B.; Yu, B. MicroRNA-486 inhibits cell proliferation, invasion and migration via down-regulating the TENM1 expressions and affecting ERK and Akt signaling pathways and epithelial-to-mesenchymal transition in papillary thyroid carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8429–8439. [Google Scholar] [PubMed]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A.; et al. MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associated symptoms. J. Clin. Investig. 2014, 124, 2651–2667. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Wang, X.; Zhang, Y.; Hui, J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 2019, 177, 23–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Wang, N.; Li, Y.; Zhang, D.; Li, Q. MicroRNA-486 Alleviates Hypoxia-Induced Damage in H9c2 Cells by Targeting NDRG2 to Inactivate JNK/C-Jun and NF-κB Signaling Pathways. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 48, 2483–2492. [Google Scholar] [CrossRef]
- Liu, Y.D.; Sun, X.; Zhang, Y.; Wu, H.J.; Wang, H.; Yang, R. Protocatechuic acid inhibits TGF-β1-induced proliferation and migration of human airway smooth muscle cells. J. Pharmacol. Sci. 2019, 139, 9–14. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Chen, X. Comparative effects of TGF-β2/Smad2 and TGF-β2/Smad3 signaling pathways on proliferation, migration, and extracellular matrix production in a human lens cell line. Exp. Eye Res. 2011, 92, 173–179. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Zhang, H.; Wang, J. MiRNA-199a-5p influences pulmonary artery hypertension via downregulating Smad3. Biochem. Biophys. Res. Commun. 2016, 473, 859–866. [Google Scholar] [CrossRef]
| Hypoxia/Normoxia | Hypoxia | Normoxia | ||
|---|---|---|---|---|
| miRNA | Fold Change | Up/Down | Seq(Normalized) | |
| hsa-miR-124-3p | −12.38 | Down | 0.13 | 1.61 |
| hsa-miR-1306-5p | −2.79 | Down | 1.01 | 2.82 |
| hsa-miR-219a-2-3p | −9.77 | Down | 0.13 | 1.27 |
| hsa-miR-338-3p | −9.78 | Down | 0.5 | 4.89 |
| hsa-miR-4804-5p | −3.25 | Down | 0.69 | 2.24 |
| hsa-miR-486-5p | 2.8 | UP | 4.42 | 1.58 |
| hsa-miR-6513-3p | 3.3 | UP | 1.32 | 0.4 |
| hsa-miR-874-3p | 2.8 | UP | 2.27 | 0.81 |
| hsa-miR-9-3p | −4.52 | Down | 1.77 | 8 |
| hsa-miR-9-5p | −5.87 | Down | 9.97 | 58.53 |
| hsa-miR-95-3p | −4.72 | Down | 0.5 | 2.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-A.; Huang, H.-C.; Wu, E.-T.; Chou, H.-W.; Chou, H.-C.; Chen, C.-Y.; Huang, S.-C.; Chen, Y.-S.; Lu, F.; Wu, M.-H.; et al. Microrna-486-5P Regulates Human Pulmonary Artery Smooth Muscle Cell Migration via Endothelin-1. Int. J. Mol. Sci. 2022, 23, 10400. https://doi.org/10.3390/ijms231810400
Yen T-A, Huang H-C, Wu E-T, Chou H-W, Chou H-C, Chen C-Y, Huang S-C, Chen Y-S, Lu F, Wu M-H, et al. Microrna-486-5P Regulates Human Pulmonary Artery Smooth Muscle Cell Migration via Endothelin-1. International Journal of Molecular Sciences. 2022; 23(18):10400. https://doi.org/10.3390/ijms231810400
Chicago/Turabian StyleYen, Ting-An, Hsin-Chung Huang, En-Ting Wu, Heng-Wen Chou, Hung-Chieh Chou, Chien-Yi Chen, Shu-Chien Huang, Yih-Sharng Chen, Frank Lu, Mei-Hwan Wu, and et al. 2022. "Microrna-486-5P Regulates Human Pulmonary Artery Smooth Muscle Cell Migration via Endothelin-1" International Journal of Molecular Sciences 23, no. 18: 10400. https://doi.org/10.3390/ijms231810400
APA StyleYen, T.-A., Huang, H.-C., Wu, E.-T., Chou, H.-W., Chou, H.-C., Chen, C.-Y., Huang, S.-C., Chen, Y.-S., Lu, F., Wu, M.-H., Tsao, P.-N., & Wang, C.-C. (2022). Microrna-486-5P Regulates Human Pulmonary Artery Smooth Muscle Cell Migration via Endothelin-1. International Journal of Molecular Sciences, 23(18), 10400. https://doi.org/10.3390/ijms231810400







