Diverse Roles of the Exon Junction Complex Factors in the Cell Cycle, Cancer, and Neurodevelopmental Disorders-Potential for Therapeutic Targeting
Abstract
1. Introduction
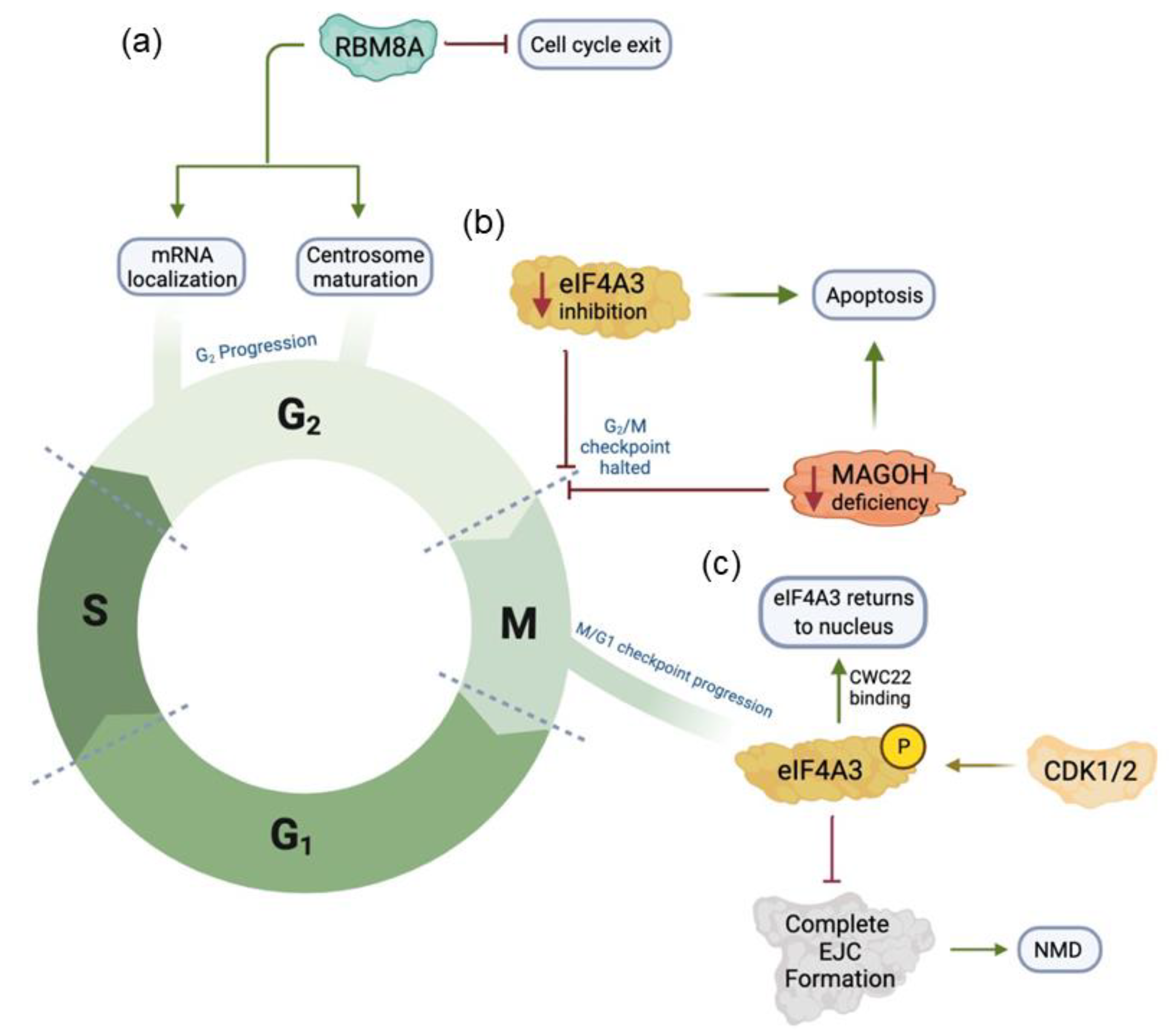
2. Core EJC Components Influence Normal Cell Cycle Progression
3. The EJC Is Implicated in a Diverse Set of Cancers
3.1. RBM8A Regulates Glioblastoma Progression
3.2. RBM8A Affects Apoptosis in Human Cell Models of Adenocarcinoma
3.3. RBM8A and eIF4A3 Contribute to the Pathogenesis of Hepatocellular Carcinoma (HCC)
4. The EJC Plays a Crucial Role in Neurodevelopmental Disorders
4.1. The EJC Controls Cell Division of Neural Stem Cells (NSCs)
4.2. EJC Modulates RNA Splicing during Development
4.3. RBM8A Contributes to Thrombocytopenia and Absent Radius (TAR) Syndrome and Neurodevelopmental Disorders
5. Targeting the EJC and NMD in Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlautmann, L.P.; Gehring, N.H. A Day in the Life of the Exon Junction Complex. Biomolecules 2020, 10, 866. [Google Scholar] [CrossRef]
- Leung, C.S.; Johnson, T.L. The exon junction complex: A multitasking guardian of the transcriptome. Mol. Cell 2018, 72, 799–801. [Google Scholar] [CrossRef]
- Boehm, V.; Gehring, N.H. Exon junction complexes: Supervising the gene expression assembly line. Trends Genet. 2016, 32, 724–735. [Google Scholar] [CrossRef]
- Le Hir, H.; Seraphin, B. EJCs at the heart of translational control. Cell 2008, 133, 213–216. [Google Scholar] [CrossRef][Green Version]
- Le Hir, H.; Andersen, G.R. Structural insights into the exon junction complex. Curr. Opin. Struct. Biol. 2008, 18, 112–119. [Google Scholar] [CrossRef]
- Le Hir, H.; Izaurralde, E.; Maquat, L.E.; Moore, M.J. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon–exon junctions. EMBO J. 2000, 19, 6860–6869. [Google Scholar] [CrossRef]
- Andersen, C.B.F.; Ballut, L.; Johansen, J.S.; Chamieh, H.; Nielsen, K.H.; Oliveira, C.L.P.; Pedersen, J.S.; Seraphin, B.; Le Hir, H.; Andersen, G.R. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science 2006, 313, 1968–1972. [Google Scholar] [CrossRef]
- Buchwald, G.; Schussler, S.; Basquin, C.; Le Hir, H.; Conti, E. Crystal structure of the human eIF4AIII-CWC22 complex shows how a DEAD-box protein is inhibited by a MIF4G domain. Proc. Natl. Acad. Sci. USA 2013, 110, E4611–E4618. [Google Scholar] [CrossRef]
- Noble, C.G.; Song, H.W. MLN51 Stimulates the RNA-Helicase Activity of eIF4AIII. PLoS ONE 2007, 2, e303. [Google Scholar] [CrossRef]
- Wang, Z.; Ballut, L.; Barbosa, I.; Le Hir, H. Exon Junction Complexes can have distinct functional flavours to regulate specific splicing events. Sci. Rep. 2018, 8, 9509. [Google Scholar] [CrossRef]
- Murachelli, A.G.; Ebert, J.; Basquin, C.; Le Hir, H.; Conti, E. The structure of the ASAP core complex reveals the existence of a Pinin-containing PSAP complex. Nat. Struct. Mol. Biol. 2012, 19, 378–386. [Google Scholar] [CrossRef]
- Viphakone, N.; Sudbery, I.; Griffith, L.; Heath, C.G.; Sims, D.; Wilson, S.A. Co-transcriptional Loading of RNA Export Factors Shapes the Human Transcriptome. Mol. Cell 2019, 75, 310–323. [Google Scholar] [CrossRef]
- Ballut, L.; Marchadier, B.; Baguet, A.; Tomasetto, C.; Seraphin, B.; Le Hir, H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat. Struct. Mol. Biol. 2005, 12, 861–869. [Google Scholar] [CrossRef]
