COVID-19, Vaccination, and Female Fertility in the Czech Republic
Abstract
1. Introduction
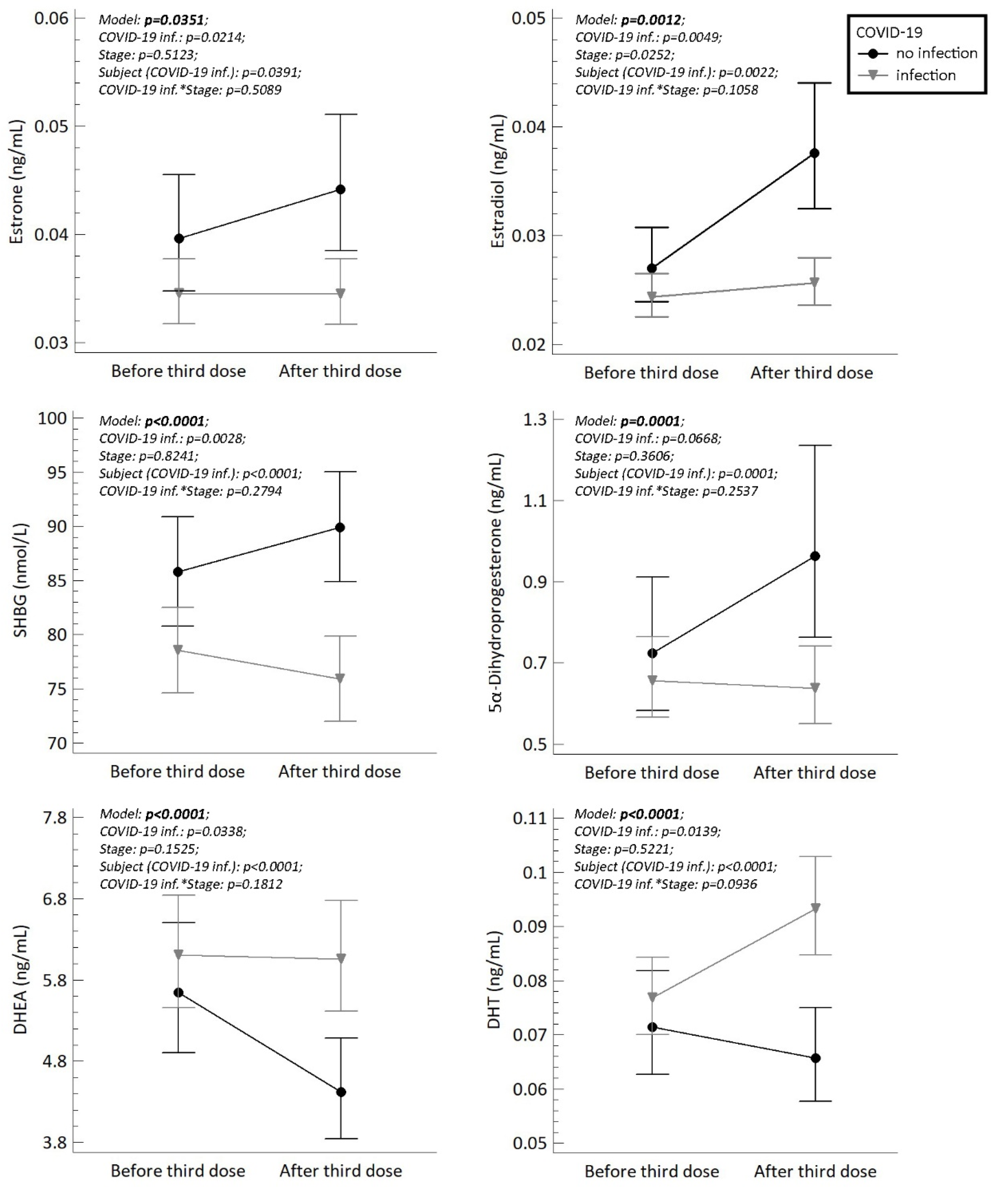
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Study Group
4.3. Determination of Steroids
4.4. Determination of LH, FSH, SHBG, AMH, Anti-SARS-CoV-2 and Anti-SARS-CoV-2S
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toth-Manikowski, S.M.; Swirsky, E.S.; Gandhi, R.; Piscitello, G. COVID-19 vaccination hesitancy among health care workers, communication, and policy-making. Am. J. Infect. Control 2021, 50, 20–25. [Google Scholar] [CrossRef]
- Schaler, L.; Wingfield, M. COVID-19 vaccine-can it affect fertility? Ir. J. Med. Sci. 2021, 1–3. [Google Scholar] [CrossRef]
- Hillson, K.; Clemens, S.C.; Madhi, S.A.; Voysey, M.; Pollard, A.J.; Minassian, A.M. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet 2021, 398, 1683–1684. [Google Scholar] [CrossRef]
- Stanovisko České Lékařské Společnosti (ČLS) Jana Evangelisty Purkyně (JEP); České Gynekologické a Porodnické Společnosti ČLS JEP. Očkování Proti Onemocnění COVID-19 u Těhotných a Kojících Žen. 2021. Available online: https://koronavirus.mzcr.cz/wp-content/uploads/2021/06/Stanovisko-k-očkován%C3%AD-proti-onemocněn%C3%AD-covid-19-u-těhotných-a-koj%C3%ADc%C3%ADch-žen.pdf (accessed on 11 September 2021).
