Lower Plasmatic Levels of Saturated Fatty Acids and a Characteristic Fatty Acid Composition in the Ovary Could Contribute to the High-Fertility Phenotype in Dummerstorf Superfertile Mice
Abstract
1. Introduction
1.1. Dummerstorf High-Fertility Mouse Lines
1.2. Lipids and Oocytes
2. Results
2.1. The FL2 Line Is Larger Than FL1, but FL1 Line Grows Faster
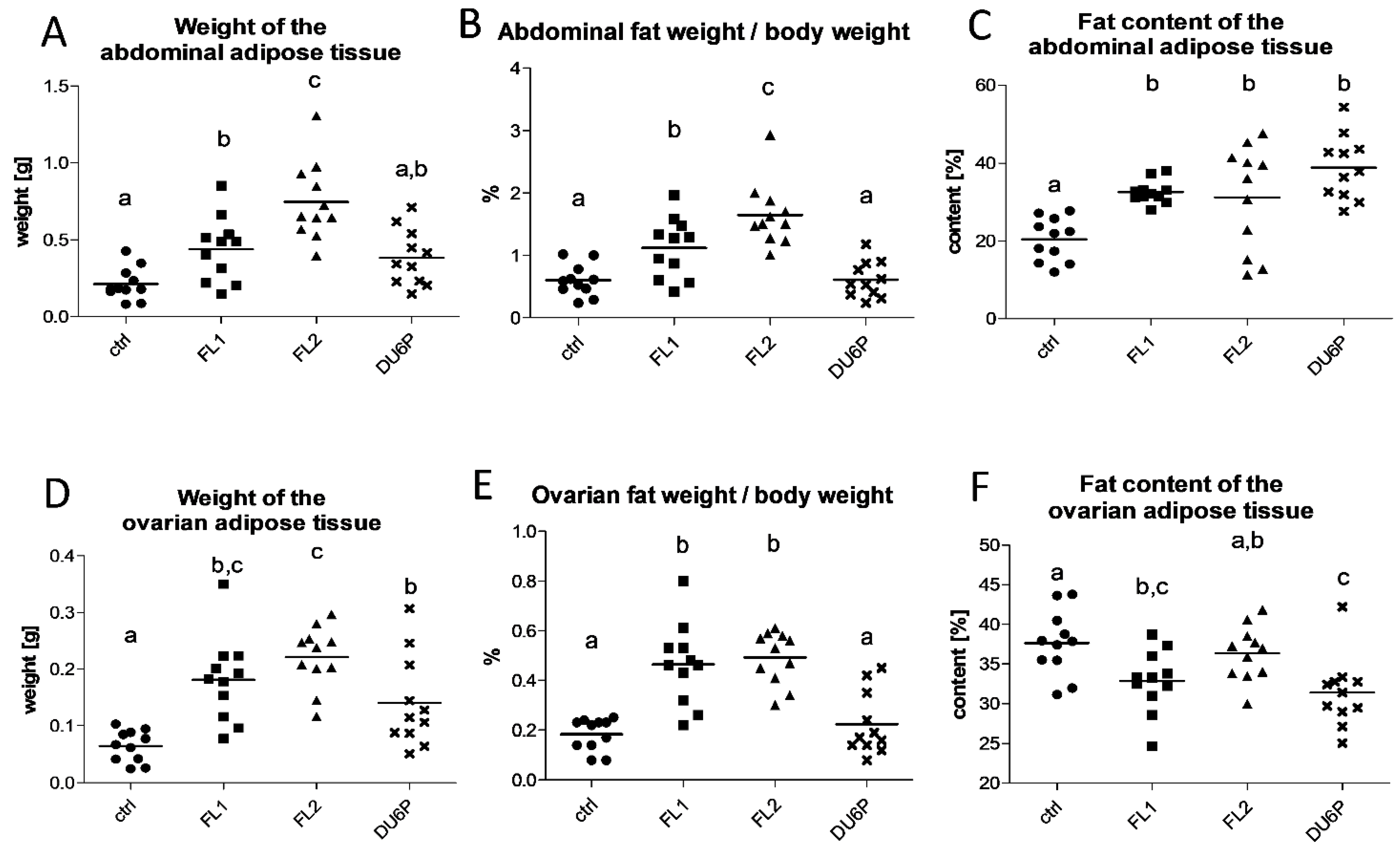
2.2. Both Fertility Lines Show a Higher Quantity of Body Fat Than Control and DU6P Mice, and Their Abdominal Fat Has a Higher Content of Lipids, but Their Ovarian Fat Has Less
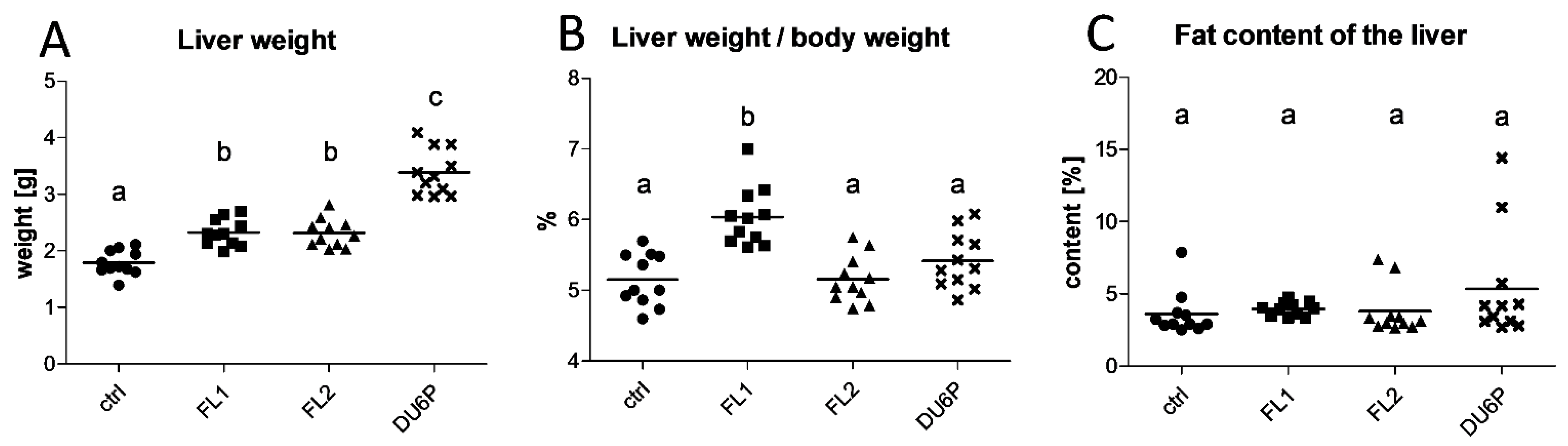
2.3. FL1 Females Have the Largest Liver Related to Their Dimension
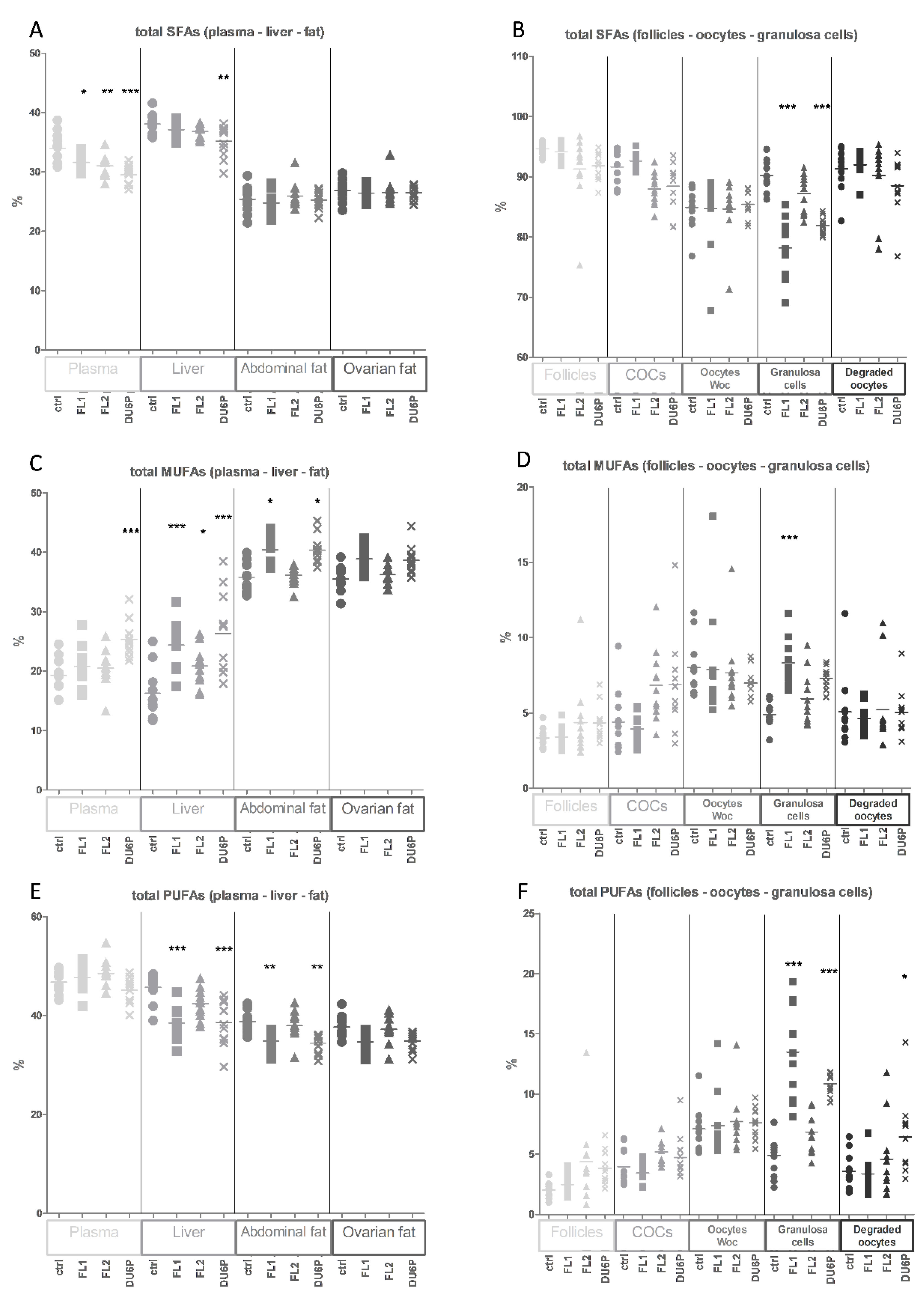
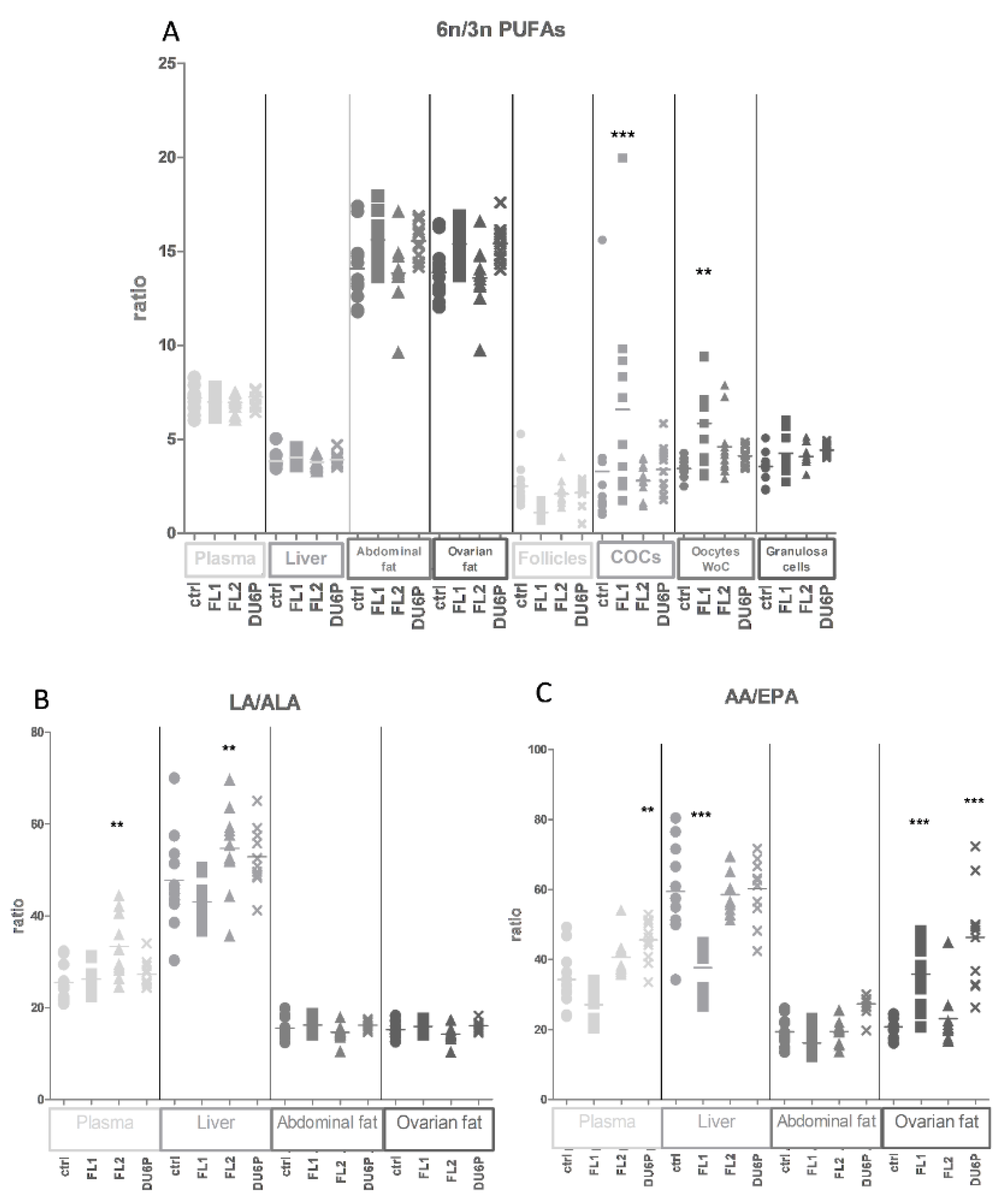
2.4. Levels of Total SFAs, MUFAs and PUFAs in All Tissues and the n-6/n-3 PUFA Ratio
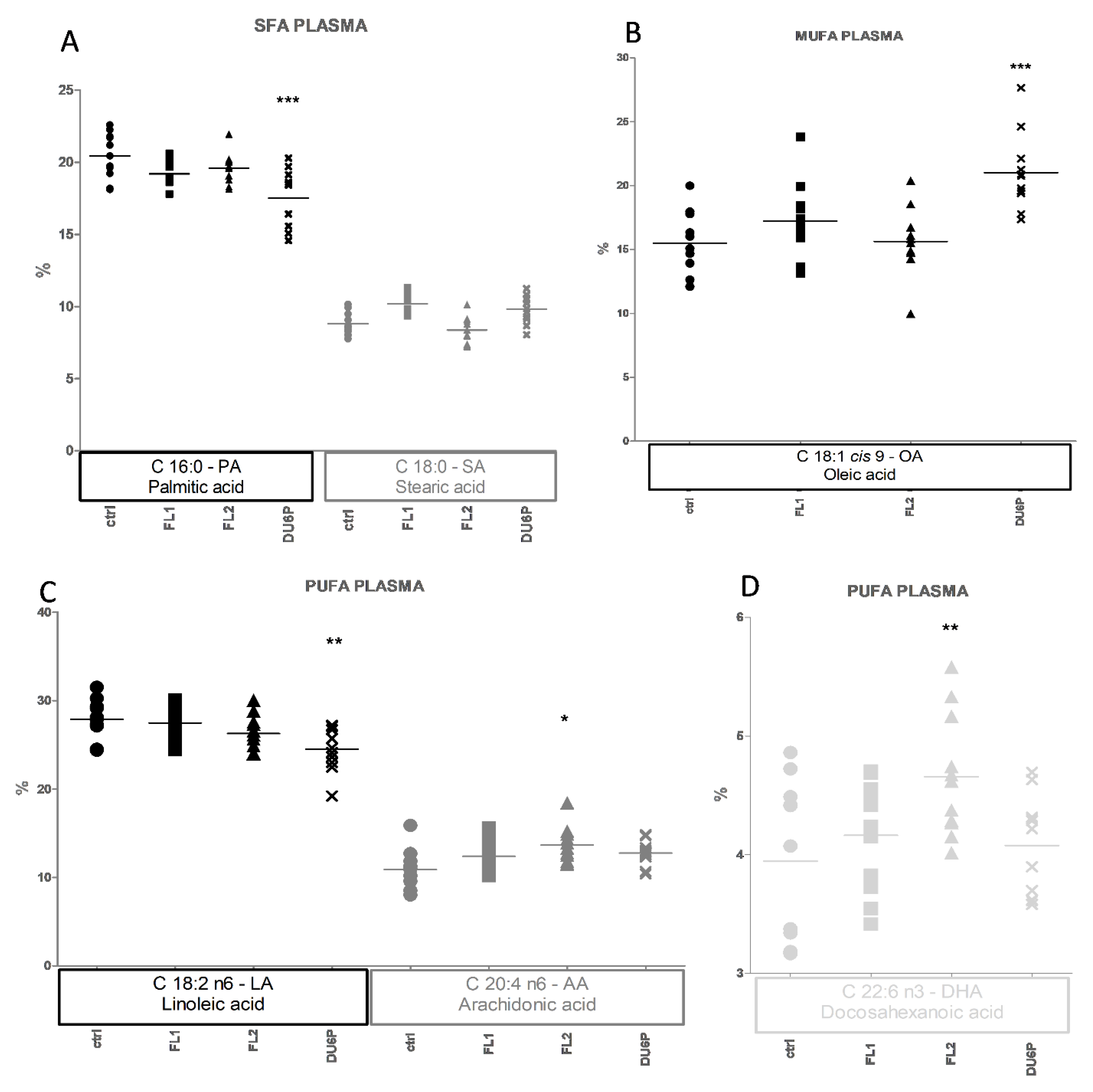
2.5. Fatty Acids in Plasma
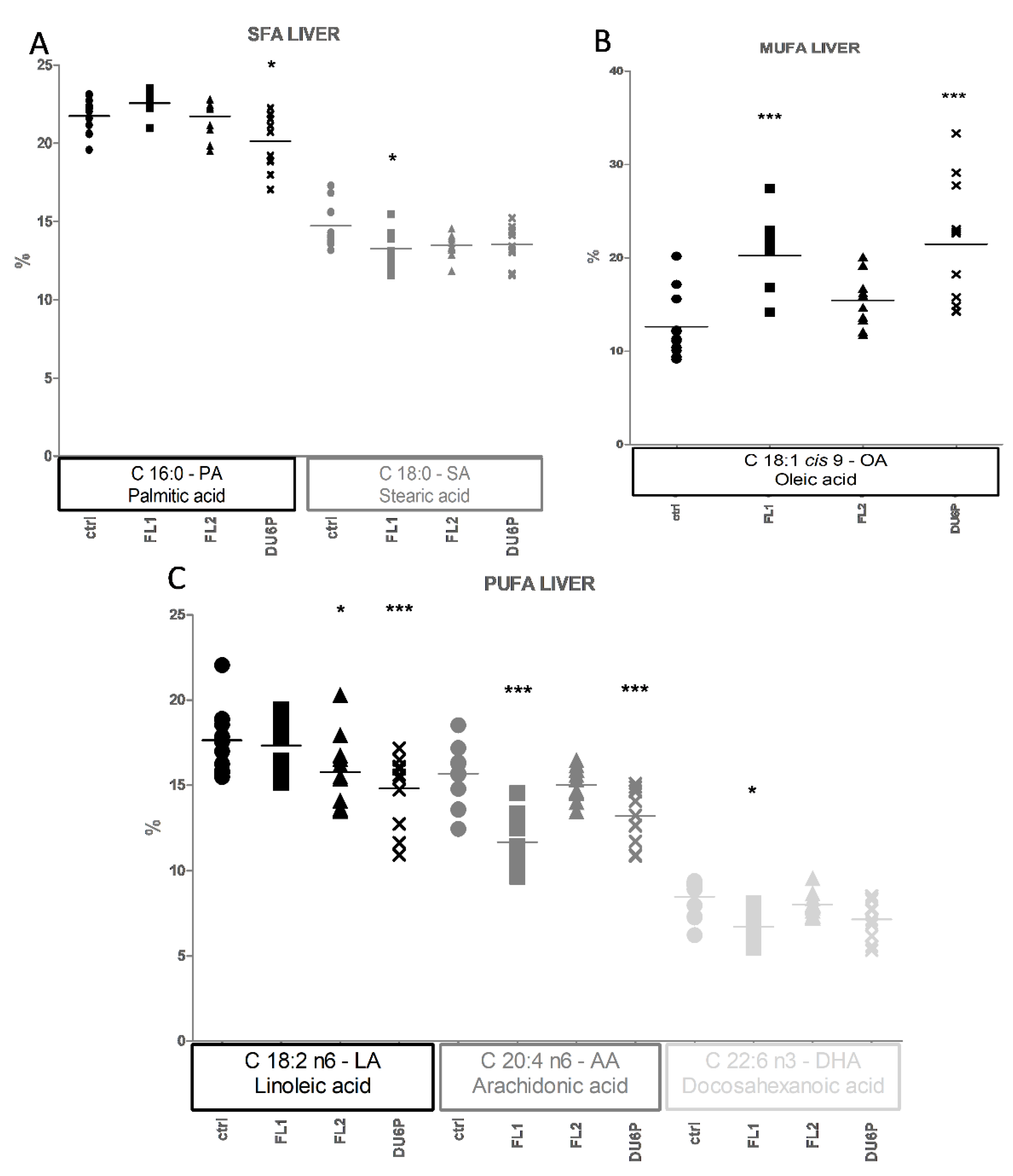
2.6. Fatty Acids in Liver
2.7. Fatty Acids in Abdominal and Ovarian Fat
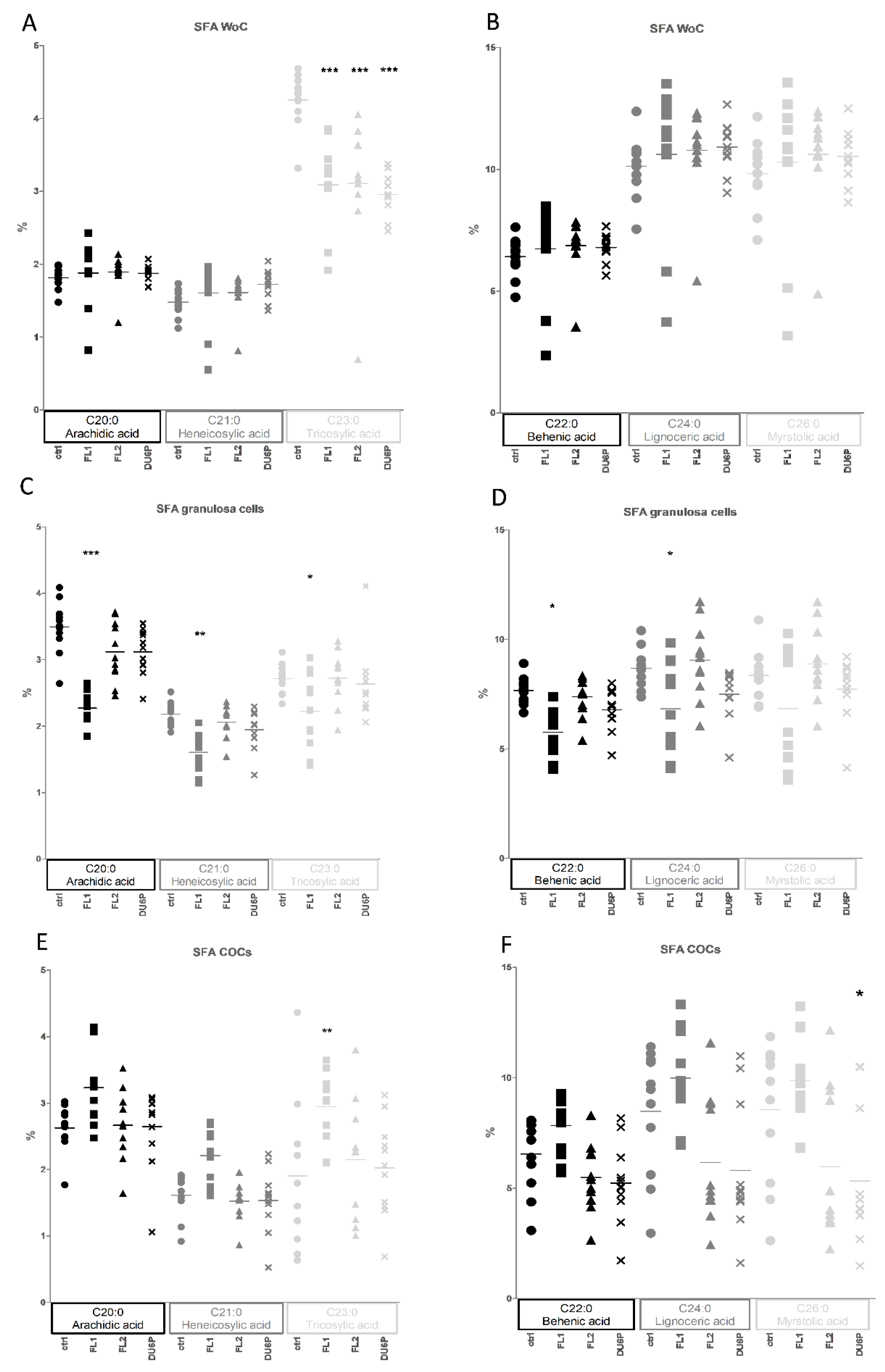
2.8. Fatty Acids in Follicles, COCs, Oocytes without Cumulus Cells and Granulosa Cells
3. Discussion
4. Material and Methods
4.1. Mouse Lines, Diet and Housing
4.2. Experimental Design and Sample Preparation
4.3. Lipid Extraction and Transesterification
4.3.1. Liver, Abdominal and Ovarian Fat
4.3.2. Plasma, Follicles, COCs, Oocytes without Cumulus Cells, Granulosa Cells and Degraded Cells
4.4. Fatty Acid Analysis
4.5. Methods of Analysis of Fatty Acids
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ALA | α-linoleic acid |
| APB | Animal Protection Board |
| BLUP | Best Linear Unbiased Prediction |
| DGLA | Dihomo-γ-linoleic acid |
| DHA | Decosahexanoic acid |
| DPA | Decosapentanoic acid |
| EPA | Eicosapentanoic acid |
| GLA | γ-linoleic acid |
