Clostridium novyi’s Alpha-Toxin Changes Proteome and Phosphoproteome of HEp-2 Cells
Abstract
1. Introduction
2. Results
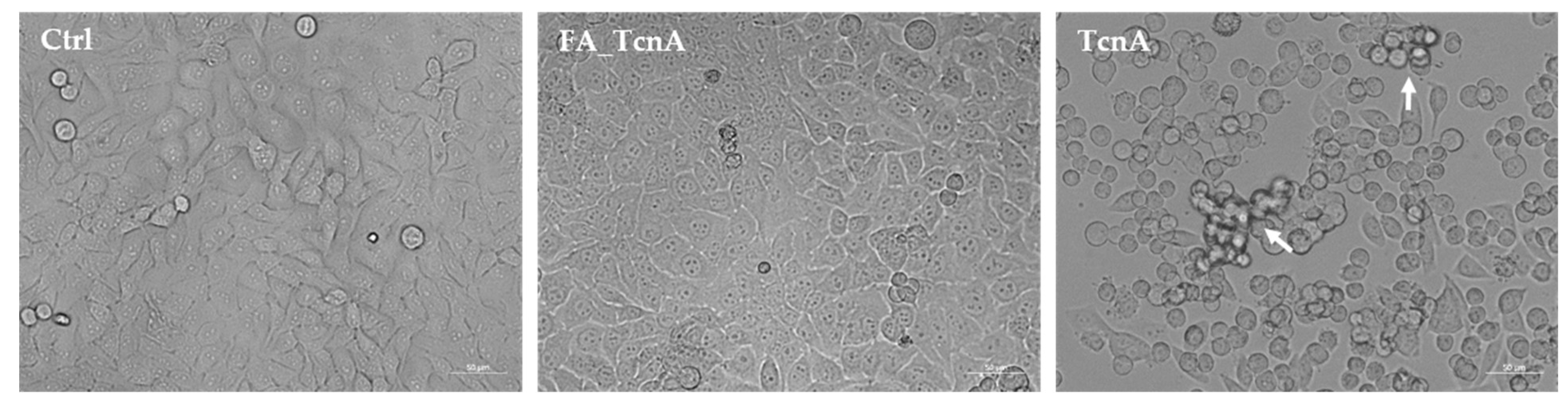
2.1. Morphological Alterations of HEp-2 Cells Treated with C. novyi’s Alpha Toxin
2.2. Generation of a Control Toxin to Elucidate TcnA Effects on Proteome and Phosphoproteome
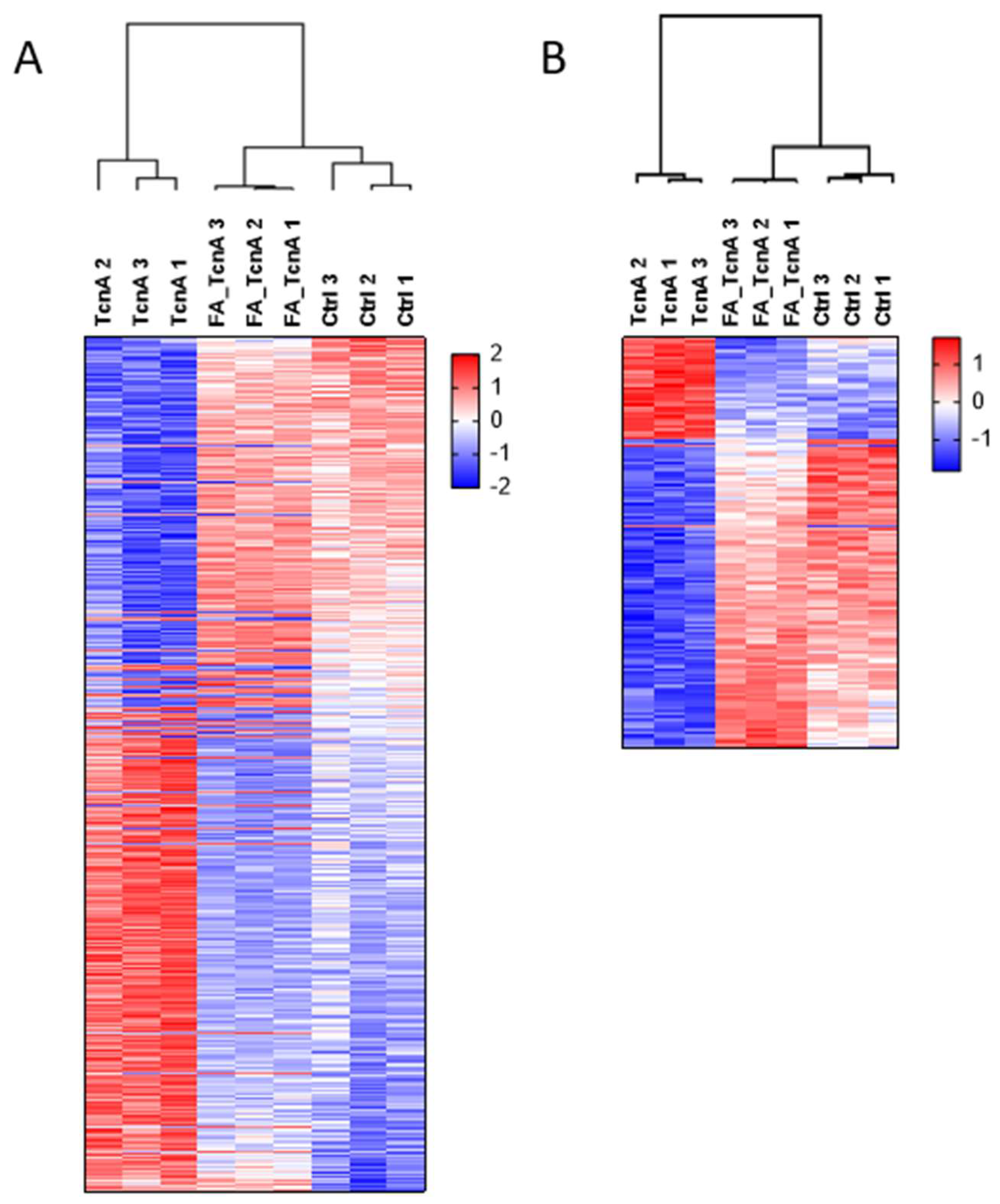
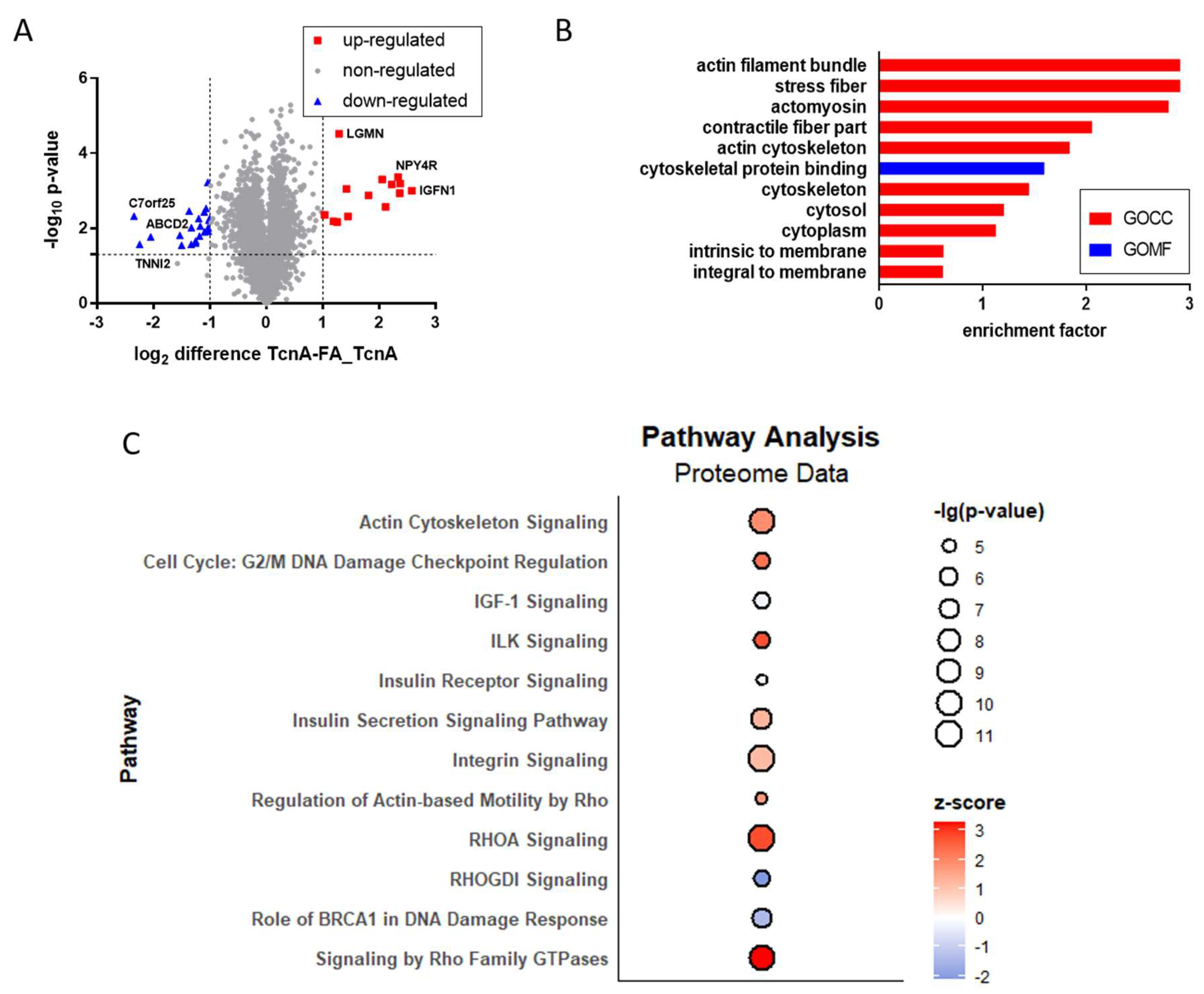
2.3. Proteomic Effects Induced by TcnA in HEp-2 Cells
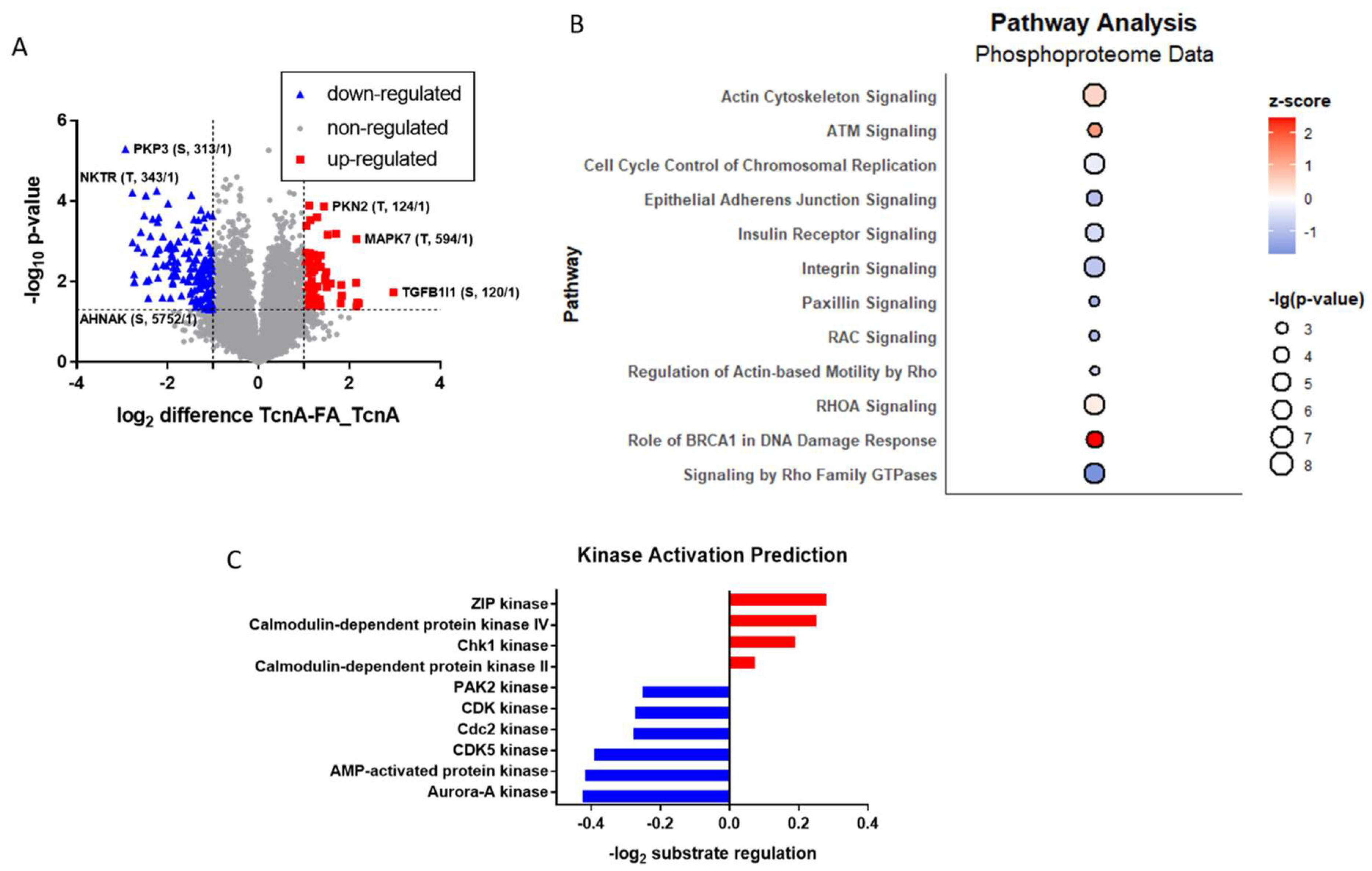
2.4. Phosphoproteomic Effects of TcnA on HEp-2 Cells
3. Discussion
4. Materials and Methods
4.1. Purification and Inactivation of Clostridium Novyi α-Toxin
4.2. Cell Culture
4.3. Toxin Treatment of HEp-2 Cells and Sample Preparation
4.4. Protein Digestion
4.5. Tandem Mass Tags Labeling and Phosphopeptide Enrichment
4.6. LC-MS Analysis
4.7. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buboltz, J.B.; Murphy-Lavoie, H.M. Gas gangrene. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Navarro, M.A.; Uzal, F.A. Pathobiology and diagnosis of clostridial hepatitis in animals. J. Vet. Diagn. Investig. 2019, 32, 192–202. [Google Scholar] [CrossRef]
- Oakley, C.L.; Warrack, G.H.; Clarke, P.H. The Toxins of Clostridium oedematiens (Cl. novyi). J. Gen. Microbiol. 1947, 1, 91–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eklund, M.W.; Poysky, F.T.; Peterson, M.E.; Meyers, J.A. Relationship of bacteriophages to alpha toxin production in Clostridium novyi types A and B. Infect. Immun. 1976, 14, 793–803. [Google Scholar] [CrossRef]
- Skarin, H.; Segerman, B. Plasmidome Interchange between Clostridium botulinum, Clostridium novyi and Clostridium haemolyticum Converts Strains of Independent Lineages into Distinctly Different Pathogens. PLoS ONE 2014, 9, e107777. