Significance of Hypermethylation of Tumor-Suppressor Genes PTGER4 and ZNF43 at CpG Sites in the Prognosis of Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Clinical Features and Follow-Up Information of Patients
2.2. Correlation between PTGER4 and ZNF43 Methylation Status and CRC
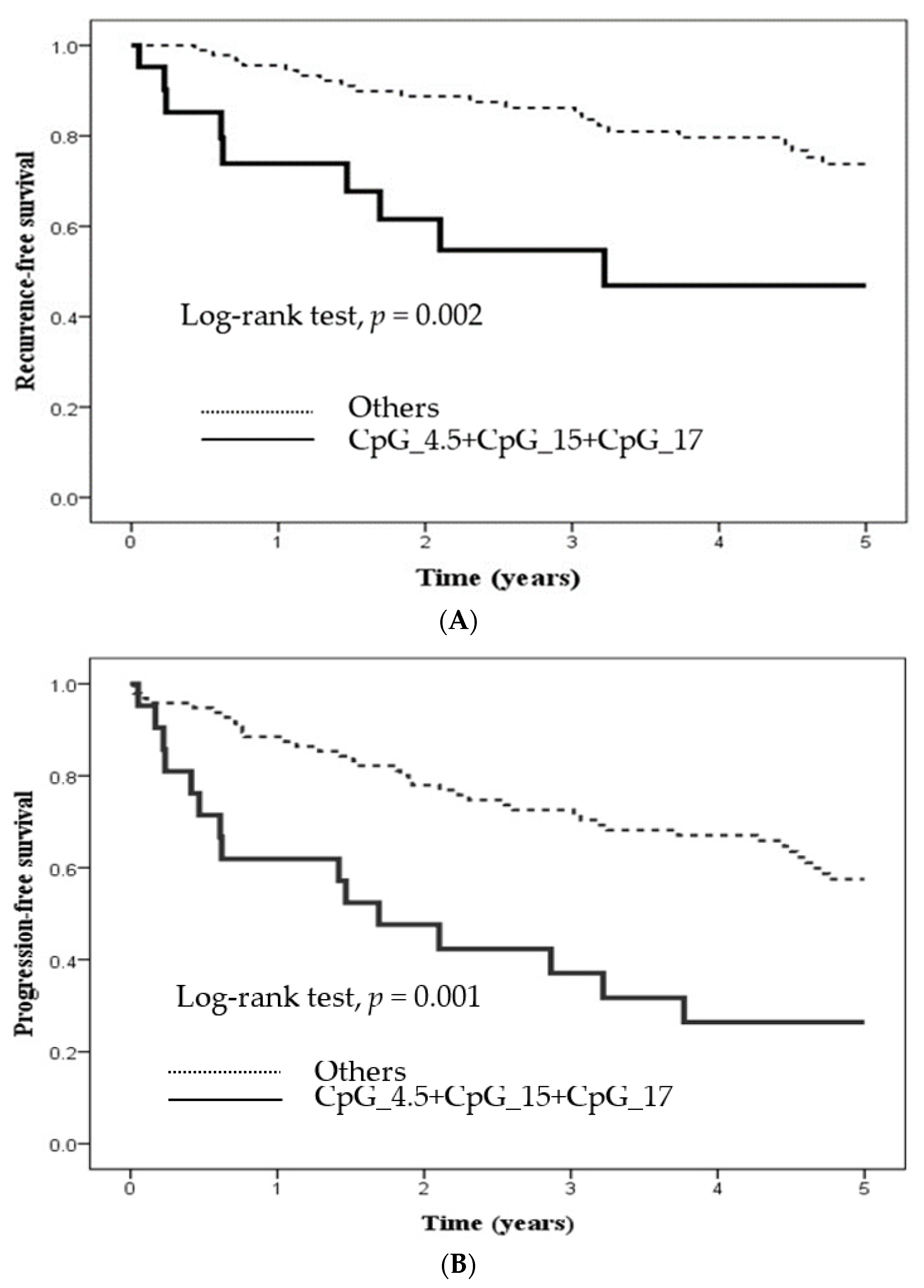
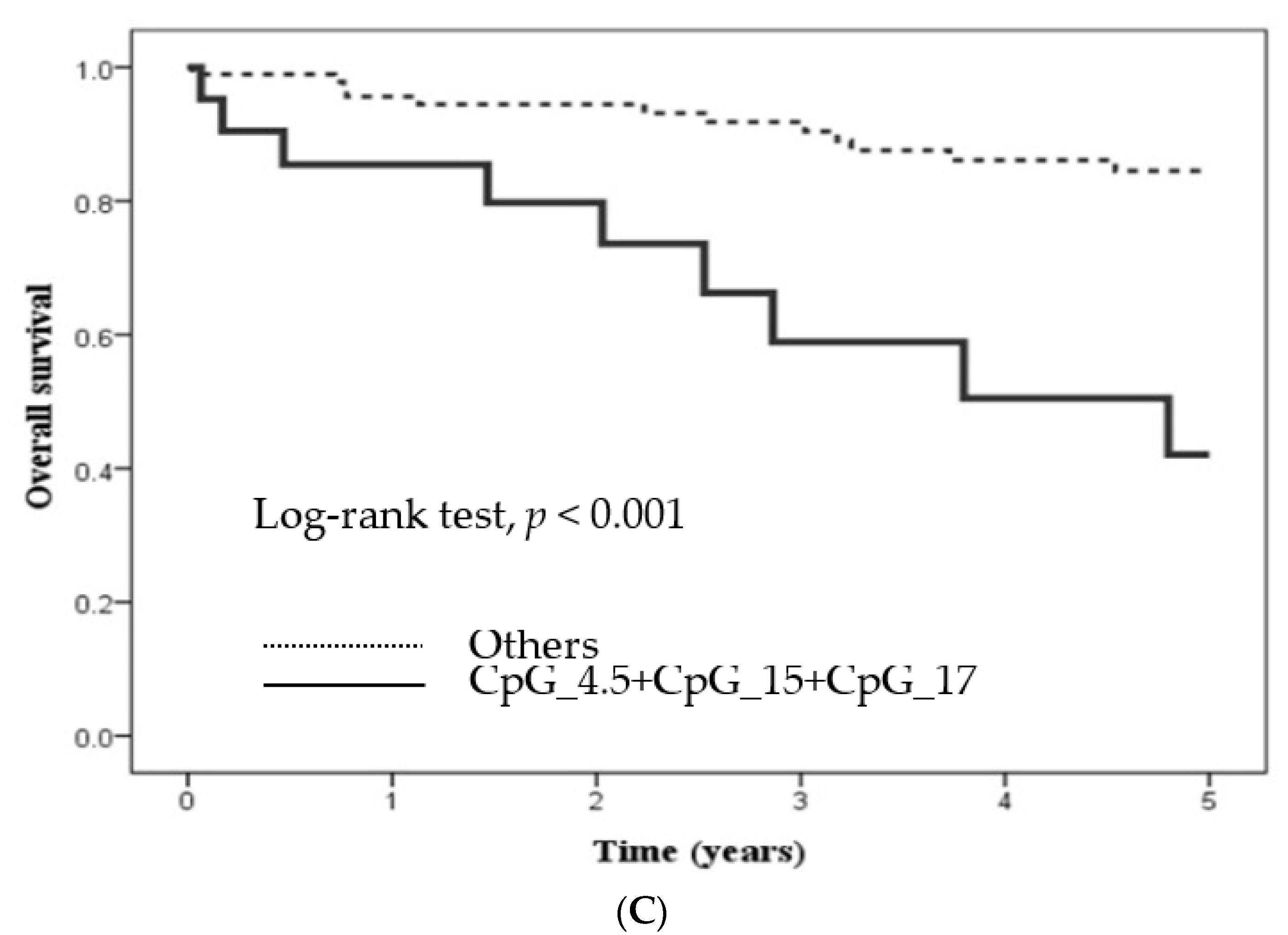
2.3. Methylation Level at Certain CpG Sites in Candidate Genes and Its Influence
3. Discussion
4. Methods and Materials
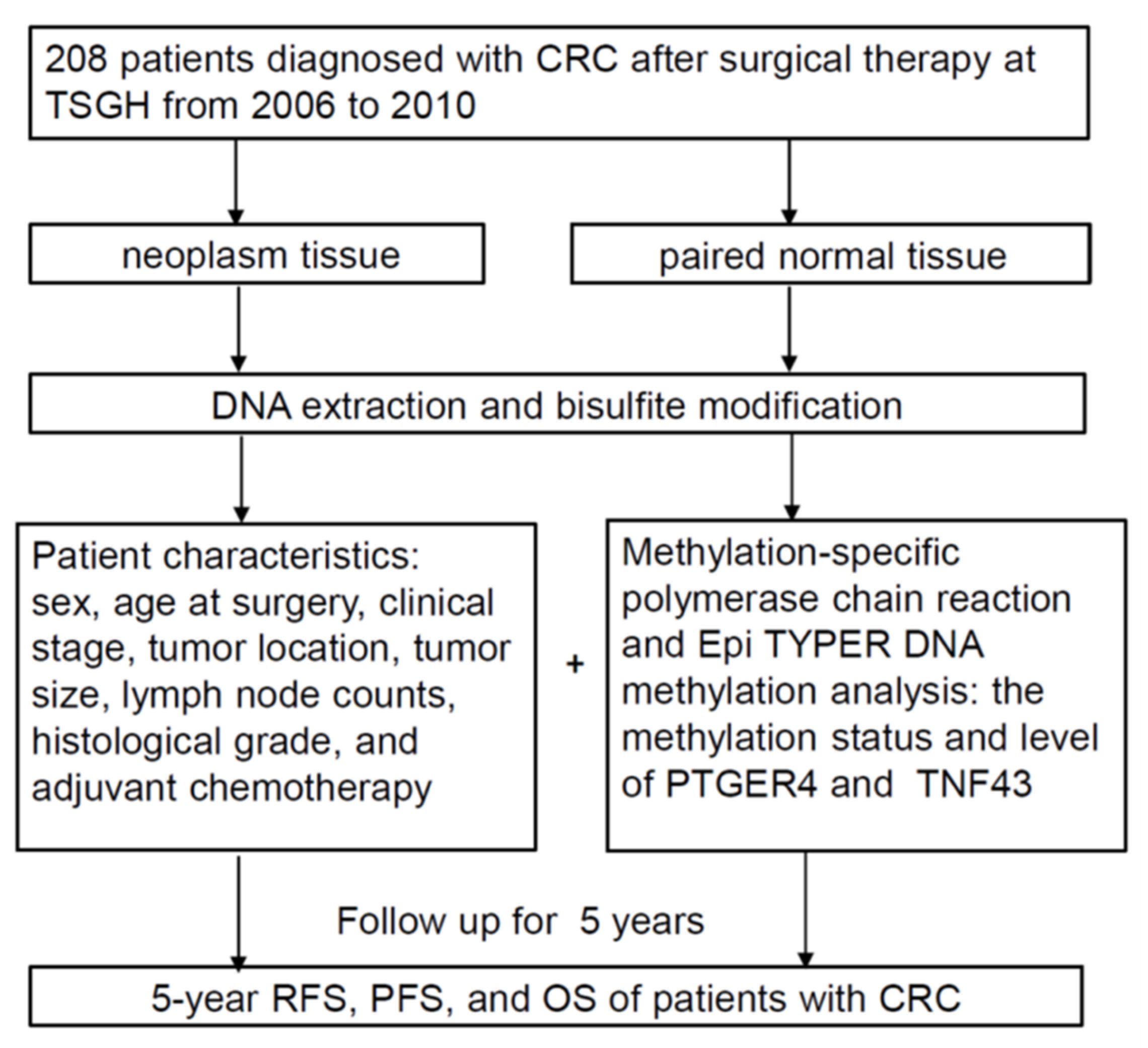
4.1. Study Design
4.2. Study Cohort and Specimens
4.3. Gene Selection
4.4. DNA Extraction and Bisulfite Modification
4.5. Qualitative and Quantitative Analysis of DNA Methylation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer—Global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Collins, G.; Wang, H.; Toh, J.W.T. Pathological Features and Prognostication in Colorectal Cancer. Curr. Oncol. 2021, 28, 5356–5383. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Cervena, K.; Siskova, A.; Buchler, T.; Vodicka, P.; Vymetalkova, V. Methylation-Based Therapies for Colorectal Cancer. Cells 2020, 9, 1540. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Rizzo, A.; Nannini, M.; Novelli, M.; Ricci, A.D.; Scioscio, V.D.; Pantaleo, M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936932. [Google Scholar] [CrossRef]
- Vera, R.; Aparicio, J.; Carballo, F.; Esteva, M.; González-Flores, E.; Santianes, J.; Santolaya, F.; Fernández-Cebrián, J.M. Recommendations for follow-up of colorectal cancer survivors. Clin. Transl. Oncol. 2019, 21, 1302–1311. [Google Scholar] [CrossRef] [Green Version]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, E.A.; Brenne, S.S.; Skorpen, F. DNA methylation markers detected in blood, stool, urine, and tissue in colorectal cancer: A systematic review of paired samples. Int. J. Colorectal Dis. 2021, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Picardo, F.; Romanelli, A.; Muinelo-Romay, L.; Mazza, T.; Fusilli, C.; Parrella, P.; Barbazán, J.; Lopez-López, R.; Barbano, R.; De Robertis, M.; et al. Diagnostic and Prognostic Value of B4GALT1 Hypermethylation and Its Clinical Significance as a Novel Circulating Cell-Free DNA Biomarker in Colorectal Cancer. Cancers 2019, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-Y.; Wang, S.-J.; Song, F.; Cheng, X.; Nan, G.; Zhao, Y.; Qian, M.-R.; Chen, X.; Li, J.-Y.; Liu, F.-L.; et al. CD147 receptor is essential for TFF3-mediated signaling regulating colorectal cancer progression. Signal Transduct. Target. Ther. 2021, 6, 268. [Google Scholar] [CrossRef]