| GVBD | Germinal vesicle breakdown |
| IVF | In vitro fertilization |
| IVM | In vitro maturation |
| LA | Linoleic acid |
| MOFs | Mono-ovulatory follicles |
| MUFAs | Monounsaturated fatty acids |
| OA | Oleic acid |
| PA | Palmitic acid |
| POFs | Polyovulatory follicles |
| PUFAs | Polyunsaturated fatty acids |
| SA | Stearic acid |
| SFAs | Saturated fatty acids |
| n-3 | Omega-3 PUFAs |
| n-6 | Omega-6 PUFAs |
References
- Schüler, L.; Bünger, L. The Reproductive Lifetime Performance of Laboratory Mouse Lines Selected for Fertility. Arch. Anim. Breed. 1982, 25, 275–281. [Google Scholar]
- Langhammer, M.; Michaelis, M.; Hoeflich, A.; Sobczak, A.; Schoen, J.; Weitzel, J.M. High-fertility phenotypes: Two outbred mouse models exhibit substantially different molecular and physiological strategies warranting improved fertility. Reproduction 2014, 147, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, M.; Michaelis, M.; Hartmann, M.F.; Wudy, S.A.; Sobczak, A.; Nürnberg, G.; Reinsch, N.; Schön, J.; Weitzel, J.M. Reproductive performance primarily depends on the female genotype in a two-factorial breeding experiment using high-fertility mouse lines. Reproduction 2017, 153, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, J.; Nürnberg, G.; Koczan, D.; Langhammer, M.; Thiesen, H.-J.; Reinsch, N. Expression profiling of a high-fertility mouse line by microarray analysis and qPCR. BMC Genom. 2008, 9, 307. [Google Scholar] [CrossRef]
- Michaelis, M.; Sobczak, A.; Koczan, D.; Langhammer, M.; Reinsch, N.; Schoen, J.; Weitzel, J.M. Selection for female traits of high fertility affects male reproductive performance and alters the testicular transcriptional profile. BMC Genom. 2017, 18, 889. [Google Scholar] [CrossRef]
- Michaelis, M.L.M.; Hoeflich, A.; Reinsch, N.; Schoen, J.; Weitzel, J.M. Initial characterization of an outbreed mouse model for male factor (in)fertility. Andrology 2013, 1, 772–778. [Google Scholar] [CrossRef]