- Han, A.R.; Lee, D.; Kim, S.K.; Choo, C.W.; Park, J.C.; Lee, J.R.; Choi, W.J.; Jun, J.H.; Rhee, J.H.; Kim, S.H.; et al. Effects and safety of COVID-19 vaccination on assisted reproductive technology and pregnancy: A comprehensive review and joint statements of the KSRM, the KSRI, and the KOSAR. Clin. Exp. Reprod. Med. 2022, 49, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Statement—COVID-19 Vaccination-Male and Female fertility, treatments to get pregnant, pregnancy. JBRA Assist. Reprod. 2022, 26, 197–198.
- Gonzalez, D.C.; Nassau, D.E.; Khodamoradi, K.; Ibrahim, E.; Blachman-Braun, R.; Ory, J.; Ramasamy, R. Sperm Parameters before and after COVID-19 mRNA Vaccination. JAMA 2021, 326, 273–274. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, S.; Dai, Z.; Hao, L.; Luan, C.; Guo, Q.; Meng, C.; Zhang, Y. Effects of COVID-19 and mRNA vaccines on human fertility. Hum. Reprod. 2021, 37, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.S.; Feil, K.; Reiser, E.; Weiss, G.; von Steuben, T.; Pinggera, G.M.; Kohn, F.M.; Toth, B. Corona and Reproduction, or Why the Corona Vaccination Does Not Result in Infertility. Geburtshilfe Frauenheilkd 2022, 82, 490–500. [Google Scholar] [CrossRef]
- Mirza, S.A.; Sheikh, A.A.E.; Barbera, M.; Ijaz, Z.; Javaid, M.A.; Shekhar, R.; Pal, S.; Sheikh, A.B. COVID-19 and the Endocrine System: A Review of the Current Information and Misinformation. Infect. Dis. Rep. 2022, 14, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Markert, U.R.; Szekeres-Bartho, J.; Schleussner, E. Adverse effects on female fertility from vaccination against COVID-19 unlikely. J. Reprod. Immunol. 2021, 148, 103428. [Google Scholar] [CrossRef]
- Bentov, Y.; Beharier, O.; Moav-Zafrir, A.; Kabessa, M.; Godin, M.; Greenfield, C.S.; Ketzinel-Gilad, M.; Ash Broder, E.; Holzer, H.E.G.; Wolf, D.; et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum. Reprod. 2021, 36, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Katsiroumpa, A.; Siskou, O.; Konstantakopoulou, O.; Katsoulas, T.; Mariolis-Sapsakos, T.; Kaitelidou, D. First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors. Vaccines 2022, 10, 1097. [Google Scholar] [CrossRef]
- Doporučení ČLS JEP (ČVS). ČLS JEP (ČSAKI) a ČLS JEP (SEM) k Přeočkování a Aplikaci Dodatečných (Třetích) Dávek Vakcíny Proti Onemocnění COVID-19. 2021. Available online: https://www.infekce.cz/zprava21-45.htm (accessed on 11 September 2021).