- Machnik, M.; Cylwa, R.; Kiełczewski, K.; Biecek, P.; Liloglou, T.; Mackiewicz, A.; Oleksiewicz, U. The expression signature of cancer-associated KRAB-ZNF factors identified in TCGA pan-cancer transcriptomic data. Mol. Oncol. 2019, 13, 701–724. [Google Scholar] [CrossRef]
- Tong, G.-J.; Zhang, G.-Y.; Liu, J.; Zheng, Z.-Z.; Chen, Y.; Niu, P.-P.; Xu, X.-T. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9, 148–161. [Google Scholar] [CrossRef]
- Gaiani, F.; Marchesi, F.; Negri, F.; Greco, L.; Malesci, A.; de’Angelis, G.L.; Laghi, L. Heterogeneity of Colorectal Cancer Progression: Molecular Gas and Brakes. Int. J. Mol. Sci. 2021, 22, 5246. [Google Scholar] [CrossRef]
- Ahluwalia, P.; Mondal, A.K.; Bloomer, C.; Fulzele, S.; Jones, K.; Ananth, S.; Gahlay, G.K.; Heneidi, S.; Rojiani, A.M.; Kota, V.; et al. Identification and Clinical Validation of a Novel 4 Gene-Signature with Prognostic Utility in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 3818. [Google Scholar] [CrossRef]
- Galamb, O.; Kalmár, A.; Péterfia, B.; Csabai, I.; Bodor, A.; Ribli, D.; Krenács, T.; Patai, Á.V.; Wichmann, B.; Barták, B.K.; et al. Aberrant DNA methylation of WNT pathway genes in the development and progression of CIMP-negative colorectal cancer. Epigenetics 2016, 11, 588–602. [Google Scholar] [CrossRef]
- Grady, W.M.; Yu, M.; Markowitz, S.D. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology 2021, 160, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.L.; Krarup, H.B.; Sunesen, K.G.; Pedersen, I.S.; Madsen, P.H.; Thorlacius-Ussing, O. Hypermethylated DNA as a biomarker for colorectal cancer: A systematic review. Colorectal Dis. 2016, 18, 549–561. [Google Scholar] [CrossRef]
- Karpisheh, V.; Nikkhoo, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Ghalamfarsa, G.; Sabz, G.; Yousefi, M.; Yousefi, B.; Jadidi-Niaragh, F. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat. 2019, 144, 106338. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Shao, J.; Washington, M.K.; DuBois, R.N. Prostaglandin E2 Increases Growth and Motility of Colorectal Carcinoma Cells. J. Biol. Chem. 2001, 276, 18075–18081. [Google Scholar] [CrossRef] [PubMed]
- Karpisheh, V.; Joshi, N.; Zekiy, A.O.; Beyzai, B.; Hojjat-Farsangi, M.; Namdar, A.; Edalati, M.; Jadidi-Niaragh, F. EP4 receptor as a novel promising therapeutic target in colon cancer. Pathol. Res. Pract. 2020, 216, 153247. [Google Scholar] [CrossRef]
- Fujino, H.; Regan, J.W. EP4 Prostanoid Receptor Coupling to a Pertussis Toxin-Sensitive Inhibitory G Protein. Mol. Pharmacol. 2006, 69, 5–10. [Google Scholar] [CrossRef]