- Bono, F.; Cook, A.G.; Grunwald, M.; Ebert, J.; Conti, E. Nuclear Import Mechanism of the EJC Component Mago-Y14 Revealed by Structural Studies of Importin 13. Mol. Cell 2010, 37, 211–222. [Google Scholar] [CrossRef]
- Kataoka, N.; Diem, M.D.; Kim, V.N.; Yong, J.; Dreyfuss, G. Magoh, a human homolog of Drosophila mago nashi protein, is a component of the splicing-dependent exon–exon junction complex. EMBO J. 2001, 20, 6424–6433. [Google Scholar] [CrossRef]
- Gehring, N.H.; Kunz, J.B.; Neu-Yilik, G.; Breit, S.; Viegas, M.H.; Hentze, M.W.; Kulozik, A.E. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements. Mol. Cell 2005, 20, 65–75. [Google Scholar] [CrossRef]
- Diem, M.D.; Chan, C.C.; Younis, I.; Dreyfuss, G. PYM binds the cytoplasmic exon-junction complex and ribosomes to enhance translation of spliced mRNAs. Nat. Struct. Mol. Biol. 2007, 14, 1173–1179. [Google Scholar] [CrossRef]
- Kim, V.N.; Yong, J.; Kataoka, N.; Abel, L.; Diem, M.D.; Dreyfuss, G. The Y14 protein communicates to the cytoplasm the position of exon–exon junctions. EMBO J. 2001, 20, 2062–2068. [Google Scholar] [CrossRef]
- Tange, T.O.; Shibuya, T.; Jurica, M.S.; Moore, M.J. Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core. RNA 2005, 11, 1869–1883. [Google Scholar] [CrossRef]
- Karousis, E.D.; Nasif, S.; Mühlemann, O. Nonsense-mediated mRNA decay: Novel mechanistic insights and biological impact. Wiley Interdiscip. Rev. RNA 2016, 7, 661–682. [Google Scholar] [CrossRef]
- Kunz, J.B.; Neu-Yilik, G.; Hentze, M.W.; Kulozik, A.E.; Gehring, N.H. Functions of hUpf3a and hUpf3b in nonsense-mediated mRNA decay and translation. RNA 2006, 12, 1015–1022. [Google Scholar] [CrossRef]
- Hug, N.; Longman, D.; Cáceres, J.F. Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Res. 2016, 44, 1483–1495. [Google Scholar] [CrossRef]
- Kishor, A.; Fritz, S.E.; Hogg, J.R. Nonsense-mediated mRNA decay: The challenge of telling right from wrong in a complex transcriptome. Wiley Interdiscip. Rev. RNA 2019, 10, e1548. [Google Scholar] [CrossRef]
- Hosoda, N.; Kim, Y.K.; Lejeune, F.; Maquat, L.E. CBP80 promotes interaction of Upf1 with Upf2 during nonsense-mediated mRNA decay in mammalian cells. Nat. Struct. Mol. Biol. 2005, 12, 893–901. [Google Scholar] [CrossRef]
- Isken, O.; Maquat, L.E. The multiple lives of NMD factors: Balancing roles in gene and genome regulation. Nat. Rev. Genet. 2008, 9, 699–712. [Google Scholar] [CrossRef]
- Woeller, C.F.; Gaspari, M.; Isken, O.; Maquat, L.E. NMD resulting from encephalomyocarditis virus IRES-directed translation initiation seems to be restricted to CBP80/20-bound mRNA. EMBO Rep. 2008, 9, 446–451. [Google Scholar] [CrossRef]
- Kashima, I.; Yamashita, A.; Izumi, N.; Kataoka, N.; Morishita, R.; Hoshino, S.; Ohno, M.; Dreyfuss, G.; Ohno, S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006, 20, 355–367. [Google Scholar] [CrossRef]
- Lopez-Perrote, A.; Castano, R.; Melero, R.; Zamarro, T.; Kurosawa, H.; Ohnishi, T.; Uchiyama, A.; Aoyagi, K.; Buchwald, G.; Kataoka, N.; et al. Human nonsense-mediated mRNA decay factor UPF2 interacts directly with eRF3 and the SURF complex. Nucleic Acids Res. 2016, 44, 1909–1923. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Izaurralde, E. Quality control of gene expression: A stepwise assembly pathway for the surveillance complex that triggers nonsense-mediated mRNA decay. Genes Dev. 2006, 20, 391–398. [Google Scholar] [CrossRef]