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kimura, I.; Yamakawa, K.; Nishida, S. Taxonomic Relationships among Clostridium novyi Types A and B, Clostridium haemolyticum and Clostridium botulinum Type C. Microbiology 1983, 129, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.C.; Poxton, I.R. Clostridium botulinum types C and D and the closely related Clostridium novyi. Rev. Med. Microbiol. 2002, 13, 75–90. [Google Scholar] [CrossRef]
- Bryant, A.E.; Stevens, D.L. Clostridial toxins in the pathogenesis of gas gangrene. In The Comprehensive Sourcebook of Bacterial Protein Toxins; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Orrell, K.E.; Melnyk, R.A. Large Clostridial Toxins: Mechanisms and Roles in Disease. Microbiol. Mol. Biol. Rev. 2021, 85, e0006421. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.F.; Petit, L.; Gibert, M.; Marvaud, J.C.; Bouchaud, C.; Popoff, M.R. Bacterial toxins modifying the actin cytoskeleton. Int. Microbiol. 1999, 2, 185–194. [Google Scholar] [CrossRef]
- Jank, T.; Aktories, K. Structure and mode of action of clostridial glucosylating toxins: The ABCD model. Trends Microbiol. 2008, 16, 222–229. [Google Scholar] [CrossRef]
- Selzer, J.; Hofmann, F.; Rex, G.; Wilm, M.; Mann, M.; Just, I.; Aktories, K. Clostridium novyi α-Toxin-catalyzed Incorporation of GlcNAc into Rho Subfamily Proteins. J. Biol. Chem. 1996, 271, 25173–25177. [Google Scholar] [CrossRef]
- Busch, C.; Schömig, K.; Hofmann, F.; Aktories, K. Characterization of the Catalytic Domain of Clostridium novyi Alpha-Toxin. Infect. Immun. 2000, 68, 6378–6383. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Geny, B. Rho/Ras-GTPase-dependent and -independent activity of clostridial glucosylating toxins. J. Med. Microbiol. 2011, 60, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Belyi, Y.; Aktories, K. Bacterial toxin and effector glycosyltransferases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Oksche, A.; Nakov, R.; Habermann, E. Morphological and biochemical study of cytoskeletal changes in cultured cells after extracellular application of Clostridium novyi alpha-toxin. Infect. Immun. 