- Wang, D.; Cabalag, C.S.; Clemons, N.J.; DuBois, R.N. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology 2021, 161, 1813–1829. [Google Scholar] [CrossRef]
- Watanabe, Y.; Murata, T.; Amakawa, M.; Miyake, Y.; Handa, T.; Konishi, K.; Matsumura, Y.; Tanaka, T.; Takeuchi, K. KAG-308, a newly-identified EP4-selective agonist shows efficacy for treating ulcerative colitis and can bring about lower risk of colorectal carcinogenesis by oral administration. Eur. J. Pharmacol. 2015, 754, 179–189. [Google Scholar] [CrossRef]
- Russo, J.; Jalkanen, A.L.; Heck, A.M.; Schmidt, C.M.; Wilusz, J.; Wilusz, C.J. Sequences encoding C2H2 zinc fingers inhibit polyadenylation and mRNA export in human cells. Sci. Rep. 2018, 8, 16995. [Google Scholar] [CrossRef]
- Emerson, R.O.; Thomas, J.H. Adaptive Evolution in Zinc Finger Transcription Factors. PLOS Genet. 2009, 5, e1000325. [Google Scholar] [CrossRef] [Green Version]
- Randolph, K.; Hyder, U.; D’Orso, I. KAP1/TRIM28: Transcriptional Activator and/or Repressor of Viral and Cellular Programs? Front. Cell. Infect. Microbiol. 2022, 12, 834636. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.M.; Bartolomei, M.S. Evolving imprinting control regions: KRAB zinc fingers hold the key. Genes Dev. 2019, 33, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, C. Downregulation of CacyBP by CRISPR/dCas9-KRAB Prevents Bladder Cancer Progression. Front. Mol. Biosci. 2021, 8, 692941. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Ling, J.; Wang, Y.; Xu, X.; Liu, Y.; Jin, C.; Ju, J.; Yuan, Y.; He, F.; et al. KRAB-containing zinc finger protein ZNF496 inhibits breast cancer cell proliferation by selectively repressing ERα activity. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 841–853. [Google Scholar] [CrossRef]
- Ye, L.; Xiang, T.; Fan, Y.; Zhang, D.; Li, L.; Zhang, C.; He, X.; Xiang, Q.; Tao, Q.; Ren, G. The 19q13 KRAB Zinc-finger protein ZFP82 suppresses the growth and invasion of esophageal carcinoma cells through inhibiting NF-κB transcription and inducing apoptosis. Epigenomics 2019, 11, 65–80. [Google Scholar] [CrossRef]
- Helleboid, P.-Y.; Heusel, M.; Duc, J.; Piot, C.; Thorball, C.W.; Coluccio, A.; Pontis, J.; Imbeault, M.; Turelli, P.; Aebersold, R.; et al. The interactome of KRAB zinc finger proteins reveals the evolutionary history of their functional diversification. EMBO J. 2019, 38, e101220. [Google Scholar] [CrossRef]
- Schultz, D.C.; Ayyanathan, K.; Negorev, D.; Maul, G.G.; Rauscher, F.J., 3rd. SETDB1: A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002, 16, 919–932. [Google Scholar] [CrossRef]