- Kervestin, S.; Jacobson, A. NMD: A multifaceted response to premature translational termination. Nat. Rev. Mol. Cell Biol. 2012, 13, 700–712. [Google Scholar] [CrossRef]
- Peccarelli, M.; Kebaara, B.W. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Eukaryot. Cell 2014, 13, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.A.; Neu-Yilik, G.; Hentze, M.W.; Kulozik, A.E. Nonsense-mediated decay approaches the clinic. Nat. Genet. 2004, 36, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, M.; Inoue, K.; Lupski, J.R. Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur. J. Hum. Genet. 2006, 14, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanagiri, M.; Schlitter, A.M.; Hentze, M.W.; Kulozik, A.E. NMD: RNA biology meets human genetic medicine. Biochem. J. 2010, 430, 365–377. [Google Scholar] [CrossRef]
- Gardner, L.B. Nonsense-mediated RNA decay regulation by cellular stress: Implications for tumorigenesis. Mol. Cancer Res. 2010, 8, 295–308. [Google Scholar] [CrossRef]
- Gehring, N.H.; Lamprinaki, S.; Hentze, M.W.; Kulozik, A.E. The Hierarchy of Exon-Junction Complex Assembly by the Spliceosome Explains Key Features of Mammalian Nonsense-Mediated mRNA Decay. PLoS Biol. 2009, 7, e1000120. [Google Scholar] [CrossRef]
- Woodward, L.A.; Mabin, J.W.; Gangras, P.; Singh, G. The exon junction complex: A lifelong guardian of mRNA fate. Wiley Interdiscip. Rev. RNA 2017, 8, e1411. [Google Scholar] [CrossRef]
- Mazloomian, A.; Araki, S.; Ohori, M.; El-Naggar, A.M.; Yap, D.; Bashashati, A.; Nakao, S.; Sorensen, P.H.; Nakanishi, A.; Shah, S.; et al. Pharmacological systems analysis defines EIF4A3 functions in cell-cycle and RNA stress granule formation. Commun. Biol. 2019, 2, 165. [Google Scholar] [CrossRef]
- Ryu, I.; Won, Y.S.; Ha, H.; Kim, E.; Park, Y.; Kim, M.K.; Kwon, D.H.; Choe, J.; Song, H.K.; Jung, H.; et al. eIF4A3 Phosphorylation by CDKs Affects NMD during the Cell Cycle. Cell Rep. 2019, 26, 2126–2139.e9. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.Y.; Cai, J.Y.; Liang, R.; Chen, G.Y.; Qin, G.; Han, X.Q.; Yuan, C.L.; Liu, Z.; Li, Y.Q.; et al. Systematic Analysis of Gene Expression Alteration and Co-Expression Network of Eukaryotic Initiation Factor 4A-3 in Cancer. J. Cancer 2018, 9, 4568–4577. [Google Scholar] [CrossRef]
- Lu, C.C.; Lee, C.C.; Tseng, C.T.; Tarn, W.Y. Y14 governs p53 expression and modulates DNA damage sensitivity. Sci. Rep. 2017, 7, srep45558. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Nakamura, Y.; Tatsuno, T.; Hashimoto, M.; Shimasaki, T.; Iwabuchi, K.; Tomosugi, N. Depletion of RNA-binding protein RBM8A (Y14) causes cell cycle deficiency and apoptosis in human cells. Exp. Biol. Med. 2013, 238, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Safieddine, A.; Coleno, E.; Salloum, S.; Imbert, A.; Traboulsi, A.M.; Kwon, O.S.; Lionneton, F.; Georget, V.; Robert, M.C.; Gostan, T.; et al. A choreography of centrosomal mRNAs reveals a conserved localization mechanism involving active polysome transport. Nat. Commun. 2021, 12, 21. [Google Scholar] [CrossRef]
- Silver, D.L.; Leeds, K.E.; Hwang, H.W.; Miller, E.E.; Pavan, W.J. The EJC component Magoh regulates proliferation and expansion of neural crest-derived melanocytes. Dev. Biol. 2013, 375, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, C.J.; McMahon, J.J.; Serdar, L.D.; Silver, D.L. Dosage-dependent requirements of Magoh for cortical interneuron generation and survival. Development 2020, 147, dev182295. [Google Scholar] [CrossRef]
