A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell
Abstract
:1. Introduction
2. Results and Discussion
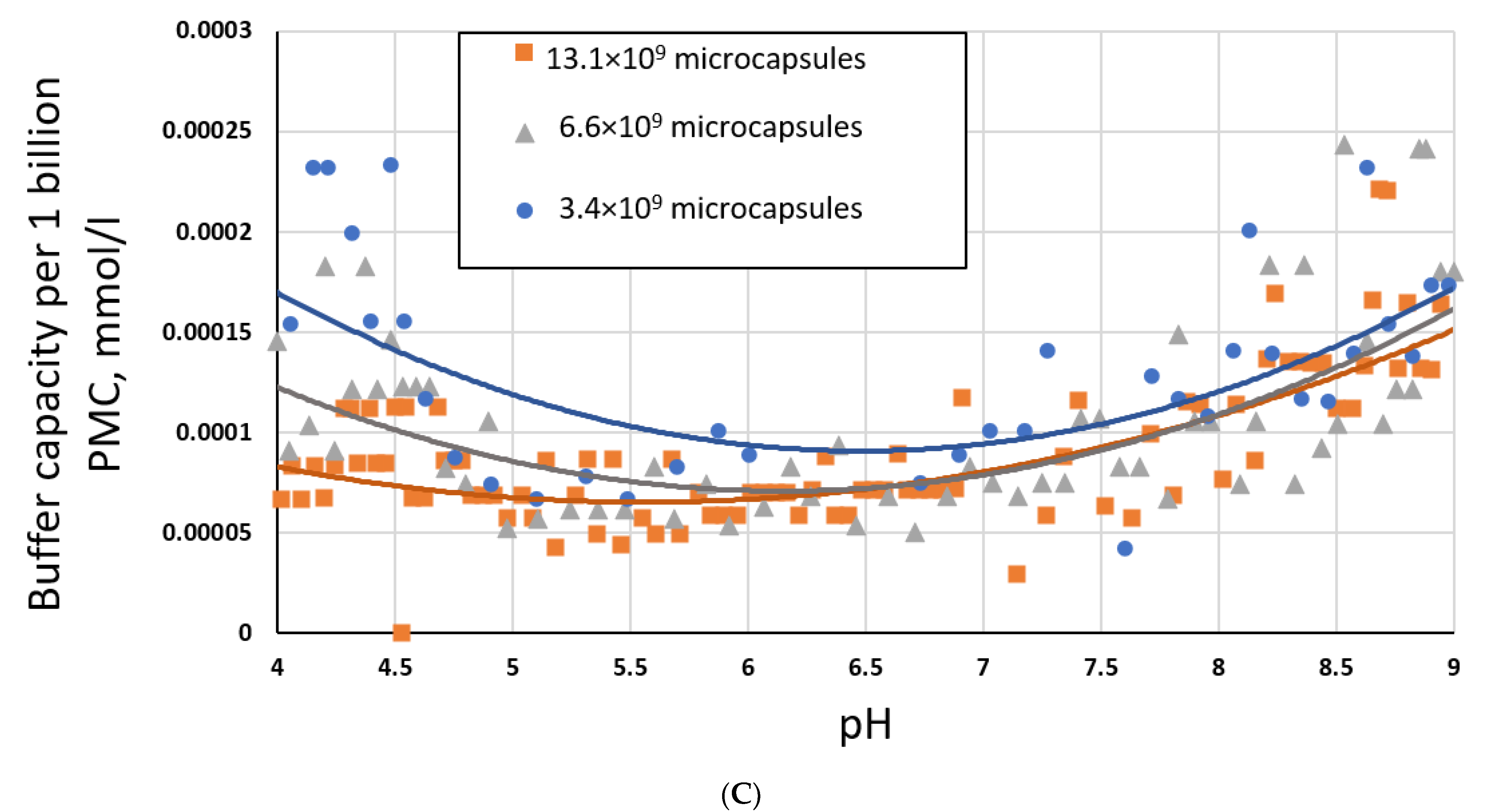
2.1. Investigation of the Buffer Capacity of PMC Depending on Their Concentration
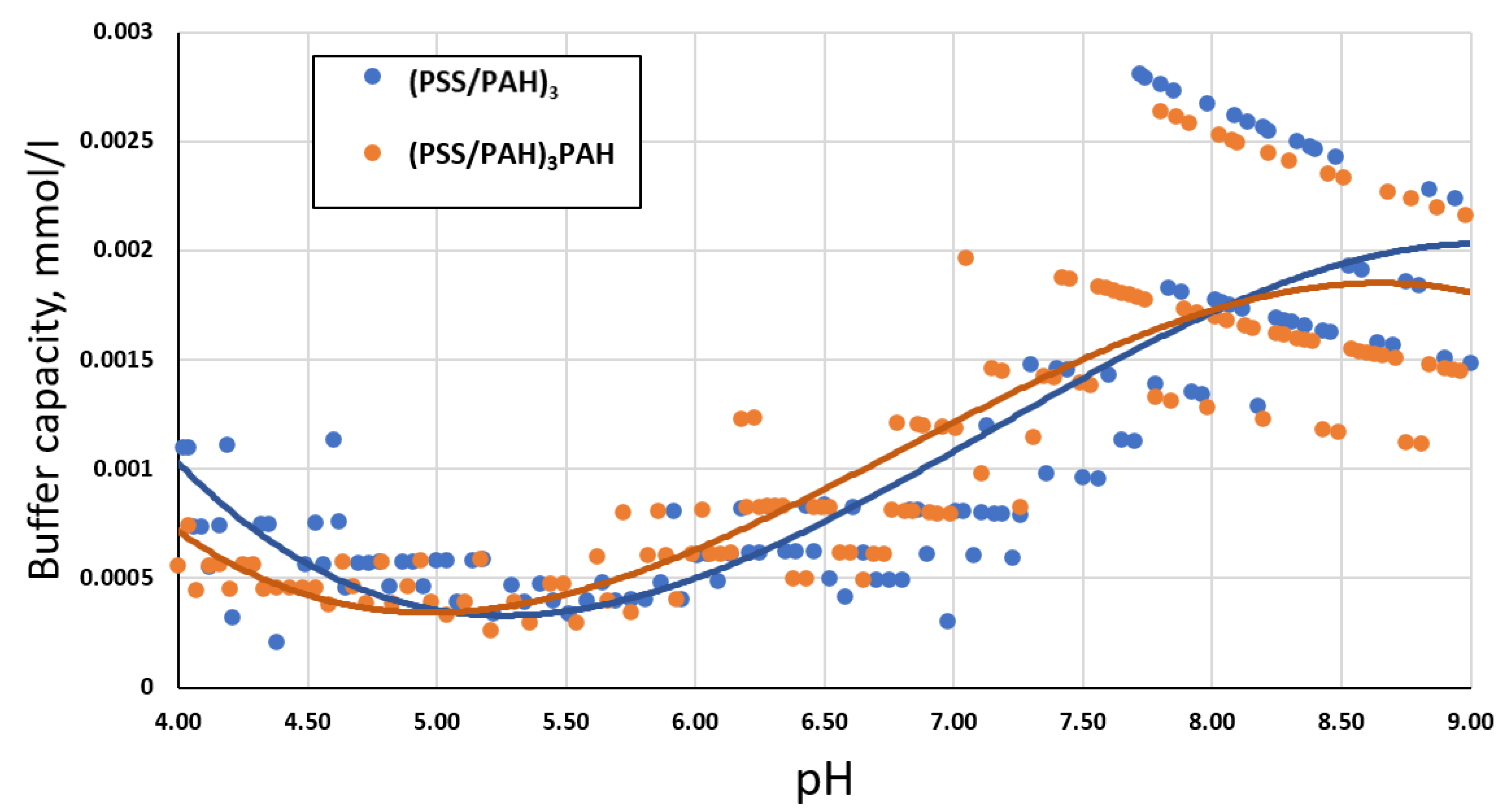
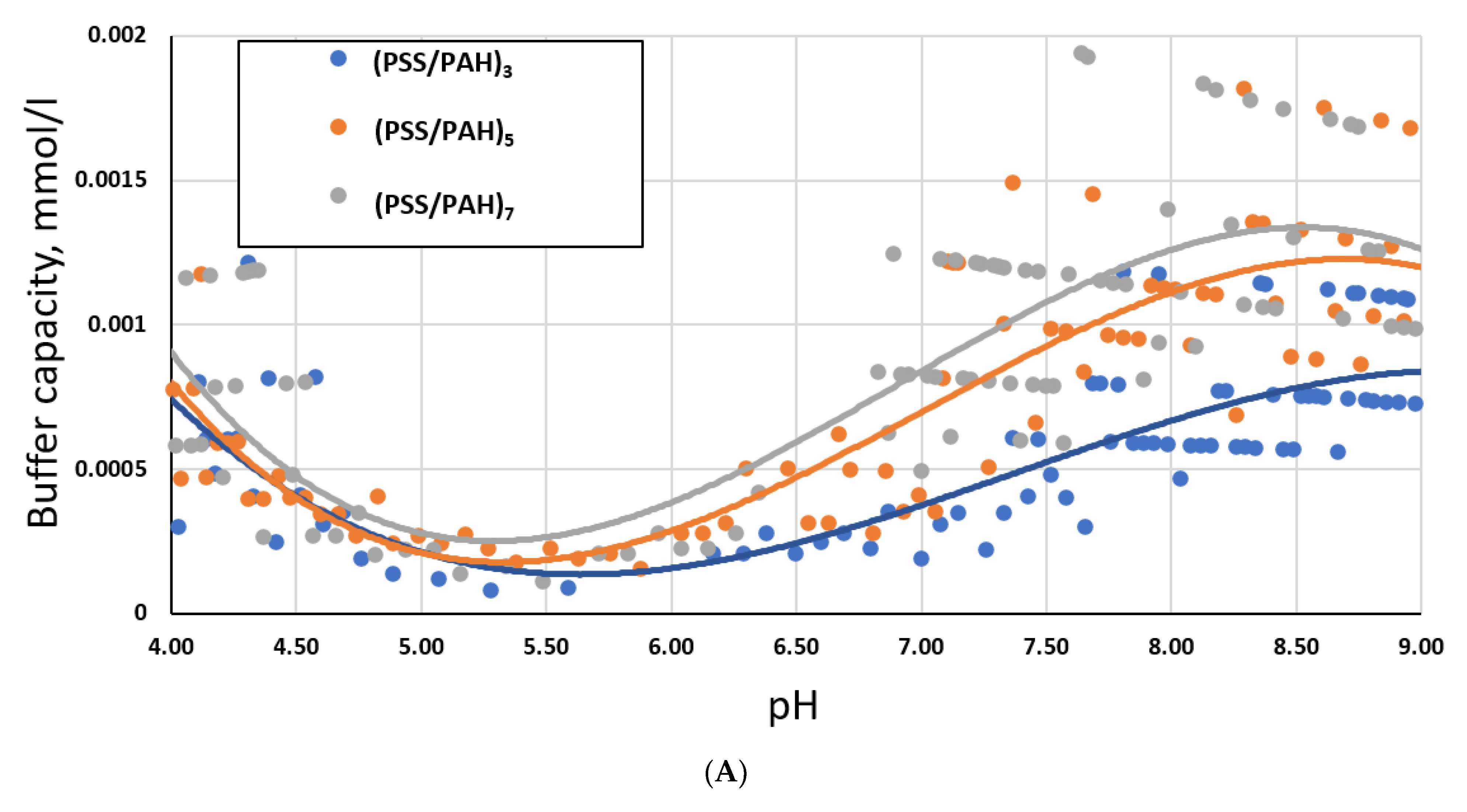
2.2. Study of the Buffer Capacity of PMC Depending on the Number of Layers of the Polyelectrolyte Shell
3. Materials and Methods
3.1. Materials
3.2. Preparation of CaCO3 Microspherulites
3.3. Preparation of Polyelectrolyte Microcapsules
3.4. Measurement of Buffering Capacity
- BC—buffer capacity
- Cacid or alkali—concentration of HCl of NaOH
- Vacid or alkali—volume of HCl of NaOH
- Vs—volume of solution
- i—number of titration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michałowska-kaczmarczyk, A.M.; Michałowski, T. Dynamic Buffer Capacity in Acid—Base Systems. J. Solut. Chem. 2015, 44, 1256–1266. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Ramazanov, B.R.; Tikhonenko, S.A. The Discovery of the Buffer Capacity of Various Types of Polyelectrolyte Microcapsules. Polymers 2021, 13, 4026. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Sukhorukov, G.B. Co-encapsulation of enzyme and sensitive dye as a tool for fabrication of microcapsule based sensor for urea measuring. Phys. Chem. Chem. Phys. 2011, 13, 11110–11117. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Dubrovskiĭ, A.V.; Moshkov, D.A.; Shabarchina, L.I.; Sukhorukov, B.I. An electron microscopy study of the structure of polyelectrolyte microcapsules containing protein and containing no protein. Biofizika 2007, 52, 850–854. [Google Scholar]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of polyelectrolyte microcapsules formed on CaCO3 microparticles and the release of a protein included by the adsorption method. Polymers 2020, 12, 520. [Google Scholar] [CrossRef]
- Lulevich, V.V.; Vinogradova, O.I. Effect of pH and Salt on the Stiffness of Polyelectrolyte Multilayer Microcapsules. Langmuir 2004, 20, 2874–2878. [Google Scholar] [CrossRef]
- Kim, B.; Vinogradova, O.I. pH-Controlled Swelling of Polyelectrolyte Multilayer Microcapsules. J. Phys. Chem. B 2004, 108, 8161–8165. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature. Int. J. Mol. Sci. 2022, 23, 6608. [Google Scholar] [CrossRef]
- Petrov, A.I.; Gavryushkin, A.V.; Sukhorukov, G.B. Effect of Temperature, pH and Shell Thickness on the Rate of Mg2+ and Ox2− Release from Multilayered Polyelectrolyte Shells Deposited onto Microcrystals of Magnesium Oxalate. J. Phys. Chem. B 2003, 107, 868–875. [Google Scholar] [CrossRef]
- Qiu, X.; Donath, E.; Möhwald, H. Permeability of Ibuprofen in Various Polyelectrolyte Multilayers. Macromol. Mater. Eng. 2001, 286, 591–597. [Google Scholar] [CrossRef]
- Krasemann, L.; Tieke, B. Selective Ion Transport across Self-Assembled Alternating Multilayers of Cationic and Anionic Polyelectrolytes. Langmuir 2000, 16, 287–290. [Google Scholar] [CrossRef]
- Krasemann, L. Self-assembled polyelectrolyte multilayer membranes with highly improved pervaporation separation of ethanol/water mixtures. J. Memb. Sci. 2001, 181, 221–228. [Google Scholar] [CrossRef]