- Cann, K.L.; Dellaire, G. Heterochromatin and the DNA damage response: The need to relax. Biochem. Cell Biol. 2011, 89, 45–60. [Google Scholar] [CrossRef]
- Venkov, C.D.; Link, A.J.; Jennings, J.L.; Plieth, D.; Inoue, T.; Nagai, K.; Xu, C.; Dimitrova, Y.N.; Rauscher, F.J., III; Neilson, E.G. A proximal activator of transcription in epithelial-mesenchymal transition. J. Clin. Investig. 2007, 117, 482–491. [Google Scholar] [CrossRef]
- Tian, C.; Xing, G.; Xie, P.; Lu, K.; Nie, J.; Wang, J.; Li, L.; Gao, M.; Zhang, L.; He, F. KRAB-type zinc-finger protein Apak specifically regulates p53-dependent apoptosis. Nat. Cell Biol. 2009, 11, 580–591. [Google Scholar] [CrossRef]
- Yang, Y.; Fiskus, W.; Yong, B.; Atadja, P.; Takahashi, Y.; Pandita, T.K.; Wang, H.-G.; Bhalla, K.N. Acetylated hsp70 and KAP1-mediated Vps34 SUMOylation is required for autophagosome creation in autophagy. Proc. Natl. Acad. Sci. USA 2013, 110, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Kel, A.; Boyarskikh, U.; Stegmaier, P.; Leskov, L.S.; Sokolov, A.V.; Yevshin, I.; Mandrik, N.; Stelmashenko, D.; Koschmann, J.; Kel-Margoulis, O.; et al. Walking pathways with positive feedback loops reveal DNA methylation biomarkers of colorectal cancer. BMC Bioinform. 2019, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- van Rijnsoever, M.; Grieu, F.; Elsaleh, H.; Joseph, D.; Iacopetta, B. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut 2002, 51, 797–802. [Google Scholar] [CrossRef]
- Lee, Y.; Dho, S.H.; Lee, J.; Hwang, J.-H.; Kim, M.; Choi, W.-Y.; Lee, J.-Y.; Lee, J.; Chang, W.; Lee, M.Y.; et al. Hypermethylation of PDX1, EN2, and MSX1 predicts the prognosis of colorectal cancer. Exp. Mol. Med. 2022, 54, 156–168. [Google Scholar] [CrossRef]
- Gladstein, S.; Damania, D.; Almassalha, L.M.; Smith, L.T.; Gupta, V.; Subramanian, H.; Rex, D.K.; Roy, H.K.; Backman, V. Correlating colorectal cancer risk with field carcinogenesis progression using partial wave spectroscopic microscopy. Cancer Med. 2018, 7, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Dampier, C.H.; Devall, M.; Jennelle, L.T.; Díez-Obrero, V.; Plummer, S.J.; Moreno, V.; Casey, G. Oncogenic Features in Histologically Normal Mucosa: Novel Insights into Field Effect from a Mega-Analysis of Colorectal Transcriptomes. Clin. Transl. Gastroenterol. 2020, 11, e00210. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef]
- Chang, J.; Vacher, J.; Yao, B.; Fan, X.; Zhang, B.; Harris, R.C.; Zhang, M.-Z. Prostaglandin E receptor 4 (EP4) promotes colonic tumorigenesis. Oncotarget 2015, 6, 33500–33511. [Google Scholar] [CrossRef]