- Pilaz, L.J.; McMahon, J.J.; Miller, E.E.; Lennox, A.L.; Suzuki, A.; Salmon, E.; Silver, D.L. Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain. Neuron 2016, 89, 83–99. [Google Scholar] [CrossRef]
- Zou, D.H.; McSweeney, C.; Sebastian, A.; Reynolds, D.J.; Dong, F.P.; Zhou, Y.J.; Deng, D.Z.; Wang, Y.G.; Liu, L.; Zhu, J.; et al. A critical role of RBM8a in proliferation and differentiation of embryonic neural progenitors. Neural Dev. 2015, 10, 16. [Google Scholar] [CrossRef]
- McSweeney, C.; Dong, F.; Chen, M.; Vitale, J.; Xu, L.; Crowley, N.; Luscher, B.; Zou, D.; Mao, Y. Full function of exon junction complex factor, Rbm8a, is critical for interneuron development. Transl. Psychiatry 2020, 10, 379. [Google Scholar] [CrossRef]
- Kim, T.J.; Choi, J.J.; Kim, W.Y.; Choi, C.H.; Lee, J.W.; Bae, D.S.; Son, D.S.; Kim, J.; Park, B.K.; Ahn, G. Gene expression profiling for the prediction of lymph node metastasis in patients with cervical cancer. Cancer Sci. 2008, 99, 31–38. [Google Scholar] [CrossRef]
- Petroziello, J.; Yamane, A.; Westendorf, L.; Thompson, M.; McDonagh, C.; Cerveny, C.; Law, C.L.; Wahl, A.; Carter, P. Suppression subtractive hybridization and expression profiling identifies a unique set of genes overexpressed in non-small-cell lung cancer. Oncogene 2004, 23, 7734–7745. [Google Scholar] [CrossRef]
- Degot, S.; Le Hir, H.; Alpy, F.; Kedinger, V.; Stoll, I.; Wendling, C.; Seraphin, B.; Rio, M.C.; Tomasetto, C. Association of the breast cancer protein MLN51 with the Exon junction complex via its speckle localizer and RNA binding module. J. Biol. Chem. 2004, 279, 33702–33715. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Huang, M.G.; Xing, L.; Yang, R.; Wang, X.S.; Jiang, R.; Zhang, L.Y.; Chen, J.X. The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer. Mol. Cancer 2020, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, X.; Wang, M.; Qiao, Y.; Xu, Y.; Yang, J.; Dong, N.; He, J.; Sun, Q.; Lv, G.; et al. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget 2016, 7, 22159–22173. [Google Scholar] [CrossRef]
- Zhang, S.; Leng, T.; Zhang, Q.; Zhao, Q.; Nie, X.; Yang, L. Sanguinarine inhibits epithelial ovarian cancer development via regulating long non-coding RNA CASC2-EIF4A3 axis and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2018, 102, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Wu, X.; Tou, L.; Zheng, J.; Zhou, D. MAGOH/MAGOHB Inhibits the Tumorigenesis of Gastric Cancer via Inactivation of b-RAF/MEK/ERK Signaling. OncoTargets Ther. 2020, 13, 12723–12735. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wei, L.; Hu, B.; Zhang, J.; Wei, J.; Qian, Z.; Zou, D. RBM8A Promotes Glioblastoma Growth and Invasion Through the Notch/STAT3 Pathway. Front. Oncol. 2021, 11, 736941. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, J.; Liu, Z.; Liu, Z.; Li, Q.; Luo, X.; Li, Y.; Ye, J.; Lin, Y. Mechanism and molecular network of RBM8A-mediated regulation of oxaliplatin resistance in hepatocellular carcinoma. Front. Oncol. 2020, 10, 585452. [Google Scholar] [CrossRef]
- Muromoto, R.; Taira, N.; Ikeda, O.; Shiga, K.; Kamitani, S.; Togi, S.; Kawakami, S.; Sekine, Y.; Nanbo, A.; Oritani, K. The exon-junction complex proteins, Y14 and MAGOH regulate STAT3 activation. Biochem. Biophys. Res. Commun. 2009, 382, 63–68. [Google Scholar] [CrossRef]