1992, 60, 3002–3006. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Luo, J.; Chen, A.; Li, X.; Pan, Z.; Wan, L.; He, L.; Li, D.; Li, Y.; et al. Sulfated glycosaminoglycans and low-density lipoprotein receptor mediate the cellular entry of Clostridium novyi alpha-toxin. Cell Res. 2021, 31, 935–938. [Google Scholar] [CrossRef]
- Bateman, N.W.; Goulding, S.P.; Shulman, N.J.; Gadok, A.K.; Szumlinski, K.K.; MacCoss, M.J.; Wu, C.C. Maximizing Peptide Identification Events in Proteomic Workflows Using Data-Dependent Acquisition (DDA). Mol. Cell. Proteom. 2014, 13, 329–338. [Google Scholar] [CrossRef]
- Engholm-Keller, K.; Larsen, M.R. Technologies and challenges in large-scale phosphoproteomics. Proteomics 2013, 13, 910–931. [Google Scholar] [CrossRef]
- Nilsson, C.L. Advances in Quantitative Phosphoproteomics. Anal. Chem. 2011, 84, 735–746. [Google Scholar] [CrossRef]
- Junemann, J.; Birgin, G.; Erdmann, J.; Schröder, A.; Just, I.; Gerhard, R.; Pich, A. Toxin A of the nosocomial pathogen Clostridium difficile induces primary effects in the proteome of HEp-2 cells. Proteom. Clin. Appl. 2016, 11, 1600031. [Google Scholar] [CrossRef]
- Erdmann, J.; Junemann, J.; Schröder, A.; Just, I.; Gerhard, R.; Pich, A. Glucosyltransferase-dependent and -independent effects of TcdB on the proteome of HEp-2 cells. Proteomics 2017, 17, 1600435. [Google Scholar] [CrossRef]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- IPATM of QIAGEN’s Ingenuity Pathway Analysis. Calculating and Interpreting the p-Values for Functions, Pathways and Lists in IPA. 2016. Available online: https://www.ingenuity.com/wp-content/themes/ingenuity-qiagen/pdf/ipa/functions-pathways-pval-whitepaper.pdf (accessed on 28 February 2022).