- Sun, M.; Ju, J.; Ding, Y.; Zhao, C.; Tian, C. The signaling pathways regulated by KRAB zinc-finger proteins in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188731. [Google Scholar] [CrossRef]
- Su, J.-Q.; Lai, P.-Y.; Hu, P.-H.; Hu, J.-M.; Chang, P.-K.; Chen, C.-Y.; Wu, J.-J.; Lin, Y.-J.; Sun, C.-A.; Yang, T.; et al. Differential DNA methylation analysis of SUMF2, ADAMTS5, and PXDN provides novel insights into colorectal cancer prognosis prediction in Taiwan. World J. Gastroenterol. 2022, 28, 825–839. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total | Methylation Status | |||
|---|---|---|---|---|---|
| PTGER4 | ZNF43 | ||||
| Normal | Tumor | Normal | Tumor | ||
| Sex | |||||
| Male | 103 (49.5) | 29 (42.0) | 41 (59.4) | 22 (31.9) | 49 (71.0) |
| Female | 105 (50.5) | 36 (48.0) | 47 (62.7) | 20 (26.7) | 57 (76.0) |
| χ2 (p value) | 0.30 (0.581) | 0.05 (0.820) | 0.26 (0.614) | 0.24 (0.625) | |
| Age at surgery | |||||
| <50 | 35 (16.8) | 12 (54.5) | 12 (54.5) | 5 (22.7) | 15 (68.2) |
| ≥50 | 173 (83.2) | 53 (43.4) | 76 (62.3) | 37 (30.3) | 91 (74.6) |
| χ2 (p value) | 0.53 (0.465) | 0.20 (0.654) | 0.22 (0.640) | 0.13 (0.715) | |
| Stage | |||||
| I + II | 106 (51.0) | 33 (45.8) | 42 (58.3) | 29 (40.3) | 53 (73.6) |
| III + IV | 102 (49.0) | 32 (44.4) | 46 (63.9) | 13 (18.1) | 53 (73.6) |
| χ2 (p value) | <0.01 (1.000) | 0.26 (0.608) | 7.56 (0.006) a | <0.01 (1.000) | |
| Tumor location 1 | |||||
| Colon | 37 (20.1) | 11 (40.7) | 14 (51.9) | 30 (28.3) | 75 (70.8) |
| Rectum | 147 (79.9) | 50 (47.2) | 66 (62.3) | 7 (25.9) | 23 (85.2) |
| χ2 (p value) | 0.15 (0.702) | 0.59 (0.443) | <0.01 (0.996) | 1.63 (0.202) | |
| Tumor size 1 | |||||
| ≤5 cm | 114 (63.0) | 40 (48.8) | 46 (56.1) | 18 (22.0) | 57 (69.5) |
| >5 cm | 67 (37.0) | 20 (40.0) | 33 (66.0) | 19 (38.0) | 40 (80.0) |
| χ2 (p value) | 0.64 (0.422) | 0.89 (0.346) | 3.21(0.073) | 1.26 (0.262) | |
| Lymph node counts 1 | |||||
| 0–11 | 34 (18.4) | 7 (35.0) | 11 (55.0) | 6 (30.0) | 15 (75.0) |
| ≥12 | 151 (81.6) | 54 (47.4) | 70 (61.4) | 31 (27.2) | 84 (73.7) |
| χ2 (p value) | 0.61 (0.435) | 0.09 (0.770) | <0.01 (1.000) | <0.01 (1.000) | |
| Histological grade 1 | |||||
| Well or Moderate | 156 (89.7) | 49 (44.5) | 66 (60.0) | 31 (28.2) | 83 (75.5) |
| Poor or undifferentiated | 18 (10.3) | 8 (53.3) | 8 (53.3) | 5 (33.3) | 9 (60.0) |
| χ2 (p value) | 0.13 (0.715) | 0.05 (0.831) | 0.01 (0.913) | 0.93 (0.336) | |
| Adjuvant chemotherapy 1 | |||||
| No | 54 (29.3) | 21 (52.5) | 26 (65.0) | 22 (23.7) | 32 (80.0) |
| Yes | 130 (70.7) | 40 (43.0) | 54 (58.1) | 15 (37.5) | 66 (71.0) |