- Liang, R.; Lin, Y.; Ye, J.Z.; Yan, X.X.; Liu, Z.H.; Li, Y.Q.; Luo, X.L.; Ye, H.H. High expression of RBM8A predicts poor patient prognosis and promotes tumor progression in hepatocellular carcinoma. Oncol. Rep. 2017, 37, 2167–2176. [Google Scholar] [CrossRef][Green Version]
- Liang, R.; Lin, Y.; Yan, X.-X.; Ye, J.-Z.; Li, Y.-Q.; Ye, H.-H. Enhanced RBM8A expression in human hepatocellular carcinoma. Int. J. Clin. Exp. Med. 2017, 10, 598–607. [Google Scholar]
- Ishigaki, Y.; Nakamura, Y.; Tatsuno, T.; Hashimoto, M.; Iwabuchi, K.; Tomosugi, N. RNA-binding protein RBM8A (Y14) and MAGOH localize to centrosome in human A549 cells. Histochem. Cell Biol. 2014, 141, 101–109. [Google Scholar] [CrossRef]
- Michelle, L.; Cloutier, A.; Toutant, J.; Shkreta, L.; Thibault, P.; Durand, M.; Garneau, D.; Gendron, D.; Lapointe, E.; Couture, S.; et al. Proteins Associated with the Exon Junction Complex Also Control the Alternative Splicing of Apoptotic Regulators. Mol. Cell. Biol. 2012, 32, 954–967. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, R.; Qiu, Y.F.; Lv, Y.F.; Zhang, J.Y.; Qin, G.; Yuan, C.L.; Liu, Z.H.; Li, Y.Q.; Zou, D.H.; et al. Expression and gene regulation network of RBM8A in hepatocellular carcinoma based on data mining. Aging 2019, 11, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, J.A.; Davidson, C.M.; Brown, N.H.; Brand, A.H. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat. Cell Biol. 2000, 2, 7–12. [Google Scholar] [CrossRef]
- Morin, X.; Bellaïche, Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell 2011, 21, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006, 441, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Konno, D.; Shioi, G.; Shitamukai, A.; Mori, A.; Kiyonari, H.; Miyata, T.; Matsuzaki, F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 2008, 10, 93–101. [Google Scholar] [CrossRef]
- Silver, D.L.; Watkins-Chow, D.E.; Schreck, K.C.; Pierfelice, T.J.; Larson, D.M.; Burnetti, A.J.; Liaw, H.J.; Myung, K.; Walsh, C.A.; Gaiano, N.; et al. The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat. Neurosci. 2010, 13, 551–558. [Google Scholar] [CrossRef]
- Mao, H.Q.; McMahon, J.J.; Tsai, Y.H.; Wang, Z.F.; Silver, D.L. Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly. PLoS Genet. 2016, 12, e1006282. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Berg, J.S.; Scaglia, F.; Belmont, J.; Bacino, C.A.; Sahoo, T.; Lalani, S.R.; Graham, B.; Lee, B.; Shinawi, M. Recurrent reciprocal 1q21. 1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat. Genet. 2008, 40, 1466–1471. [Google Scholar] [CrossRef]
- Mefford, H.C.; Sharp, A.J.; Baker, C.; Itsara, A.; Jiang, Z.; Buysse, K.; Huang, S.; Maloney, V.K.; Crolla, J.A.; Baralle, D. Recurrent rearrangements of chromosome 1q21. 1 and variable pediatric phenotypes. N. Engl. J. Med. 2008, 359, 1685–1699. [Google Scholar] [CrossRef]