- Eneh, C.I.; Kastinen, T.; Oka, S.; Batys, P.; Sammalkorpi, M.; Lutkenhaus, J.L. Quantification of Water–Ion Pair Interactions in Polyelectrolyte Multilayers Using a Quartz Crystal Microbalance Method. ACS Polym. Au 2022, 2, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Bernabini, C.; Morgan, H. Single-Colloidal Particle Impedance Spectroscopy: Complete Equivalent Circuit Analysis of Polyelectrolyte Microcapsules. Langmuir 2010, 26, 3821–3828. [Google Scholar] [CrossRef]
- Bordi, F.; Colby, R.H.; Cametti, C.; De Lorenzo, L.; Gili, T. Electrical Conductivity of Polyelectrolyte Solutions in the Semidilute and Concentrated Regime: The Role of Counterion Condensation. J. Phys. Chem. B 2002, 106, 6887–6893. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Park, T.-E.; Jiang, T.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Tuning the Buffering Capacity of Polyethylenimine with Glycerol Molecules for Efficient Gene Delivery: Staying In or Out of the Endosomes. Macromol. Biosci. 2015, 15, 622–635. [Google Scholar] [CrossRef]
- Saikaew, R.; Meesorn, W.; Zoppe, J.O.; Weder, C.; Dubas, S.T. Influence of the Salt Concentration on the Properties of Salt-Free Polyelectrolyte Complex Membranes. Macromol. Mater. Eng. 2019, 304, 1900245. [Google Scholar] [CrossRef]
- Ali, S.; Bleuel, M.; Prabhu, V.M. Lower Critical Solution Temperature in Polyelectrolyte Complex Coacervates. ACS Macro Lett. 2019, 8, 289–293. [Google Scholar] [CrossRef]