- Jing, Y.; Li, R.-Q.; Wang, H.-R.; Chen, H.-R.; Liu, Y.-B.; Yang, G.; Fei, C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373. [Google Scholar] [CrossRef]
- Zupin, L.; Pascolo, L.; Zito, G.; Ricci, G.; Crovella, S. SARS-CoV-2 and the next generations: Which impact on reproductive tissues? J. Assist. Reprod. Genet. 2020, 37, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Morelli, F.; Meirelles, L.E.F.; de Souza, M.V.F.; Mari, N.L.; Mesquita, C.S.S.; Dartibale, C.B.; Damke, G.; Damke, E.; da Silva, V.R.S.; Souza, R.P.; et al. COVID-19 Infection in the Human Reproductive Tract of Men and Nonpregnant Women. Am. J. Trop. Med. Hyg. 2021, 104, 814–825. [Google Scholar] [CrossRef]
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Song, H.; Seddighzadeh, B.; Cooperberg, M.R.; Huang, F.W. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur. Urol. 2020, 78, 296–298. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. SARS-CoV-2 infection, oxidative stress and male reproductive hormones: Can testicular-adrenal crosstalk be ruled-out? J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20200205. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, L.; Jia, G.; Guo, R.; Leng, Q. Single-cell expression profiles of ACE2 and TMPRSS2 reveals potential vertical transmission and fetus infection of SARS-CoV-2. Aging 2020, 12, 19880–19897. [Google Scholar] [CrossRef] [PubMed]
- Stanley, K.E.; Thomas, E.; Leaver, M.; Wells, D. Coronavirus disease-19 and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertil. Steril. 2020, 114, 33–43. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Massari, F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020, 16, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Q.; Ren, X.; Hu, J.; Li, Z.; Long, R.; Xi, Q.; Zhu, L.; Jin, L. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. eClinicalMedicine 2021, 38, 101013. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Gornet, M.; Sims, H.; Kisanga, E.; Knight, Z.; Segars, J. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and its effect on gametogenesis and early pregnancy. Am. J. Reprod. Immunol. 2020, 84, e13351. [Google Scholar] [CrossRef] [PubMed]
- Knizatova, N.; Massanyi, M.; Roychoudhury, S.; Guha, P.; Greifova, H.; Tokarova, K.; Jambor, T.; Massanyi, P.; Lukac, N. Is there impact of the SARS-CoV-2 pandemic on steroidogenesis and fertility? Physiol. Res. 2021, 70 (Suppl. 2), S161–S175. [Google Scholar] [CrossRef]
- Freire Santana, M.; Borba, M.G.S.; Baia-da-Silva, D.C.; Val, F.; Alexandre, M.A.A.; Brito-Sousa, J.D.; Melo, G.C.; Queiroga, M.V.O.; Leao Farias, M.E.; Camilo, C.C.; et al. Case Report: Adrenal Pathology Findings in Severe COVID-19: An Autopsy Study. Am. J. Trop. Med. Hyg. 2020, 103, 1604–1607. [Google Scholar] [CrossRef]
- Orvieto, R.; Noach-Hirsh, M.; Segev-Zahav, A.; Haas, J.; Nahum, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod. Biol. Endocrinol. 2021, 19, 69. [Google Scholar] [CrossRef]
- Bowman, C.J.; Bouressam, M.; Campion, S.N.; Cappon, G.D.; Catlin, N.R.; Cutler, M.W.; Diekmann, J.; Rohde, C.M.; Sellers, R.S.; Lindemann, C. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod. Toxicol. 2021, 103, 28–35. [Google Scholar] [CrossRef]