- Kataoka, N.; Dreyfuss, G. A simple whole cell lysate system for in vitro splicing reveals a stepwise assembly of the exon-exon junction complex. J. Biol. Chem. 2004, 279, 7009–7013. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, H.; Gatfield, D.; Izaurralde, E.; Moore, M.J. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001, 20, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.K.; Black, D.L.; Zheng, S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 2016, 17, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Roignant, J.Y.; Treisman, J.E. Exon Junction Complex Subunits Are Required to Splice Drosophila MAP Kinase, a Large Heterochromatic Gene. Cell 2010, 143, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Liu, A.; Li, R.; Su, Y.; Du, J.; Li, C.; Zhu, A.J. The exon junction complex regulates the splicing of cell polarity gene dlg1 to control Wingless signaling in development. eLife 2016, 5, e17200. [Google Scholar] [CrossRef]
- Obrdlik, A.; Lin, G.; Haberman, N.; Ule, J.; Ephrussi, A. The Transcriptome-wide Landscape and Modalities of EJC Binding in Adult Drosophila. Cell Rep. 2019, 28, 1219–1236.e11. [Google Scholar] [CrossRef]
- Contreras, J.; Begley, V.; Marsella, L.; Villalobo, E. The splicing of tiny introns of Paramecium is controlled by MAGO. Gene 2018, 663, 101–109. [Google Scholar] [CrossRef]
- Zheng, S. Alternative splicing and nonsense-mediated mRNA decay enforce neural specific gene expression. Int. J. Dev. Neurosci. 2016, 55, 102–108. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, M.; Stoilov, P.; Chen, L.; Zheng, S. Developmental Attenuation of Neuronal Apoptosis by Neural-Specific Splicing of Bak1 Microexon. Neuron 2020, 107, 1180–1196.e8. [Google Scholar] [CrossRef]
- Su, C.-H.; Liao, W.-J.; Ke, W.-C.; Yang, R.-B.; Tarn, W.-Y. The Y14-p53 regulatory circuit in megakaryocyte differentiation and thrombocytopenia. iScience 2021, 24, 103368. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.H.; Li, R.J.; Huang, X.H.; Chen, G.Y.; Liu, Y.; Meng, Y.S.; Wang, Y.M.; Wu, Y.; Mao, Y.W. Identification of molecular correlations of RBM8A with autophagy in Alzheimer’s disease. Aging 2019, 11, 11673–11685. [Google Scholar] [CrossRef]
- Pastor, F.; Kolonias, D.; Giangrande, P.H.; Gilboa, E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature 2010, 465, 227–230. [Google Scholar] [CrossRef]
- Tan, K.; Stupack, D.G.; Wilkinson, M.F. Nonsense-mediated RNA decay: An emerging modulator of malignancy. Nat. Rev. Cancer 2022, 22, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, R.G.; Supek, F.; Lehner, B. The rules and impact of nonsense-mediated mRNA decay in human cancers. Nat. Genet. 2016, 48, 1112–1118. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, T.; Cary, D.R.; Iwatani-Yoshihara, M.; Kamada, Y.; Kawamoto, T.; Aparicio, S.; Nakanishi, A.; Imaeda, Y. Discovery of Novel 1,4-Diacylpiperazines as Selective and Cell-Active eIF4A3 Inhibitors. J. Med. Chem. 2017, 60, 3335–3351. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Lim, E.L.; Xu, H.; Liu, P.; Al-Bakir, M.; Wong, Y.N.S.; Rowan, A.; Funt, S.A.; Merghoub, T. Escape from nonsense-mediated decay associates with anti-tumor immunogenicity. Nat. Commun. 2020, 11, 3800. [Google Scholar] [CrossRef]
- Bruno, I.G.; Karam, R.; Huang, L.L.; Bhardwaj, A.; Lou, C.H.; Shum, E.Y.; Song, H.W.; Corbett, M.A.; Gifford, W.D.; Gecz, J.; et al. Identification of a MicroRNA that Activates Gene Expression by Repressing Nonsense-Mediated RNA Decay. Mol. Cell 2011, 42, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.S.; Werkmeister, E.; Gonzalez-Hilarion, S.; Leroy, C.; Gruenert, D.C.; Lafont, F.; Tulasne, D.; Lejeune, F. Premature termination codon readthrough in human cells occurs in novel cytoplasmic foci and requires UPF proteins. J. Cell Sci. 2017, 130, 3009–3022. [Google Scholar] [CrossRef]