- Spitschak, M.; Langhammer, M.; Schneider, F.; Renne, U.; Vanselow, J. Two high-fertility mouse lines show differences in component fertility traits after long-term selection. Reprod. Fertil. Dev. 2007, 19, 815–821. [Google Scholar] [CrossRef]
- Powell-Braxton, L.; Hollingshead, P.; Giltinan, D.; Pitts-Meek, S.; Stewart, T. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann. N. Y. Acad. Sci. 1993, 692, 300–301. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, W.-H.; Moser, H.; Valle, D.; Gould, S.J. Isolation of the human PEX12 gene, mutated in group 3 of the peroxisome biogenesis disorders. Nat. Genet. 1997, 15, 385–388. [Google Scholar] [CrossRef]
- Divall, S.A.; Williams, T.R.; Carver, S.E.; Koch, L.; Brüning, J.C.; Kahn, C.R.; Wondisford, F.; Radovick, S.; Wolfe, A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Investig. 2010, 120, 2900–2909. [Google Scholar] [CrossRef]
- Chehab, F.F. 20 years of leptin: Leptin and reproduction: Past milestones, present undertakings, and future endeavors. J. Endocrinol. 2014, 223, T37–T48. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.R.; Donthireddy, L.; Adhikary, D.; Chakraborty, S. Long-term high fat diet has a profound effect on body weight, hormone levels, and estrous cycle in mice. Med. Sci. Monit. 2016, 22, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Malik, I.; Durairajanayagam, D. Leptin and its actions on reproduction in males. Asian J. Androl. 2019, 21, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Calanni-Pileri, M.; Weitzel, J.M.; Langhammer, M.; Wytrwat, E.; Michaelis, M. Altered insulin, leptin and ghrelin hormone levels and atypical estrous cycle lengths in two highly-fertile mouse lines. Reprod. Domest. Anim. 2022, 57, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Calanni-Pileri, M.; Weitzel, J.M.; Langhammer, M.; Michaelis, M. Higher quality rather than superior quantity of oocytes determine the amount of fertilizable oocytes in two outbred Dummerstorf high-fertility mouse lines. Reprod. Domest. Anim. 2022. [Google Scholar] [CrossRef]
- Loewenstein, J.E.; Cohen, A.I. Dry mass, lipid content and protein content of the intact and zona-free mouse ovum. J. Embryol. Exp. Morphol. 1964, 12, 113–121. [Google Scholar] [CrossRef]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Lanzendorf, S.E.; Ratts, V.S.; Moley, K.H. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011, 95, 1970–1974. [Google Scholar] [CrossRef]
- Matorras, R.; Ruiz, J.I.; Mendoza, R.; Ruiz, N.; Sanjurjo, P.; Rodriguez-Escudero, F.J. Fatty acid composition of fertilization-failed human oocytes. Hum. Reprod. 1998, 13, 2227–2230. [Google Scholar] [CrossRef][Green Version]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. Reproduction 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Caldironi, H.A.; Alonso, T.S. Lipidic characterization of full-grown amphibian oocytes and their plasma mem-brane-enriched fractions. Lipids 1996, 31, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Hannaert, J.C.; Hoorens, A.; Eizirik, D.L.; Pipeleers, D.G. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 2001, 50, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.-Y.; Dunning, K.; Yang, X.; Russell, D.L.; Lane, M.; Norman, R.; Robker, R. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology 2010, 151, 5438–5445. [Google Scholar] [CrossRef] [PubMed]
- Aardema, H.; Vos, P.L.; Lolicato, F.; Roelen, B.A.; Knijn, H.M.; Vaandrager, A.B.; Helms, J.B.; Gadella, B.M. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 2011, 85, 62–69. [Google Scholar] [CrossRef]