| χ2 (p value) | 0.67 (0.414) | 0.31 (0.614) | 2.03 (0.155) | 0.76 (0.384) | |
| 5-year recurrence | |||||
| No | 149 (71.6) | 48 (45.3) | 22 (57.9) | 32 (31.1) | 74 (71.8) |
| Yes | 59 (28.4) | 17 (44.7) | 66 (62.3) | 10 (24.4) | 32 (78.0) |
| χ2 (p value) | <0.01 (1.000) | 0.08 (0.779) | 0.35 (0.554) | 0.31 (0.580) | |
| 5-year progression | |||||
| No | 116 (55.8) | 36 (46.2) | 45 (57.7) | 28 (29.5) | 68 (71.6) |
| Yes | 92 (44.2) | 29 (43.9) | 43 (65.2) | 14 (28.6) | 38 (77.6) |
| χ2 (p value) | <0.01 (0.922) | 0.55 (0.457) | <0.01 (1.000) | 0.33 (0.568) | |
| 5-year all-cause death | |||||
| No | 168 (80.8) | 59 (49.6) | 69 (58.0) | 33 (27.7) | 88 (73.9) |
| Yes | 40 (19.2) | 6 (24.0) | 19 (76.0) | 9 (36.0) | 18 (72.0) |
| χ2 (p value) | 4.48 (0.034) a | 2.12 (0.146) | 0.34 (0.559) | <0.01 (1.000) | |
| Methylation Status | |||
|---|---|---|---|
| PTGER4 a | ZNF43 a | ||
| Normal | Tumor | Normal | Tumor |
| 65 (45.1) | 88 (61.1) | 42 (29.2) | 106 (73.6) |
| Normal | Tumor | p Value 3 | |||||
|---|---|---|---|---|---|---|---|
| n 1 | Median (Q1–Q3) (%) | Mean ± SD 2 (%) | n 1 | Median (Q1–Q3) (%) | Mean ± SD 2 (%) | ||
| PTGER4 | |||||||
| CpG_1.2 | 143 | 6 (3–8) | 6.78 ± 2.4 | 144 | 6 (4.3–8) | 7.37 ± 7.0 | 0.322 |
| CpG_3 | 144 | 4 (3–6) | 4.74 ± 2.7 | 140 | 3 (2–4) | 4.64 ± 8.3 | <0.001 |
| CpG_4.5 | 141 | 11 (8–14) | 12.06 ± 6.5 | 141 | 10 (7–13) | 11.24 ± 7.5 | 0.044 |
| CpG_6.7 | 142 | 6 (5–8) | 6.65 ± 3.2 | 144 | 5 (3.3–7) | 6.38 ± 7.4 | <0.001 |
| CpG_9.10 | 143 | 9 (8–12) | 10.14 ± 4.4 | 143 | 7 (5–10) | 8.71 ± 8.4 | <0.001 |
| CpG_11 | 144 | 10.5 (9–13) | 11.56 ± 5.0 | 144 | 8 (6–11.8) | 10.10 ± 8.5 | <0.001 |
| CpG_13 | 144 | 16 (13–18) | 16.42 ± 5.8 | 144 | 11.5 (9–15) | 13.07 ± 7.6 | <0.001 |
| CpG_15 | 142 | 16 (12–21) | 18.05 ± 10.8 | 139 | 11 (6–16) | 13.24 ± 11.1 | <0.001 |
| CpG_16 | 141 | 29 (26–33) | 29.27 ± 5.8 | 143 | 23 (18–30) | 25.36 ± 10.0 | <0.001 |
| CpG_17 | 84 | 18 (14–22) | 18.21 ± 6.9 | 85 | 14 (10–18) | 15.05 ± 8.2 | 0.052 |
| CpG_18 | 143 | 27 (23–32) | 28.31 ± 8.5 | 144 | 20 (16–29) | 24.10 ± 13.8 | <0.001 |
| ZNF43 | |||||||
| CpG_2 | 143 | 4 (3.0–6.0) | 5.17 ± 0.25 | 143 | 9 (4.0–29.0) | 18.10 ± 1.56 | <0.001 |
| CpG_3 | 144 | 3 (2.0–3.0) | 3.15 ± 0.26 | 144 | 9 (3.0–36.0) | 20.39 ± 1.84 | <0.001 |
| CpG_4 | 131 | 1 (1.0–3.0) | 1.98 ± 0.24 | 140 | 11 (2.0–36.0) | 20.31 ± 1.87 | <0.001 |
| CpG_5 | 144 | 6 (4.0–9.0) | 8.99 ± 0.77 | 143 | 16 (6.0–38.0) | 23.08 ± 1.77 | <0.001 |
| CpG_6 | 84 | 3 (2.0–4.8) | 4.07 ± 0.37 | 115 | 16 (4.0–39.0) | 22.65 ± 1.82 | <0.001 |
| CpG_7.8 | 144 | 4 (4.0–5.0) | 4.60 ± 0.22 | 144 | 9 (4.0–35.0) | 21.24 ± 1.76 | <0.001 |