- Ingenuity® Systems. Ingenuity Downstream Effects Analysis in IPA®. 2012. Available online: http://pages.ingenuity.com/rs/ingenuity/images/0812%20downstream_effects_analysis_whitepaper.pdf (accessed on 28 February 2022).
- Gerhard, R.; Nottrott, S.; Schoentaube, J.; Tatge, H.; Olling, A.; Just, I. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J. Med. Microbiol. 2008, 57, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Wohlan, K.; Goy, S.; Olling, A.; Srivaratharajan, S.; Tatge, H.; Genth, H.; Gerhard, R. Pyknotic cell death induced by Clostridium difficile TcdB: Chromatin condensation and nuclear blister are induced independently of the glucosyltransferase activity. Cell. Microbiol. 2014, 16, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S. Asparaginylendopeptidase: An enzyme probably responsible to post-translational proteolysis and transpeptidation of proconcanavalin A. Seikagaku J. Jpn. Biochem. Soc. 1993, 65, 185–189. [Google Scholar]
- Dall, E.; Brandstetter, H. Structure and function of legumain in health and disease. Biochimie 2016, 122, 126–150. [Google Scholar] [CrossRef]
- Rotari, V.I.; Dando, P.M.; Barrett, A.J. Legumain Forms from Plants and Animals Differ in Their Specificity. Biol. Chem. 2001, 382, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Dando, P.M.; Stevens, R.A.E.; Fortunato, M.; Barrett, A. Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 1998, 335, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.H.; Johansen, H.T.; Pettersen, S.J.; Solberg, R.; Brix, K.; Flatmark, K.; Maelandsmo, G.M. Nuclear Legumain Activity in Colorectal Cancer. PLoS ONE 2013, 8, e52980. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, Y.; Xu, C.; Liu, Q.; Peng, B.; Kaufmann, G.F.; Chen, X.; Lan, B.; Wei, C.; Lu, D.; et al. Functional Role of Asparaginyl Endopeptidase Ubiquitination by TRAF6 in Tumor Invasion and Metastasis. JNCI J. Natl. Cancer Inst. 2014, 106, dju012. [Google Scholar] [CrossRef]
- Smith, R.; Johansen, H.T.; Nilsen, H.; Haugen, M.H.; Pettersen, S.J.; Mælandsmo, G.M.; Abrahamson, M.; Solberg, R. Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie 2012, 94, 2590–2599. [Google Scholar] [CrossRef]
- Solberg, R.; Smith, R.; Almlöf, M.; Tewolde, E.; Nilsen, H.; Johansen, H.T. Legumain expression, activity and secretion are increased during monocyte-to-macrophage differentiation and inhibited by atorvastatin. Biol. Chem. 2014, 396, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, R.; Vizovisek, M.; Turk, D.; Turk, B.; Fonović, M. Protease cleavage site fingerprinting by label-free in-gel degradomics reveals pH -dependent specificity switch of legumain. EMBO J. 2017, 36, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Dall, E.; Brandstetter, H. Mechanistic and structural studies on legumain explain its zymogenicity, distinct activation pathways, and regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 10940–10945. [Google Scholar] [CrossRef] [PubMed]
- Dall, E.; Stanojlovic, V.; Demir, F.; Briza, P.; Dahms, S.O.; Huesgen, P.F.; Cabrele, C.; Brandstetter, H. The Peptide Ligase Activity of Human Legumain Depends on Fold Stabilization and Balanced Substrate Affinities. ACS Catal. 2021, 11, 11885–11896. [Google Scholar] [CrossRef]
- Manoury, B.; Hewitt, E.; Morrice, N.; Dando, P.M.; Barrett, A.J.; Watts, C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature 1998, 396, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.J.; Barton, P.J. Structural characterization of the human fast skeletal muscle troponin I gene (TNNI2). Gene 2000, 242, 313–320. [Google Scholar] [CrossRef]
- Farah, C.S.; Reinach, F.C. The troponin complex and regulation of muscle contraction. FASEB J. 1995, 9, 755–767. [Google Scholar] [CrossRef]
- Sawaki, K.; Kanda, M.; Miwa, T.; Umeda, S.; Tanaka, H.; Tanaka, C.; Kobayashi, D.; Suenaga, M.; Hattori, N.; Hayashi, M.; et al. Troponin I2 as a Specific Biomarker for Prediction of Peritoneal Metastasis in Gastric Cancer. Ann. Surg. Oncol. 2018, 25, 2083–2090. [Google Scholar] [CrossRef]