- Aardema, H.; Lolicato, F.; van de Lest, C.H.; Brouwers, J.F.; Vaandrager, A.B.; Van Tol, H.T.; Roelen, B.A.; Vos, P.L.; Helms, J.B.; Gadella, B.M. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intra-cellular lipid storage. Biol. Reprod. 2013, 88, 164. [Google Scholar] [CrossRef]
- Lolicato, F.; Brouwers, J.F.; van de Lest, C.H.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.; Helms, J.B.; Gadella, B.M. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biol. Reprod. 2015, 92, 16. [Google Scholar] [CrossRef]
- Stanhiser, J.; Jukic, A.M.Z.; Steiner, A.Z. Serum omega-3 and omega-6 fatty acid concentrations and natural fertility. Hum. Reprod. 2020, 35, 950–957. [Google Scholar] [CrossRef]
- Stoffel, W.; Schmidt-Soltau, I.; Binczek, E.; Thomas, A.; Thevis, M.; Wegner, I. Dietary omega3-and omega6-polyunsaturated fatty acids reconstitute fertility of juvenile and adult Fads2-deficient mice. Mol. Metab. 2020, 36, 100974. [Google Scholar] [CrossRef]
- Jungheim, E.S.; Frolova, A.I.; Jiang, H.; Riley, J.K. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J. Clin. Endocrinol. Metab. 2013, 98, E1364–E1368. [Google Scholar] [CrossRef]
- Downs, S.M.; Mosey, J.L.; Klinger, J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol. Reprod. Dev. 2009, 76, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.M. Deconstructing mammalian reproduction: Using knockouts to define fertility pathways. Reproduction 2006, 131, 207–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matzuk, M.M.; Lamb, D.J. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 2002, 4, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Kubandová-Babeľová, J.; Kšiňanová, M.; Waczulíková, I.; Fabianová, K.; Koppel, J. Overweight and fertility: What we can learn from an intergenerational mouse obesity model. Int. J. Environ. Res. Public Health 2022, 19, 7918. [Google Scholar] [CrossRef]