- Stebbings, R.; Maguire, S.; Armour, G.; Jones, C.; Goodman, J.; Maguire, A.K.; Tang, C.M.; Skellett, V.; Harris, J. Developmental and reproductive safety of AZD1222 (ChAdOx1 nCoV-19) in mice. Reprod. Toxicol. 2021, 104, 134–142. [Google Scholar] [CrossRef]
- Mohr-Sasson, A.; Haas, J.; Abuhasira, S.; Sivan, M.; Doitch Amdurski, H.; Dadon, T.; Blumenfeld, S.; Derazne, E.; Hemi, R.; Orvieto, R.; et al. The effect of COVID-19 mRNA vaccine on serum anti-Mullerian hormone levels. Hum. Reprod. 2022, 37, 534–541. [Google Scholar] [CrossRef]
- Kolanska, K.; Hours, A.; Jonquiere, L.; Mathieu d’Argent, E.; Dabi, Y.; Dupont, C.; Touboul, C.; Antoine, J.M.; Chabbert-Buffet, N.; Darai, E. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod. Biomed. Online 2021, 43, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.F.; Zaami, S.; Pallottini, M.; Perelli, F.; Vidiri, A.; Marinelli, E.; Straface, G.; Signore, F.; Scambia, G.; Marchi, L. Flu and Tdap Maternal Immunization Hesitancy in Times of COVID-19: An Italian Survey on Multiethnic Sample. Vaccines 2021, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.F.; Marchi, L.; Aquilini, D.; Brunelli, T.; Vasarri, P.L. Passive immunity in newborn from SARS-CoV-2-infected mother. J. Med. Virol. 2021, 93, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.F.; Carabaneanu, A.I.; Perelli, F.; Matarrese, D.; Brunelli, T.; Casprini, P.; Vasarri, P.L. Universal screening for SARS-CoV-2 in pregnant women admitted for delivery: How to manage antibody testing? J. Mattern. Fetal Neonatal Med. 2022, 35, 3005–3006. [Google Scholar] [CrossRef]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Ferri, A.; Colucci, P.; Viggiani, L.; Matera, I.; Ostuni, A. Are the Follicular Fluid Characteristics of Recovered Coronavirus Disease 2019 Patients Different from Those of Vaccinated Women Approaching in vitro Fertilization? Front. Physiol. 2022, 13, 840109. [Google Scholar] [CrossRef]
- Odeh-Natour, R.; Shapira, M.; Estrada, D.; Freimann, S.; Tal, Y.; Atzmon, Y.; Bilgory, A.; Aslih, N.; Abu-Raya, Y.S.; Shalom-Paz, E. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am. J. Reprod. Immunol. 2022, 87, e13530. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Findlay, J.K.; Liew, S.H.; Simpson, E.R.; Korach, K.S. Estrogen signaling in the regulation of female reproductive functions. Handb. Exp. Pharmacol. 2010, 198, 29–35. [Google Scholar]
- Li, K.; Chen, G.; Hou, H.; Liao, Q.; Chen, J.; Bai, H.; Lee, S.; Wang, C.; Li, H.; Cheng, L.; et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod. Biomed. Online 2021, 42, 260–267. [Google Scholar] [CrossRef]
- Cattrini, C.; Bersanelli, M.; Latocca, M.M.; Conte, B.; Vallome, G.; Boccardo, F. Sex Hormones and Hormone Therapy during COVID-19 Pandemic: Implications for Patients with Cancer. Cancers 2020, 12, 2325. [Google Scholar] [CrossRef]
- Traish, A.M. Sex steroids and COVID-19 mortality in women. Trends Endocrinol. Metab. 2021, 32, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K.; Jakubek, M.; Drábek, J. Chrání estrogeny před těžkým průběhem COVID-19? Vesmír 2021, 100, 596. [Google Scholar]