- Herzberg, O.; Moult, J.; James, M.N. A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J. Biol. Chem. 1986, 261, 2638–2644. [Google Scholar] [CrossRef]
- Fu, Z.; Liang, X.; Shi, L.; Tang, L.; Chen, D.; Liu, A.; Shao, C. SYT8 promotes pancreatic cancer progression via the TNNI2/ERRα/SIRT1 signaling pathway. Cell Death Discov. 2021, 7, 390. [Google Scholar] [CrossRef]
- Gerhard, R.; Tatge, H.; Genth, H.; Thum, T.; Borlak, J.; Fritz, G.; Just, I. Clostridium difficile Toxin A Induces Expression of the Stress-induced Early Gene Product RhoB. J. Biol. Chem. 2005, 280, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Genth, H.; Dreger, S.C.; Huelsenbeck, J.; Just, I. Clostridium difficile toxins: More than mere inhibitors of Rho proteins. Int. J. Biochem. Cell Biol. 2008, 40, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.A.; Lindquist, S. The Function of Heat-Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef] [PubMed]
- Kamalvand, G.; Pinard, G.; Ali-Khan, Z. Heme-oxygenase-1 response, a marker of oxidative stress, in a mouse model of AA amyloidosis. Amyloid 2003, 10, 151–159. [Google Scholar] [CrossRef]
- Frädrich, C.; Beer, L.-A.; Gerhard, R. Reactive Oxygen Species as Additional Determinants for Cytotoxicity of Clostridium difficile Toxins A and B. Toxins 2016, 8, 25. [Google Scholar] [CrossRef]
- Physiol, A.J.; Cell, L.; Physiol, M.; Petrache, I.; Otterbein, L.E.; Alam, J.; Wiegand, G.W.; Augustine, M.K.; Petrache, I.; Otterbein LE, O.E.; et al. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L312–L319. [Google Scholar]
- Cruse, I.; Maines, M.D. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J. Biol. Chem. 1988, 263, 3348–3353. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Sheikh, S.Z.; Maharshak, N.; Steinbach, E.C.; Russo, S.M.; Kobayashi, T.; Mackey, L.C.; Hansen, J.J.; Moeser, A.; Rawls, J.; et al. Carbon Monoxide and Heme Oxygenase-1 Prevent Intestinal Inflammation in Mice by Promoting Bacterial Clearance. Gastroenterology 2013, 144, 789–798. [Google Scholar] [CrossRef]
- Rana, N.; McLean, S.; Mann, B.E.; Poole, R.K. Interaction of the carbon monoxide-releasing molecule Ru(CO)3Cl(glycinate) (CORM-3) with Salmonella enterica serovar Typhimurium: In situ measurements of carbon monoxide binding by integrating cavity dual-beam spectrophotometry. Microbiology 2014, 160, 2771–2779. [Google Scholar] [CrossRef]
- Sebastián, V.P.; Salazar, G.; Coronado-Arrázola, I.; Schultz, B.M.; Vallejos, O.P.; Berkowitz, L.; Álvarez-Lobos, M.M.; Riedel, C.; Kalergis, A.; Bueno, S.M. Heme Oxygenase-1 as a Modulator of Intestinal Inflammation Development and Progression. Front. Immunol. 2018, 9, 1956. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Hashida, M.; Takakura, Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 2009, 61, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Janco, M.; Bonello, T.T.; Byun, A.; Coster, A.C.F.; Lebhar, H.; Dedova, I.; Gunning, P.W.; Böcking, T. The impact of tropomyosins on actin filament assembly is isoform specific. BioArchitecture 2016, 6, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Guen, V.J.; Gamble, C.; Perez, D.E.; Bourassa, S.; Zappel, H.; Gärtner, J.; Lees, J.A.; Colas, P. STAR syndrome-associated CDK10/Cyclin M regulates actin network architecture and ciliogenesis. Cell Cycle 2016, 15, 678–688. [Google Scholar] [CrossRef]
- Kubouchi, K.; Mukai, H. PKN2 is involved in aggregation and spheroid formation of fibroblasts in suspension culture by regulating cell motility and N-cadherin expression. Biochem. Biophys. Rep. 2021, 25, 100895. [Google Scholar] [CrossRef]
- Ruby, M.A.; Riedl, I.; Massart, J.; Åhlin, M.; Zierath, J.R. Protein kinase N2 regulates AMP kinase signaling and insulin responsiveness of glucose metabolism in skeletal muscle. Am. J. Physiol. Metab. 2017, 313, E483–E491. [Google Scholar] [CrossRef]