| RFS | PFS | OS | ||||
|---|---|---|---|---|---|---|
| cHR (95% CI) | aHR (95% CI) 1 | cHR (95% CI) | aHR (95% CI) 1 | cHR (95% CI) | aHR (95% CI) 2 | |
| PTGER4 in tumor tissue | ||||||
| all CpG sites | ||||||
| hypomethylation | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| hypermethylation | 1.22 (0.64–2.30) | 1.03 (0.51–2.08) | 1.47 (0.91–2.40) | 1.33 (0.76–2.31) | 2.18 (0.94–5.04) | 1.77 (0.74–4.24) |
| p value | 0.550 | 0.945 | 0.121 | 0.263 | 0.070 | 0.201 |
| CpG_4.5 a | ||||||
| hypomethylation | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| hypermethylation | 1.36 (0.71–2.58) | 1.04 (0.51–2.12) | 1.60 (0.97–2.63) | 1.33 (0.76–2.30) | 1.98 (0.85–4.58) | 1.72 (0.72–4.09) |
| p value | 0.354 | 0.917 | 0.064 | 0.317 | 0.112 | 0.222 |
| CpG_15 a | ||||||
| hypomethylation | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| hypermethylation | 1.86 (0.94–3.66) | 1.06 (0.48–2.33) | 1.39 (0.85–2.29) | 1.07 (0.60–1.90) | 2.11 (0.91–4.88) | 1.55 (0.63–3.79) |
| p value | 0.075 | 0.895 | 0.195 | 0.829 | 0.082 | 0.339 |
| CpG_17 a | ||||||
| hypomethylation | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| hypermethylation | 1.87 (0.79–4.41) | 2.08 (0.79–5.48) | 1.60 (0.84–3.04) | 1.85 (0.89–3.84) | 2.60 (0.83–8.17) | 2.55 (0.80–8.05) |
| p value | 0.155 | 0.137 | 0.156 | 0.099 | 0.103 | 0.112 |
| ZNF43 in normal tissue | ||||||
| CpG_5 | ||||||
| hypomethylation | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| hypermethylation | 1.81 (0.94–3.49) | 2.33 (1.07–5.08) b | 1.78 (0.98–3.23) | 2.42 (1.19–4.91) b | 1.16 (0.52–2.58) | 1.13 (0.48–2.67) |
| p value | 0.077 | 0.014 | 0.059 | 0.014 | 0.716 | 0.775 |
| n 1 | RFS | PFS | OS | ||||
|---|---|---|---|---|---|---|---|
| cHR (95% CI) | aHR (95% CI) 2 | cHR (95% CI) | aHR (95% CI) 3 | cHR (95% CI) | aHR (95% CI) 4 | ||
| CpG_4.5 + CpG_15 hypermethylation | |||||||
| No | 90 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 49 | 2.14 (1.12–4.07) a | 1.54 (0.74–3.18) | 1.73 (1.06–2.84) a | 1.29 (0.73–2.28) | 2.63 (1.19–5.80) a | 2.19 (0.95–5.05) |
| p value | 0.021 | 0.248 | 0.030 | 0.385 | 0.016 | 0.066 | |
| CpG_4.5 + CpG_17 hypermethylation | |||||||
| No | 79 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 26 | 2.92 (1.37–6.22) a | 3.44 (1.53–7.72) a,b | 2.74 (1.53–4.92) a,b | 2.45 (1.26–4.76) a | 4.11 (1.62–10.4) a,b | 3.79 (1.46–9.88) a |
| p value | 0.005 | 0.003 | 0.001 | 0.008 | 0.003 | 0.006 | |
| CpG_15 + CpG_17 hypermethylation | |||||||