- Sundstrom-Poromaa, I.; Comasco, E.; Sumner, R.; Luders, E. Progesterone-Friend or foe? Front. Neuroendocrinol. 2020, 59, 100856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nadeau, M.; Faucher, F.; Lescelleur, O.; Biron, S.; Daris, M.; Rheaume, C.; Luu-The, V.; Tchernof, A. Progesterone metabolism in adipose cells. Mol. Cell. Endocrinol. 2009, 298, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Nogara, A.; Merico, M.; Ferlin, A.; Foresta, C. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane. Steroids 1999, 64, 168–175. [Google Scholar] [CrossRef]
- Anderson, G.D.; Odegard, P.S. Pharmacokinetics of estrogen and progesterone in chronic kidney disease. Adv. Chronic Kidney Dis. 2004, 11, 357–360. [Google Scholar] [CrossRef]
- Kancheva, R.; Hill, M.; Cibula, D.; Vcelakova, H.; Kancheva, L.; Vrbikova, J.; Fait, T.; Parizek, A.; Starka, L. Relationships of circulating pregnanolone isomers and their polar conjugates to the status of sex, menstrual cycle, and pregnancy. J. Endocrinol. 2007, 195, 67–78. [Google Scholar] [CrossRef]
- Hill, M.; Cibula, D.; Havlikova, H.; Kancheva, L.; Fait, T.; Kancheva, R.; Parizek, A.; Starka, L. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J. Steroid Biochem. Mol. Biol. 2007, 105, 166–175. [Google Scholar] [CrossRef]
- Hirst, J.J.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Neuroactive steroids in pregnancy: Key regulatory and protective roles in the foetal brain. J. Steroid Biochem. Mol. Biol. 2014, 139, 144–153. [Google Scholar] [CrossRef]
- Duarte-Neto, A.N.; Monteiro, R.A.A.; da Silva, L.F.F.; Malheiros, D.; de Oliveira, E.P.; Theodoro-Filho, J.; Pinho, J.R.R.; Gomes-Gouvea, M.S.; Salles, A.P.M.; de Oliveira, I.R.S.; et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020, 77, 186–197. [Google Scholar] [CrossRef]
- Stárka, L.; Dušková, M. Androgeny v nákaze koronavirem SARS-CoV-2. Diabetol. Metab. Endokrinol. Vyziv. 2021, 2, 74–77. [Google Scholar]
- Male, V. Menstrual changes after COVID-19 vaccination. BMJ 2021, 374, n2211. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Hosono, A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res. 2018, 5, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Shilen, A.; Heslin, K.M.; Ishimwe, P.; Allen, A.M.; Jacobs, E.T.; Farland, L.V. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am. J. Obstet. Gynecol. 2022, 226, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova Sosvorova, L.; Chlupacova, T.; Vitku, J.; Vlk, M.; Heracek, J.; Starka, L.; Saman, D.; Simkova, M.; Hampl, R. Determination of selected bisphenols, parabens and estrogens in human plasma using LC-MS/MS. Talanta 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Simkova, M.; Kolatorova, L.; Drasar, P.; Vitku, J. An LC-MS/MS method for the simultaneous quantification of 32 steroids in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1201–1202, 123294. [Google Scholar] [CrossRef]
- Vitku, J.; Chlupacova, T.; Sosvorova, L.; Hampl, R.; Hill, M.; Heracek, J.; Bicikova, M.; Starka, L. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015, 140, 62–67. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
| Characteristics | n (%) | Characteristics | n (%) |
|---|---|---|---|
| COVID-19 vaccine manufacturer-1st and 2nd dose | COVID-19 infection before vaccination | ||
| Pfizer/BioNTech | 22 (88) | Yes-all mild disease course | 6 (24) |
| Moderna | 3 (12) | No | 19 (76) |
| Reaction after the 1st dose of vaccine | MC changes after the 1st dose of vaccine | ||