- Heldstab, S.A.; Van Schaik, C.P.; Isler, K. Getting fat or getting help? How female mammals cope with energetic constraints on reproduction. Front. Zool. 2017, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, M.L.; Mensegue, M.F.; Pirola, C.J. The association between high fat diet around gestation and metabolic syndrome-related phenotypes in rats: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5086. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, W.; Wang, S.; Liu, R.; Loor, J.J.; Dong, Z.; Zhao, Y.; Ma, X.; Xia, C.; Xu, C. Lipid accumulation and injury in primary calf hepatocytes challenged with different long-chain fatty acids. Front. Vet. Sci. 2020, 7, 547047. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Yamachika, S.; Zhang, Z.; Taniguchi, K.; Umemura, A.; Hatzivassiliou, G.; Roose-Girma, M.; Reina-Campos, M.; Duran, A.; et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J. Hepatol. 2020, 72, 1182–1195. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Chi, M.M.-Y.; Schulte, M.B.; Moley, K.H. The fatty acid beta-oxidation pathway is important for decidualization of endometrial stromal cells in both humans and mice. Biol. Reprod. 2014, 90, 34. [Google Scholar] [CrossRef] [PubMed]
- Czumaj, A.; Śledziński, T. Biological role of unsaturated fatty acid desaturases in health and disease. Nutrients 2020, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimha, A.B.; Puvvada, M.; Hammerschmidt, M.; Michel, M. Leptin modulates oocyte maturation by a central and a direct pathway in zebrafish. J. Endocrinol. 