- Fatscher, T.; Boehm, V.; Weiche, B.; Gehring, N.H. The interaction of cytoplasmic poly(A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay. RNA 2014, 20, 1579–1592. [Google Scholar] [CrossRef]
- Toma, K.G.; Rebbapragada, I.; Durand, S.; Lykke-Andersen, J. Identification of elements in human long 3′ UTRs that inhibit nonsense-mediated decay. RNA 2015, 21, 887–897. [Google Scholar] [CrossRef]
- Kishor, A.; Ge, Z.; Hogg, J.R. hnRNP L-dependent protection of normal mRNAs from NMD subverts quality control in B cell lymphoma. EMBO J. 2019, 38, e99128. [Google Scholar] [CrossRef]
- Fatscher, T.; Gehring, N.H. Harnessing short poly(A)-binding protein-interacting peptides for the suppression of nonsense-mediated mRNA decay. Sci. Rep. 2016, 6, 37311. [Google Scholar] [CrossRef]
- Kamelgarn, M.; Chen, J.; Kuang, L.; Jin, H.; Kasarskis, E.J.; Zhu, H. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc. Natl. Acad. Sci. USA 2018, 115, E11904–E11913. [Google Scholar] [CrossRef]
- Dang, Y.; Low, W.-K.; Xu, J.; Gehring, N.H.; Dietz, H.C.; Romo, D.; Liu, J.O. Inhibition of Nonsense-mediated mRNA Decay by the Natural Product Pateamine A through Eukaryotic Initiation Factor 4AIII*. J. Biol. Chem. 2009, 284, 23613–23621. [Google Scholar] [CrossRef]
- Durand, S.; Cougot, N.; Mahuteau-Betzer, F.; Nguyen, C.-H.; Grierson, D.S.; Bertrand, E.; Tazi, J.; Lejeune, F. Inhibition of nonsense-mediated mRNA decay (NMD) by a new chemical molecule reveals the dynamic of NMD factors in P-bodies. J. Cell Biol. 2007, 178, 1145–1160. [Google Scholar] [CrossRef]
- Martin, L.; Grigoryan, A.; Wang, D.; Wang, J.; Breda, L.; Rivella, S.; Cardozo, T.; Gardner, L.B. Identification and Characterization of Small Molecules That Inhibit Nonsense-Mediated RNA Decay and Suppress Nonsense p53 Mutations. Cancer Res. 2014, 74, 3104–3113. [Google Scholar] [CrossRef]
- Bhuvanagiri, M.; Lewis, J.; Putzker, K.; Becker, J.P.; Leicht, S.; Krijgsveld, J.; Batra, R.; Turnwald, B.; Jovanovic, B.; Hauer, C.; et al. 5-azacytidine inhibits nonsense-mediated decay in a MYC-dependent fashion. EMBO Mol. Med. 2014, 6, 1593–1609. [Google Scholar] [CrossRef]
- Iwatani-Yoshihara, M.; Ito, M.; Ishibashi, Y.; Oki, H.; Tanaka, T.; Morishita, D.; Ito, T.; Kimura, H.; Imaeda, Y.; Aparicio, S.; et al. Discovery and Characterization of a Eukaryotic Initiation Factor 4A-3-Selective Inhibitor That Suppresses Nonsense-Mediated mRNA Decay. ACS Chem. Biol. 2017, 12, 1760–1768. [Google Scholar] [CrossRef]
- Huang, L.; Low, A.; Damle, S.S.; Keenan, M.M.; Kuntz, S.; Murray, S.F.; Monia, B.P.; Guo, S. Antisense suppression of the nonsense mediated decay factor Upf3b as a potential treatment for diseases caused by nonsense mutations. Genome Biol. 2018, 19, 4. [Google Scholar] [CrossRef]
- Nickless, A.; Jackson, E.; Marasa, J.; Nugent, P.; Mercer, R.W.; Piwnica-Worms, D.; You, Z. Intracellular calcium regulates nonsense-mediated mRNA decay. Nat. Med. 2014, 20, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Puri, R.; Liu, K.; Nunez, I.; Chen, L.; Zheng, S. Molecular profiling of individual FDA-approved clinical drugs identifies modulators of nonsense-mediated mRNA decay. Mol. Ther. Nucleic Acids 2022, 27, 304–318. [Google Scholar] [CrossRef] [PubMed]
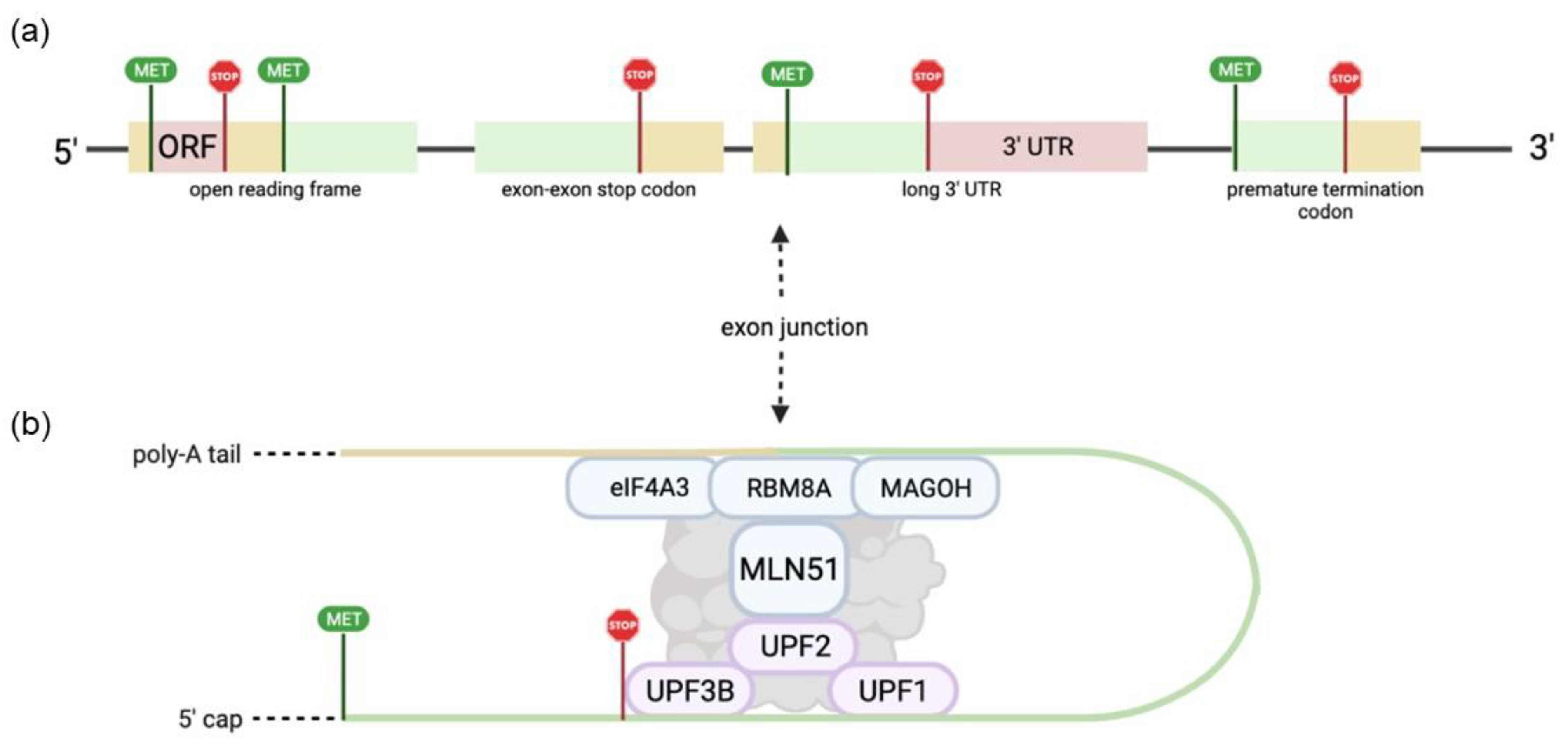
| EJC and NMD Factors | Functional Role | References |
|---|---|---|