| Fatigue | 7 (28) | MC shortening | 1 (4) |
| Injection site pain | 14 (56) | MC prolongation | 1 (4) |
| Elevated temperature | 1 (4) | Bleeding out of the cycle | 0 (0) |
| Headache | 2 (8) | Headache | 0 (0) |
| Chills | 0 (0) | Premenstrual syndrome | 0 (0) |
| Vertigo | 0 (0) | Ovulation pain | 0 (0) |
| None | 8 (32) | None | 23 (92) |
| Reaction after the 2nd dose of vaccine | MC changes after the 2nd dose of vaccine | ||
| Fatigue | 16 (64) | MC shortening | 1 (4) |
| Injection site pain | 12 (48) | MC prolongation | 1 (4) |
| Elevated temperature | 3 (48) | Bleeding out of the cycle | 0 (0) |
| Headache | 5 (20) | Headache | 0 (0) |
| Chills | 1 (4) | Premenstrual syndrome | 0 (0) |
| Vertigo | 1 (4) | Ovulation pain | 0 (0) |
| None | 8 (32) | None | 23 (92) |
| COVID-19 vaccine manufacturer-3rd dose | COVID-19 infection during the study | ||
| Pfizer/BioNTech | 15 (60) | Yes-all mild disease course | 6 (24) (4 Omicron) |
| Moderna | 10 (40) | No | 19 (76) |
| Reaction after the 3rd dose of vaccine | MC after the 3rd dose of vaccine | ||
| Fatigue | 12 (48) | MC shortening | 2 (8) |
| Injection site pain | 15 (60) | MC prolongation | 5 (20) |
| Elevated temperature | 3 (12) | Bleeding out of the cycle | 1 (4) |
| Headache | 4 (16) | Headache | 0 (0) |
| Chills | 0 (0) | Premenstrual syndrome | 0 (0) |
| Vertigo | 0 (0) | Ovulation pain | 0 (0) |
| None | 5 (20) | None | 16 (64) |
| Analyte (Steroids in ng/mL) | Before the 3rd Dose of COVID-19 Vaccine | After the 3rd Dose of COVID-19 Vaccine | p-Value |
|---|---|---|---|
| Estrone | 0.035 (0.029, 0.046) | 0.0375 (0.031, 0.044) | 0.424 |
| Estradiol | 0.024 (0.021, 0.032) | 0.0299 (0.023, 0.040) | 0.424 |
| Estriol | 0.003 (0.001, 0.005) | 0.004 (0.002, 0.007) | 0.23 |
| Progesterone | 0.097 (0.052, 0.146) | 0.103 (0.063, 0.132) | 0.838 |
| 17-OH-Progesterone | 0.315 (0.283, 0.446) | 0.396 (0.268, 0.556) | 0.214 |
| 5α-Dihydroprogesterone | 0.656 (0.346, 1.106) | 0.696 (0.396, 1.611) | 0.831 |
| Pregnenolone | 0.928 (0.755, 1.291) | 0.97 (0.620, 1.708) | 0.953 |
| 17-OH-Pregnenolone | 1.322 (0.888, 2.320) | 1.137 (0.522, 2.527) | 0.838 |
| DHEA | 6.807 (4.071, 9.248) | 5.114 (3.900, 9.404) | 0.689 |
| 7α-OH-DHEA | 0.252 (0.115, 0.376) | 0.188 (0.115, 0.382) | 0.359 |
| 7β-OH-DHEA | 0.092 (0.067, 0.115) | 0.079 (0.060, 0.132) | 0.383 |
| 7-oxo-DHEA | 0.032 (0.024, 0.055) | 0.028 (0.024, 0.068) | 0.264 |
| Testosterone | 0.224 (0.166, 0.263) | 0.215 (0.176, 0.266) | 0.368 |
| DHT | 0.078 (0.058, 0.096) | 0.077 (0.054, 0.121) | 0.689 |
| Androstenedione | 0.89 (0.646, 1.045) | 0.793 (0.669, 1.219) | 0.424 |
| 11β-OH-Androstenedione | 1.21 (0.899, 1.702) | 1.01 (0.738, 1.532) | 0.23 |
| 11-OH-Testosterone | 0.135 (0.112, 0.166) | 0.127 (0.091, 0.174) | 0.54 |
| 11-Keto-testosterone | 0.307 (0.224, 0.395) | 0.318 (0.208, 0.405) | 0.525 |
| Cortisol | 144 (128, 153) | 131 (114, 167) | 0.424 |
| Cortisone | 29.9 (26, 34.8) | 27.5 (23.0, 35.7) | 0.935 |
| Corticosterone | 2.404 (1.440, 4.007) | 2.038 (1.634, 3.646) | 0.4 |
| 11-Deoxycortisol | 0.339 (0.217, 0.451) | 0.311 (0.190, 0.479) | 0.567 |
| 21-Deoxycortisol | 0.026 (0.017, 0.049) | 0.029 (0.012, 0.055) | 0.831 |
| 11-Deoxycorticosterone | 0.035 (0.026, 0.053) | 0.043 (0.025, 0.057) | 0.831 |