2022, 254, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alm, H.; Kuhlmann, S.; Langhammer, M.; Tuchscherer, A.; Torner, H.; Reinsch, N. Occurrence of polyovular follicles in mouse lines selected for high fecundity. J. Reprod. Dev. 2010, 56, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Ramaiyan, B.; Zarei, M.; Acharya, P.; Talahalli, R.R. Dietary n-3 but not n-6 fatty acids modulate anthropometry and fertility indices in high-fat diet fed rats: A two-generation study. J. Food Sci. Technol. 2020, 58, 349–355. [Google Scholar] [CrossRef]
- Pleiner, J.S.G.; Mittermayer, F.; Bayerle-Eder, M.; Roden, M.; Wolzt, M. FFA-induced endothelial dysfunction can be corrected by vitamin C. J. Clin. Endocrinol. Metab. 2002, 87, 2913–2917. [Google Scholar] [CrossRef] [PubMed]
- Abdollahifar, M.-A.; Azad, N.; Sajadi, E.; Mofarahe, Z.S.; Zare, F.; Moradi, A.; Rezaee, F.; Gholamin, M.; Abdi, S. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat. Cell Biol. 2019, 52, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sawitzky, M.; Zeissler, A.; Langhammer, M.; Bielohuby, M.; Stock, P.; Hammon, H.M.; Görs, S.; Metges, C.C.; Stoehr, B.J.M.; Bidlingmaier, M.; et al. Phenotype selection reveals coevolution of muscle glycogen and protein and PTEN as a gate keeper for the accretion of muscle mass in adult female mice. PLoS ONE 2012, 7, e39711. [Google Scholar] [CrossRef] [PubMed]
- Bünger, L.; Renne, U.; Dietl, G.; Kuhla, S. Long-term selection for protein amount over 70 generations in mice. Genet. Res. 1998, 72, 93–109. [Google Scholar] [CrossRef]
- Tönißen, K.; Pfuhl, R.; Franz, G.P.; Dannenberger, D.; Bochert, R.; Grunow, B. Impact of spawning season on fillet quality of wild pikeperch (Sander lucioperca). Eur. Food Res. Technol. 2022, 248, 1277–1285. [Google Scholar] [CrossRef]
- Dannenberger, D.N.G.; Nuernberg, K.; Will, K.; Schauer, N.; Schmicke, M. Effects of diets supplemented with n–3 or n–6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. J. Food Compos. Anal. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Kalbe, C.; Priepke, A.; Nurnberg, G.; Dannenberger, D. Effects of long-term microalgae supplementation on muscle microstructure, meat quality and fatty acid composition in growing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 103, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acid Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calanni-Pileri, M.; Weitzel, J.M.; Dannenberger, D.; Langhammer, M.; Michaelis, M. Lower Plasmatic Levels of Saturated Fatty Acids and a Characteristic Fatty Acid Composition in the Ovary Could Contribute to the High-Fertility Phenotype in Dummerstorf Superfertile Mice. Int. J. Mol. Sci. 2022, 23, 10245. https://doi.org/10.3390/ijms231810245
Calanni-Pileri M, Weitzel JM, Dannenberger D, Langhammer M, Michaelis M. Lower Plasmatic Levels of Saturated Fatty Acids and a Characteristic Fatty Acid Composition in the Ovary Could Contribute to the High-Fertility Phenotype in Dummerstorf Superfertile Mice. International Journal of Molecular Sciences. 2022; 23(18):10245. https://doi.org/10.3390/ijms231810245
Chicago/Turabian StyleCalanni-Pileri, Michela, Joachim M. Weitzel, Dirk Dannenberger, Martina Langhammer, and Marten Michaelis. 2022. "Lower Plasmatic Levels of Saturated Fatty Acids and a Characteristic Fatty Acid Composition in the Ovary Could Contribute to the High-Fertility Phenotype in Dummerstorf Superfertile Mice" International Journal of Molecular Sciences 23, no. 18: 10245. https://doi.org/10.3390/ijms231810245
APA StyleCalanni-Pileri, M., Weitzel, J. M., Dannenberger, D., Langhammer, M., & Michaelis, M. (2022). Lower Plasmatic Levels of Saturated Fatty Acids and a Characteristic Fatty Acid Composition in the Ovary Could Contribute to the High-Fertility Phenotype in Dummerstorf Superfertile Mice. International Journal of Molecular Sciences, 23(18), 10245. https://doi.org/10.3390/ijms231810245