| ACIN1 | Alternative splicing | [10,11] |
| ALYREF | mRNA localization | [12] |
| CWC22 | Alternative splicing | [8,13] |
| eIF4A3 | Invariable EJC core component ubiquitous in all EJC functions | [8,13] |
| IPO13 | mRNA localization | [14] |
| MAGOH | Invariable EJC core component ubiquitous in all EJC functions | [15] |
| MLN51 | Alternative splicing, mRNA localization, mRNA translation, and NMD | [10,16] |
| PNN | Alternative Splicing | [10,11] |
| PYM | mRNA translation and EJC disassembly | [17] |
| RBM8A | Invariable EJC core component ubiquitous in all EJC functions | [18] |
| RNPS1 | Alternative splicing and mRNA translation | [19] |
| SAP18 | Alternative Splicing | [10,11] |
| SMG6 | NMD | [20] |
| UPF1 | NMD | [20] |
| UPF2 | NMD | [20] |
| UPF3A | mRNA translation and NMD | [21] |
| UPF3B | mRNA translation and NMD | [21] |
| EJC Component | Dysfunction | References |
|---|---|---|
| eIF4A3 | Deficiency results in G2/M cell cycle arrest, chromosome segregation abnormalities, and apoptosis. Mutations of eIF4A3 specifically impact Tumor Factor-α pathway. | [38,40] |
| RBM8A | Deficiency results in G2/M cell cycle arrest, multinucleated cells with abnormal nuclear structure, and genome instability. Improper mRNA localization to mature centrosome has also been observed. | [41,42,43] |
| MAGOH | Deficiency inhibits melanoblast proliferation via arrest of cell cycle at G2/M and stalled mitosis in interneuron progenitors. Also results in apoptosis of radial glial cell progenitors. | [44,45,46] |
| EJC Associated Cancers | EJC Component(s) Implicated | References |
|---|---|---|
| Breast Cancer | MLN51, eIF4A3 | [51,52] |
| Cervical cancer | RBM8A | [49] |
| Colorectal Cancer | eIF4A3 | [53] |
| Gastric Cancer | MAGOH | [55] |
| Glioblastoma | RBM8A | [56] |
| Hepatocellular carcinoma | RBM8A, MAGOH, eIF4A3 | [38,57] |
| Lung Adenocarcinoma | RBM8A | [42,50] |
| Non-small-cell lung carcinoma | RBM8A | [50] |
| Ovarian Cancer | eIF4A3 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, H.; Rupkey, J.; Asthana, S.; Yoon, J.; Patel, S.; Mott, J.; Pei, Z.; Mao, Y. Diverse Roles of the Exon Junction Complex Factors in the Cell Cycle, Cancer, and Neurodevelopmental Disorders-Potential for Therapeutic Targeting. Int. J. Mol. Sci. 2022, 23, 10375. https://doi.org/10.3390/ijms231810375
Martin H, Rupkey J, Asthana S, Yoon J, Patel S, Mott J, Pei Z, Mao Y. Diverse Roles of the Exon Junction Complex Factors in the Cell Cycle, Cancer, and Neurodevelopmental Disorders-Potential for Therapeutic Targeting. International Journal of Molecular Sciences. 2022; 23(18):10375. https://doi.org/10.3390/ijms231810375
Chicago/Turabian StyleMartin, Hannah, Julian Rupkey, Shravan Asthana, Joy Yoon, Shray Patel, Jennifer Mott, Zifei Pei, and Yingwei Mao. 2022. "Diverse Roles of the Exon Junction Complex Factors in the Cell Cycle, Cancer, and Neurodevelopmental Disorders-Potential for Therapeutic Targeting" International Journal of Molecular Sciences 23, no. 18: 10375. https://doi.org/10.3390/ijms231810375
APA StyleMartin, H., Rupkey, J., Asthana, S., Yoon, J., Patel, S., Mott, J., Pei, Z., & Mao, Y. (2022). Diverse Roles of the Exon Junction Complex Factors in the Cell Cycle, Cancer, and Neurodevelopmental Disorders-Potential for Therapeutic Targeting. International Journal of Molecular Sciences, 23(18), 10375. https://doi.org/10.3390/ijms231810375