- Schmidt, A.; Durgan, J.; Magalhães, A.; Hall, A. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 2007, 26, 1624–1636. [Google Scholar] [CrossRef]
- Bonné, S.; Gilbert, B.; Hatzfeld, M.; Chen, X.; Green, K.J.; Van Roy, F. Defining desmosomal plakophilin-3 interactions. J. Cell Biol. 2003, 161, 403–416. [Google Scholar] [CrossRef]
- Gosavi, P.; Kundu, S.T.; Khapare, N.; Sehgal, L.; Karkhanis, M.S.; Dalal, S.N. E-cadherin and plakoglobin recruit plakophilin3 to the cell border to initiate desmosome assembly. Experientia 2010, 68, 1439–1454. [Google Scholar] [CrossRef] [PubMed]
- Neuholz, M.E. Der Einfluss der Protein-Kinase-C auf Die Regulation der Desmosomalen Proteine in Mauskeratinozyten. Ph.D. Thesis, Martin-Luther-University Halle-Wittenberg, Halle (Saale), Germany, 2021. [Google Scholar] [CrossRef]
- Keil, R.; Rietscher, K.; Hatzfeld, M. Antagonistic Regulation of Intercellular Cohesion by Plakophilins 1 and 3. J. Investig. Dermatol. 2016, 136, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Kešo, R.; Breuninger, S.; Hofmann, S.; Henn, M.; Röhrig, T.; Ströbel, P.; Stoecklin, G.; Hofmann, I. Plakophilins 1 and 3 Bind to FXR1 and Thereby Influence the mRNA Stability of Desmosomal Proteins. Mol. Cell. Biol. 2014, 34, 4244–4256. [Google Scholar] [CrossRef]
- Neuber, S.; Jäger, S.; Meyer, M.; Wischmann, V.; Koch, P.J.; Moll, R.; Schmidt, A. c-Src mediated tyrosine phosphorylation of plakophilin 3 as a new mechanism to control desmosome composition in cells exposed to oxidative stress. Cell Tissue Res. 2014, 359, 799–816. [Google Scholar] [CrossRef] [PubMed]
- López-Colomé, A.M.; Lee-Rivera, I.; Benavides-Hidalgo, R.; López, E. Paxillin: A crossroad in pathological cell migration. J. Hematol. Oncol. 2017, 10, 50. [Google Scholar] [CrossRef]
- Nah, A.S.; Chay, K.O. Roles of paxillin phosphorylation in IL-3 withdrawal-induced Ba/F3 cell apoptosis. Genes Genom. 2019, 41, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rhee, S.H.; Pothoulakis, C.; LaMont, J.T. Clostridium difficile toxin A binds colonocyte Src causing dephosphorylation of focal adhesion kinase and paxillin. Exp. Cell Res. 2009, 315, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Genth, H.; Pauillac, S.; Schelle, I.; Bouvet, P.; Bouchier, C.; Varela-Chavez, C.; Just, I.; Popoff, M.R. Haemorrhagic toxin and lethal toxin from Clostridium sordellii strain vpi9048: Molecular characterization and comparative analysis of substrate specificity of the large clostridial glucosylating toxins. Cell. Microbiol. 2014, 16, 1706–1721. [Google Scholar] [CrossRef]
- Brown, M.C.; Cary, L.A.; Jamieson, J.S.; Cooper, J.A.; Turner, C.E. Src and FAK Kinases Cooperate to Phosphorylate Paxillin Kinase Linker, Stimulate Its Focal Adhesion Localization, and Regulate Cell Spreading and Protrusiveness. Mol. Biol. Cell 2005, 16, 4316–4328. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wittki, S.; Bräu, T.; Dreyer, F.S.; Krätzel, K.; Dindorf, J.; Johnston, I.C.; Gross, S.; Kremmer, E.; Zeidler, R.; et al. HIV Nef, Paxillin, and Pak1/2 Regulate Activation and Secretion of TACE/ADAM10 Proteases. Mol. Cell 2013, 49, 668–679. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef]
- Colbran, R.J. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem. J. 2004, 378, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kögel, D.; Reimertz, C.; Mech, P.; Poppe, M.; Frühwald, M.C.; Engemann, H.; Scheidtmann, K.H.; Prehn, J.H.M. Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: Requirement of the mitochondrial apoptosis pathway. Br. J. Cancer 2001, 85, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.A.; Modi, P.K.; Ramesh, P.; Sidransky, D.; Gowda, H.; Prasad, T.S.K.; Chatterjee, A. Molecular Profiling Associated with Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2)-Mediated Carcinogenesis in Gastric Cancer. J. Proteome Res. 2021, 20, 2687–2703. [Google Scholar] [CrossRef]