| LH (IU/L) | 6.36 (4.80, 7.42) | 6.11 (5.70, 7.50) | 0.424 |
| FSH (IU/L) | 6.2 (5.8, 8.6) | 6.49 (5.045, 8.660) | 0.424 |
| SHBG (nmol/L) | 67.4 (49.7, 105.3) | 70.17 (56.76, 97.50) | 0.75 |
| AMH (ng/mL) | 3.25 (1.46, 5.09) | 3.03 (1.68, 5.04) | 0.689 |
| AFC | 23 (19.5, 28.75) | 24 (21.50, 28.75) | 0.19 |
| Characteristics | n (%) | Characteristics | n (%) |
|---|---|---|---|
| Age (years) | BMI | ||
| <25 | 6 (24) | <18.5 | 2 (8) |
| 25–35 | 13 (52) | 18.5–25 | 15 (60) |
| >35 | 6 (24) | >25 | 8 (32) |
| Mean (±STD) | 30(6.8) | Mean (±STD) | 24.1 (5.3) |
| Average length of MC | First MC | ||
| <26 | 2 (8) | <11 | 2 (8) |
| 26–30 | 17 (68) | 11–13 | 18 (72) |
| >30 | 6 (24) | >13 | 5 (20) |
| Mean (±STD) | 28.8 (2.8) | Mean (±STD) | 12.5 (1.4) |
| Smoking | Alcohol | ||
| Yes | 2 (8) | Regularly | 2 (8) |
| No | 19 (76) | Occasionally | 21 (84) |
| In the past | 4 (16) | None | 2 (8) |
| Eating habits | Physical activity | ||
| Common | 19 (76) | Common | 16 (64) |
| Vegetarian/vegan | 3 (12) | Fitness sport | 7 (28) |
| Intermittent fasting | 2 (8) | Competitive sport | 2 (8) |
| Diets | 1 (4) | ||
| Contraception in the past | Pregnancy in the past | ||
| Yes | 15 (60) | Yes | 4 (16) |
| No | 10 (40) | −3 women have children | |
| No | 21 (84) | ||
| Method | Units | Measuring Range | Limit of Detection | Reference Range/ Data Interpretation |
|---|---|---|---|---|
| Elecsys®LH | IU/L | 0.100–200 | 0.1 | women in follicular phase: 2.4–12.6 |
| Elecsys®FSH | IU/L | 0.100–200 | <0.100 | women in follicular phase: 3.5–12.5 |
| Elecsys®AMH | ng/mL | 0.01–23 | 0.01 | women 20–24 years: 1.22–11.7 women 25–29 years: 0.890–9.85 women 30–34 years: 0.576–8.13 women 35–39 years 0.147–7.49 women 40–44 years: 0.027–5.47 |
| Elecsys®SHBG | nmol/L | 0.350–200 | 0.35 | 43–95 |
| Elecsys®Anti-SARS-CoV-2 | COI | qualitative | 99.5% of specificity | ≥1.0 indicates positive |
| Elecsys®Anti-SARS-CoV-2 S | U/mL | 0.40–250 | 0.35 | ≥0.8 indicates positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolatorova, L.; Adamcova, K.; Vitku, J.; Horackova, L.; Simkova, M.; Hornova, M.; Vosatkova, M.; Vaisova, V.; Parizek, A.; Duskova, M. COVID-19, Vaccination, and Female Fertility in the Czech Republic. Int. J. Mol. Sci. 2022, 23, 10909. https://doi.org/10.3390/ijms231810909
Kolatorova L, Adamcova K, Vitku J, Horackova L, Simkova M, Hornova M, Vosatkova M, Vaisova V, Parizek A, Duskova M. COVID-19, Vaccination, and Female Fertility in the Czech Republic. International Journal of Molecular Sciences. 2022; 23(18):10909. https://doi.org/10.3390/ijms231810909
Chicago/Turabian StyleKolatorova, Lucie, Karolina Adamcova, Jana Vitku, Lenka Horackova, Marketa Simkova, Marketa Hornova, Michala Vosatkova, Veronika Vaisova, Antonin Parizek, and Michaela Duskova. 2022. "COVID-19, Vaccination, and Female Fertility in the Czech Republic" International Journal of Molecular Sciences 23, no. 18: 10909. https://doi.org/10.3390/ijms231810909
APA StyleKolatorova, L., Adamcova, K., Vitku, J., Horackova, L., Simkova, M., Hornova, M., Vosatkova, M., Vaisova, V., Parizek, A., & Duskova, M. (2022). COVID-19, Vaccination, and Female Fertility in the Czech Republic. International Journal of Molecular Sciences, 23(18), 10909. https://doi.org/10.3390/ijms231810909