- Gallops, C.E.; Yu, C.; Ziebarth, J.D.; Wang, Y. Effect of the Protonation Level and Ionic Strength on the Structure of Linear Polyethyleneimine. ACS Omega 2019, 4, 7255–7264. [Google Scholar] [CrossRef]
- Haložan, D.; Déjugnat, C.; Brumen, M.; Sukhorukov, G.B. Entrapment of a Weak Polyanion and H + /Na + Exchange in Confined Polyelectrolyte Microcapsules. J. Chem. Inf. Model. 2005, 45, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Kreft, O.; Javier, A.M.; Sukhorukov, G.B.; Parak, W.J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 2007, 17, 4471. [Google Scholar] [CrossRef]
- Tang, Q.; Denton, A.R. Ion density deviations in polyelectrolyte microcapsules: Influence on biosensors. Phys. Chem. Chem. Phys. 2014, 16, 20924–20931. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Giannaros, P.; Al-Tabbaa, A. The effect of varying volume fraction of microcapsules on fresh, mechanical and self-healing properties of mortars. Constr. Build. Mater. 2016, 122, 577–593. [Google Scholar] [CrossRef]
- Mooney, M. The viscosity of a concentrated suspension of spherical particles. J. Colloid Sci. 1951, 6, 162–170. [Google Scholar] [CrossRef]
- Chong, J.S.; Christiansen, E.B.; Baer, A.D. Rheology of concentrated suspensions. J. Appl. Polym. Sci. 1971, 15, 2007–2021. [Google Scholar] [CrossRef]
- Faroughi, S.A.; Huber, C. Crowding-based rheological model for suspensions of rigid bimodal-sized particles with interfering size ratios. Phys. Rev. E 2014, 90, 052303. [Google Scholar] [CrossRef]