- Naz, H.; Islam, A.; Ahmad, F.; Hassan, M.I. Calcium/calmodulin-dependent protein kinase IV: A multifunctional enzyme and potential therapeutic target. Prog. Biophys. Mol. Biol. 2016, 121, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Lacher, M.D.; Pincheira, R.; Zhu, Z.; Camoretti-Mercado, B.; Matli, M.; Warren, R.S.; Castro, A.F. Rheb activates AMPK and reduces p27Kip1 levels in Tsc2-null cells via mTORC1-independent mechanisms: Implications for cell proliferation and tumorigenesis. Oncogene 2010, 29, 6543–6556. [Google Scholar] [CrossRef]
- Motoshima, H.; Goldstein, B.J.; Igata, M.; Araki, E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 2006, 574, 63–71. [Google Scholar] [CrossRef]
- Shen, Y.; Sherman, J.W.; Chen, X.; Wang, R. Phosphorylation of CDC25C by AMP-activated protein kinase mediates a metabolic checkpoint during cell-cycle G2/M-phase transition. J. Biol. Chem. 2018, 293, 5185–5199. [Google Scholar] [CrossRef]
- Gubern, A.; Joaquin, M.; Marquès, M.; Maseres, P.; Garcia-Garcia, J.; Amat, R.; González-Nuñez, D.; Oliva, B.; Real, F.X.; de Nadal, E.; et al. The N-Terminal Phosphorylation of RB by p38 Bypasses Its Inactivation by CDKs and Prevents Proliferation in Cancer Cells. Mol. Cell 2016, 64, 25–36. [Google Scholar] [CrossRef]
- Obaya, A.; Sedivy, J. Regulation of cyclin-Cdk activity in mammalian cells. Experientia 2002, 59, 126–142. [Google Scholar] [CrossRef]
- May, M.; Schelle, I.; Brakebusch, C.; Rottner, K.; Genth, H. Rac1-dependent recruitment of PAK2 to G2phase centrosomes and their roles in the regulation of mitotic entry. Cell Cycle 2014, 13, 2210–2220. [Google Scholar] [CrossRef]
- Adhikari, B.; Bozilovic, J.; Diebold, M.; Schwarz, J.D.; Hofstetter, J.; Schröder, M.; Wanior, M.; Narain, A.; Vogt, M.; Stankovic, N.D.; et al. PROTAC-mediated degradation reveals a non-catalytic function of AURORA-A kinase. Nat. Chem. Biol. 2020, 16, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Genth, H.; Hofmann, F.; Selzer, J.; Rex, G.; Aktories, K.; Just, I. Difference in Protein Substrate Specificity between Hemorrhagic Toxin and Lethal Toxin from Clostridium sordellii. Biochem. Biophys. Res. Commun. 1996, 229, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, F.; Gerhard, R.; Pich, A. The Binary Toxin of Clostridioides difficile Alters the Proteome and Phosphoproteome of HEp-2 Cells. Front. Microbiol. 2021, 12, 725612. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Michalski, A.; Mann, M. Software Lock Mass by Two-Dimensional Minimization of Peptide Mass Errors. J. Am. Soc. Mass Spectrom. 2011, 22, 1373–1380. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef]
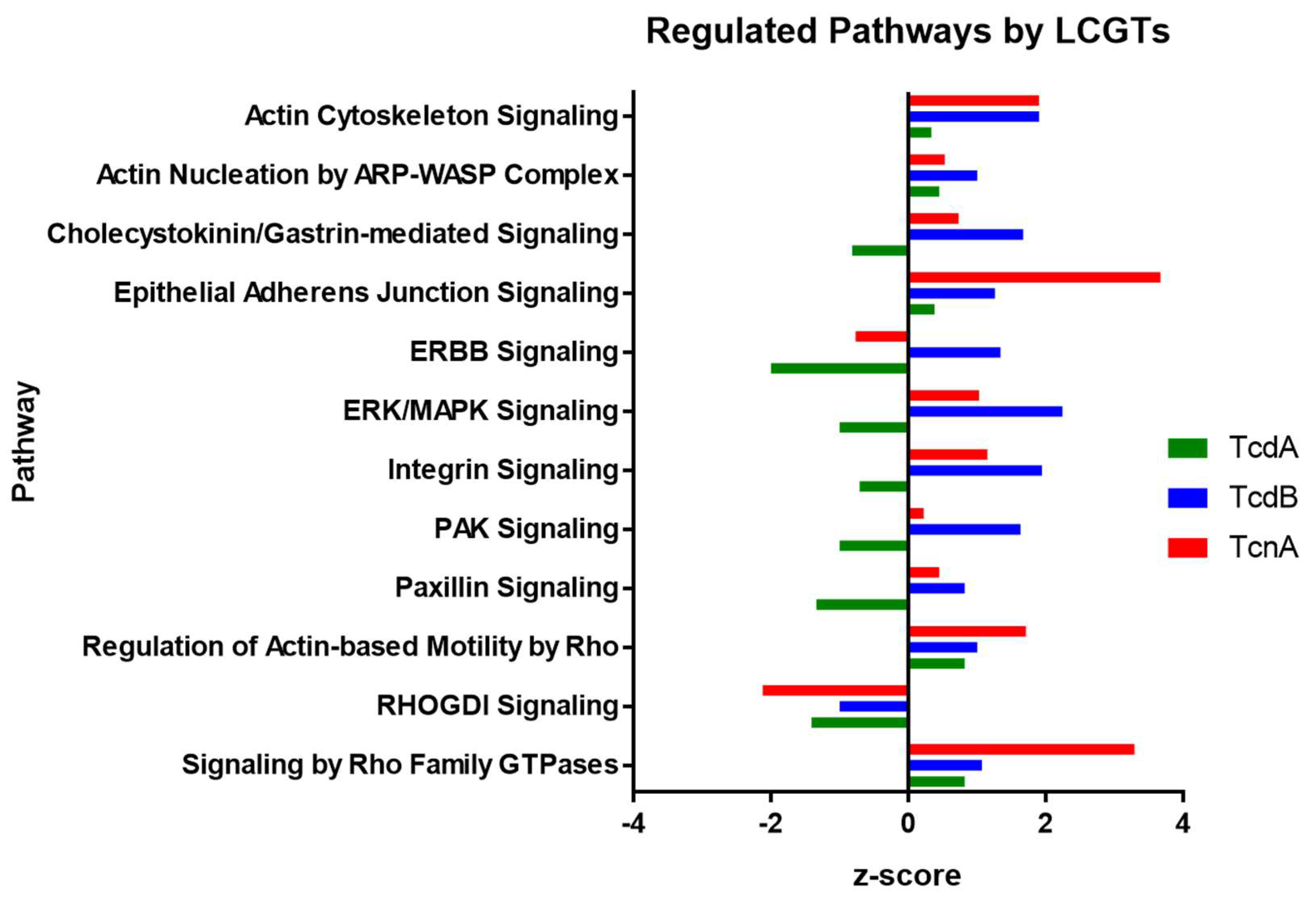
| Pathway | z-Score | ||
|---|---|---|---|
| TcdA | TcdB | TcnA | |
| Signaling by Rho Family GTPases | 0.82 | 1.07 | 3.29 |
| Epithelial Adherens Junction Signaling | 0.38 | 1.26 | 3.67 |
| IL-8 Signaling | 0.82 | 2.11 | 2.12 |
| Protein Kinase A Signaling | 0.82 | 1.9 | 2.11 |
| Actin Cytoskeleton Signaling | 0.33 | 1.9 | 1.9 |
| Regulation of Actin-based Motility by Rho | 0.82 | 1 | 1.71 |
| Ephrin Receptor Signaling | −1.13 | 1.67 | 1.46 |
| Leukocyte Extravasation Signaling | −1.13 | 1.67 | 1.3 |
| mTOR Signaling | 0.45 | 1.41 | 1.22 |
| Integrin Signaling | −0.71 | 1.94 | 1.15 |
| Hepatic Fibrosis Signaling Pathway | −0.63 | 1.29 | 1.07 |
| ERK/MAPK Signaling | −1 | 2.24 | 1.03 |
| Cholecystokinin/Gastrin-mediated Signaling | −0.82 | 1.67 | 0.73 |
| Cardiac Hypertrophy Signaling | 0.82 | 1.67 | 0.67 |
| Actin Nucleation by ARP-WASP Complex | 0.45 | 1 | 0.53 |
| Paxillin Signaling | −1.34 | 0.82 | 0.45 |
| PAK Signaling | −1 | 1.63 | 0.22 |
| HER-2 Signaling in Breast Cancer | −1 | 1.63 | −0.18 |
| Glioma Invasiveness Signaling | 0.45 | 0.82 | −0.24 |