| No | 79 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 34 | 1.91 (0.89–4.11) | 1.71 (0.73–4.01) | 1.56 (0.87–2.78) | 1.42 (0.74–2.74) | 2.89 (1.17–7.11) a | 2.36 (0.93–5.97) |
| p value | 0.099 | 0.214 | 0.133 | 0.295 | 0.021 | 0.071 | |
| CpG_4.5 + CpG_15 + CpG_17 hypermethylation | |||||||
| No | 96 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Yes | 21 | 3.18 (1.45–6.97) a,b | 3.26 (1.38–7.73) a | 2.77 (1.52–5.05) a,b | 2.35 (1.17–4.71) a | 4.79 (2.00–11.4) a,b | 4.32 (1.8–10.5) a,b |
| p value | 0.004 | 0.007 | 0.001 | 0.016 | <0.001 | 0.001 | |
| Genes | Forward Primer (5′→3′) | Annealing Temperature (°C) | Product Size (bp) | |
|---|---|---|---|---|
| PTGER4 | M | F: GTTTTATTTCGTTCGTGGTGA | 58.6 | 247 |
| R: AAAAAAAAAACCCAAACTTCC | ||||
| U | F: GGGTTGGGGTTTTATTTGGTT | 64.3 | 315 | |
| R: CAACAAACTCCCCTCCACATC | ||||
| Q | F: ATTTTTTTGGTGGTGTTTATTTGTT a | 57.9 | 440 | |
| R: TCAAATTTTACAATTCACAATTCACA a | ||||
| ZNF43 | M | F: GGAGGAAGTTTTGTTTGAAAAGGC | 61.3 | 323 |
| R: TTCTAAACTTCCGAAAAATCCTAAC | ||||
| U | F: GAAGTTTTGTTTGAAAAGGTGG | 59.5 | 328 | |
| R: ACCATTTCTAAACTTCCAAAA | ||||
| Q | F: AAGGTTAAAGGTAAATATTTTTTGGG a | 60.8 | 267 | |
| R: TCCAACTACAACCAAAAACAAAAAC a | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Wu, J.-J.; Lin, Y.-J.; Hsu, C.-H.; Hu, J.-M.; Chang, P.-K.; Sun, C.-A.; Yang, T.; Su, J.-Q.; Chou, Y.-C. Significance of Hypermethylation of Tumor-Suppressor Genes PTGER4 and ZNF43 at CpG Sites in the Prognosis of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 10225. https://doi.org/10.3390/ijms231810225
Chen C-Y, Wu J-J, Lin Y-J, Hsu C-H, Hu J-M, Chang P-K, Sun C-A, Yang T, Su J-Q, Chou Y-C. Significance of Hypermethylation of Tumor-Suppressor Genes PTGER4 and ZNF43 at CpG Sites in the Prognosis of Colorectal Cancer. International Journal of Molecular Sciences. 2022; 23(18):10225. https://doi.org/10.3390/ijms231810225
Chicago/Turabian StyleChen, Chao-Yang, Jia-Jheng Wu, Yu-Jyun Lin, Chih-Hsiung Hsu, Je-Ming Hu, Pi-Kai Chang, Chien-An Sun, Tsan Yang, Jing-Quan Su, and Yu-Ching Chou. 2022. "Significance of Hypermethylation of Tumor-Suppressor Genes PTGER4 and ZNF43 at CpG Sites in the Prognosis of Colorectal Cancer" International Journal of Molecular Sciences 23, no. 18: 10225. https://doi.org/10.3390/ijms231810225
APA StyleChen, C.-Y., Wu, J.-J., Lin, Y.-J., Hsu, C.-H., Hu, J.-M., Chang, P.-K., Sun, C.-A., Yang, T., Su, J.-Q., & Chou, Y.-C. (2022). Significance of Hypermethylation of Tumor-Suppressor Genes PTGER4 and ZNF43 at CpG Sites in the Prognosis of Colorectal Cancer. International Journal of Molecular Sciences, 23(18), 10225. https://doi.org/10.3390/ijms231810225