- Peyratout, C.S.; Dähne, L. Tailor-Made Polyelectrolyte Microcapsules: From Multilayers to Smart Containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual Salt and pH Induced Changes in Polyethylenimine Solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef]
- Vlachy, V. Polyelectrolyte hydration: Theory and experiment. Pure Appl. Chem. 2008, 80, 1253–1266. [Google Scholar] [CrossRef]
- Estrela-Lopis, I.; Leporatti, S.; Clemens, D.; Donath, E. Polyelectrolyte multilayer hollow capsules studied by small-angle neutron scattering (SANS). Soft Matter 2009, 5, 214–219. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan–DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musin, E.V.; Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell. Int. J. Mol. Sci. 2022, 23, 9917. https://doi.org/10.3390/ijms23179917
Musin EV, Dubrovskii AV, Kim AL, Tikhonenko SA. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell. International Journal of Molecular Sciences. 2022; 23(17):9917. https://doi.org/10.3390/ijms23179917
Chicago/Turabian StyleMusin, Egor V., Alexey V. Dubrovskii, Aleksandr L. Kim, and Sergey A. Tikhonenko. 2022. "A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell" International Journal of Molecular Sciences 23, no. 17: 9917. https://doi.org/10.3390/ijms23179917
APA StyleMusin, E. V., Dubrovskii, A. V., Kim, A. L., & Tikhonenko, S. A. (2022). A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Concentration and the Number of Layers of the Polyelectrolyte Shell. International Journal of Molecular Sciences, 23(17), 9917. https://doi.org/10.3390/ijms23179917