| HGF Signaling | −1.34 | 1.41 | −0.24 |
| ERBB Signaling | −2 | 1.34 | −0.77 |
| HIPPO signaling | −0.82 | −1 | −1.15 |
| RHOGDI Signaling | −1.41 | −1 | −2.12 |
| Experiment | Up-Regulated | Down-Regulated | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene Name | Protein ID | p-Value | Log2 Fold-Change | Gene Name | Protein ID | p-Value | Log2 Fold-Change | |
| TcnA | IGFN1 | Q86VF2-5 | 9.9 × 10−4 | 2.58 | C7orf25 | Q9BPX7 | 4.8 × 10−3 | −2.34 |
| TcnA | NKTR | P30414 | 6.4 × 10−4 | 2.37 | TNNI2 | P48788-2 | 2.7 × 10−2 | −2.24 |
| TcnA | FBRSL1 | Q9HCM7 | 1.2 × 10−3 | 2.36 | ABCD2 | Q9UBJ2 | 1.7 × 10−2 | −2.05 |
| TcnA | NPY4R | P0DQD5 | 4.3 × 10−4 | 2.33 | SMU1 | Q2TAY7-2 | 1.5 × 10−2 | −1.53 |
| TcnA | INTS1 | Q8N201 | 6.8 × 10−4 | 2.22 | C1QTNF6 | Q9BXI9-1 | 2.8 × 10−2 | −1.50 |
| TcnA | MTCL1 | Q9Y4B5-2 | 2.7 × 10−3 | 2.11 | MIF | P14174 | 3.5 × 10−3 | −1.37 |
| TcnA | TAOK1 | Q7L7X3 | 5.0 × 10−4 | 2.05 | CCDC129 | Q6ZRS4-2 | 2.7 × 10−2 | −1.33 |
| TcnA | MYLPF | Q96A32 | 1.3 × 10−3 | 1.81 | SCAF4 | O95104-2 | 9.7 × 10−3 | −1.33 |
| TcnA | C12orf29 | Q8N999-3 | 4.8 × 10−3 | 1.45 | NUDT9 | Q9BW91-2 | 2.5 × 10−2 | −1.25 |
| TcnA | SLC6A12 | P48065 | 8.8 × 10−4 | 1.42 | HSP90AB2P | Q58FF8 | 2.2 × 10−2 | −1.25 |
| TcdB | RHOB | P62745 | 1.4 × 10−2 | 2.25 | CDC42EP1 | Q00587 | 2.1 × 10−3 | −2.48 |
| TcdB | CTSL | P07711 | 1.1 × 10−4 | 1.94 | RND3 | P61587 | 4.5 × 10−3 | −1.63 |
| TcdB | JUN | P05412 | 5.6 × 10−4 | 1.73 | YAP1 | P46937 | 7.6 × 10−6 | −1.55 |
| TcdB | HMOX1 | P09601 | 2.0 × 10−2 | 0.83 | CDC42EP2 | O14613 | 1.2 × 10−2 | −1.30 |
| TcdB | MYO10 | Q9HD67 | 1.8 × 10−2 | 0.77 | CYR61 | O00622 | 7.2 × 10−3 | −1.23 |
| TcdB | SDCBP | O00560 | 3.2 × 10−4 | 0.77 | TPM2 | Q5TCU3 | 1.3 × 10−2 | −1.05 |
| TcdB | SEC14L1 | Q92503 | 6.7 × 10−3 | 0.76 | TPM2 | P07951-2 | 1.6 × 10−2 | −0.86 |
| TcdB | SDCBP | O00560-2 | 2.2 × 10−2 | 0.71 | TPM3 | Q5VU63 | 8.1 × 10−3 | −0.74 |
| TcdB | STAU1 | O95793 | 2.5 × 10−2 | 0.63 | F3 | P13726 | 3.3 × 10−2 | −0.69 |
| TcdB | RASSF5 | Q8WWW0 | 3.3 × 10−2 | 0.61 | SLC1A3 | P43003 | 1.9 × 10−2 | −0.67 |
| TcdA | RHOB | P62745 | 1.8 × 10−4 | 2.72 | YAP1 | P46937 | 4.5 × 10−4 | −1.71 |
| TcdA | HMOX1 | P09601 | 4.4 × 10−2 | 0.93 | TPM2 | P07951-2 | 1.2 × 10−5 | −1.14 |
| TcdA | APP | E9PEV0 | 1.0 × 10−3 | 0.67 | TPM1 | P09493-3 | 7.6 × 10−4 | −1.07 |
| TcdA | TIMP3 | B1AJV7 | 3.4 × 10−2 | 0.61 | CLDN1 | O95832 | 5.2 × 10−3 | −0.78 |
| TcdA | TM4SF1 | F8WF27 | 2.5 × 10−3 | 0.61 | SLC1A3 | P43003-2 | 3.6 × 10−2 | −0.74 |
| TcdA | TUBB2A | Q13885 | 3.3 × 10−2 | 0.55 | AFAP1 | Q8N556 | 2.6 × 10−3 | −0.73 |
| TcdA | CD63 | F8VNT9 | 6.7 × 10−3 | 0.51 | FHL2 | J3KNW4 | 2.1 × 10−3 | −0.63 |
| TcdA | RRM2 | P31350 | 7.2 × 10−3 | 0.51 | MPRIP | Q6WCQ1 | 1.9 × 10−4 | −0.51 |
| TcdA | S100A16 | Q96FQ6 | 1.4 × 10−3 | 0.49 | STAT3 | K7ENL3 | 4.2 × 10−3 | −0.46 |
| TcdA | ITGA5 | P08648 | 5.0 × 10−3 | 0.46 | PRKCI | P41743 | 3.1 × 10−2 | −0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweitzer, T.; Genth, H.; Pich, A. Clostridium novyi’s Alpha-Toxin Changes Proteome and Phosphoproteome of HEp-2 Cells. Int. J. Mol. Sci. 2022, 23, 9939. https://doi.org/10.3390/ijms23179939
Schweitzer T, Genth H, Pich A. Clostridium novyi’s Alpha-Toxin Changes Proteome and Phosphoproteome of HEp-2 Cells. International Journal of Molecular Sciences. 2022; 23(17):9939. https://doi.org/10.3390/ijms23179939
Chicago/Turabian StyleSchweitzer, Theresa, Harald Genth, and Andreas Pich. 2022. "Clostridium novyi’s Alpha-Toxin Changes Proteome and Phosphoproteome of HEp-2 Cells" International Journal of Molecular Sciences 23, no. 17: 9939. https://doi.org/10.3390/ijms23179939
APA StyleSchweitzer, T., Genth, H., & Pich, A. (2022). Clostridium novyi’s Alpha-Toxin Changes Proteome and Phosphoproteome of HEp-2 Cells. International Journal of Molecular Sciences, 23(17), 9939. https://doi.org/10.3390/ijms23179939





