The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress
Abstract
1. Introduction
2. Results
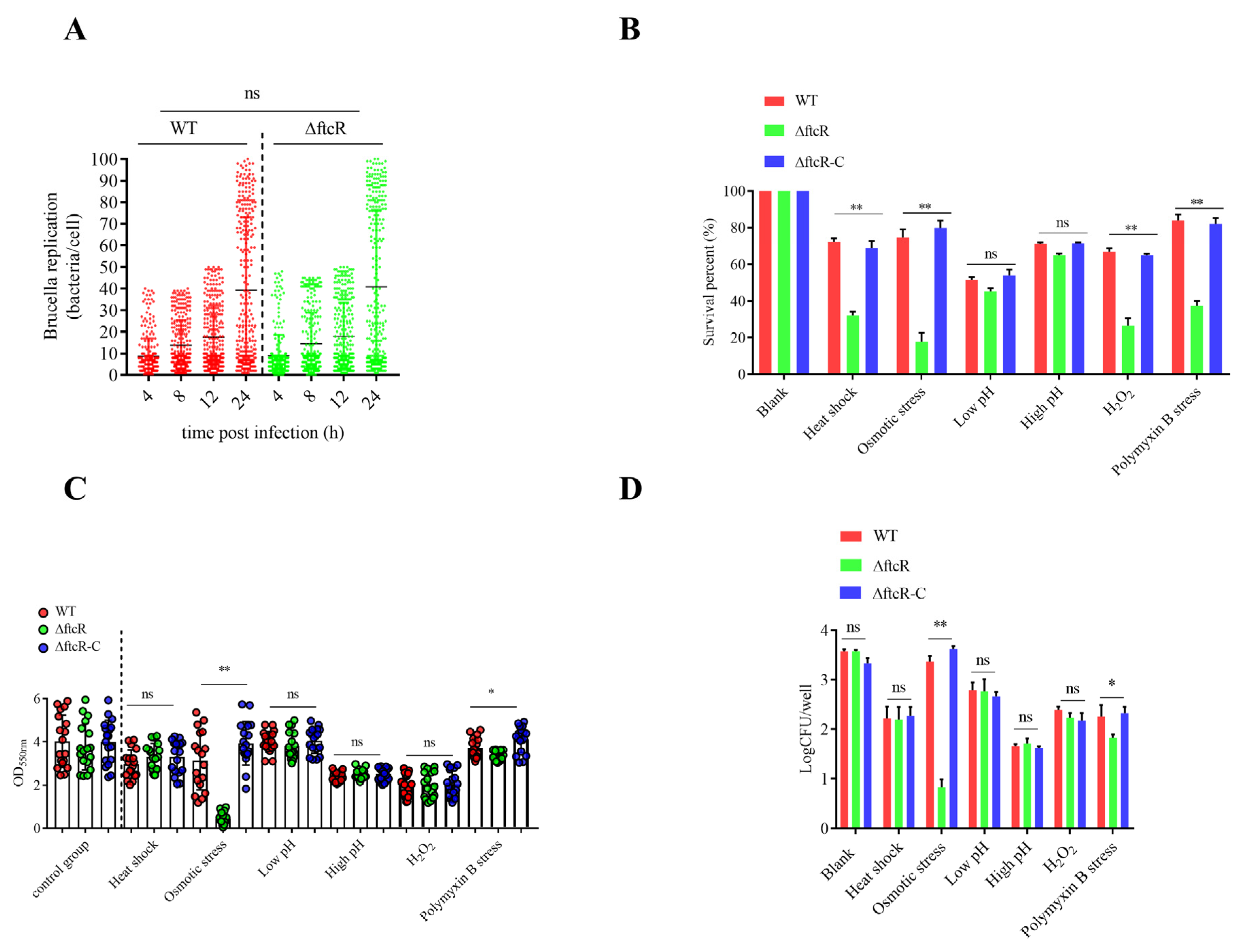
2.1. FtcR Is Required for B. melitensis 16M’s Survival in Hostile Environments in Planktonic and Biofilm States
2.2. Scriptional Autoregulatory Properties of FtcR and Indepess
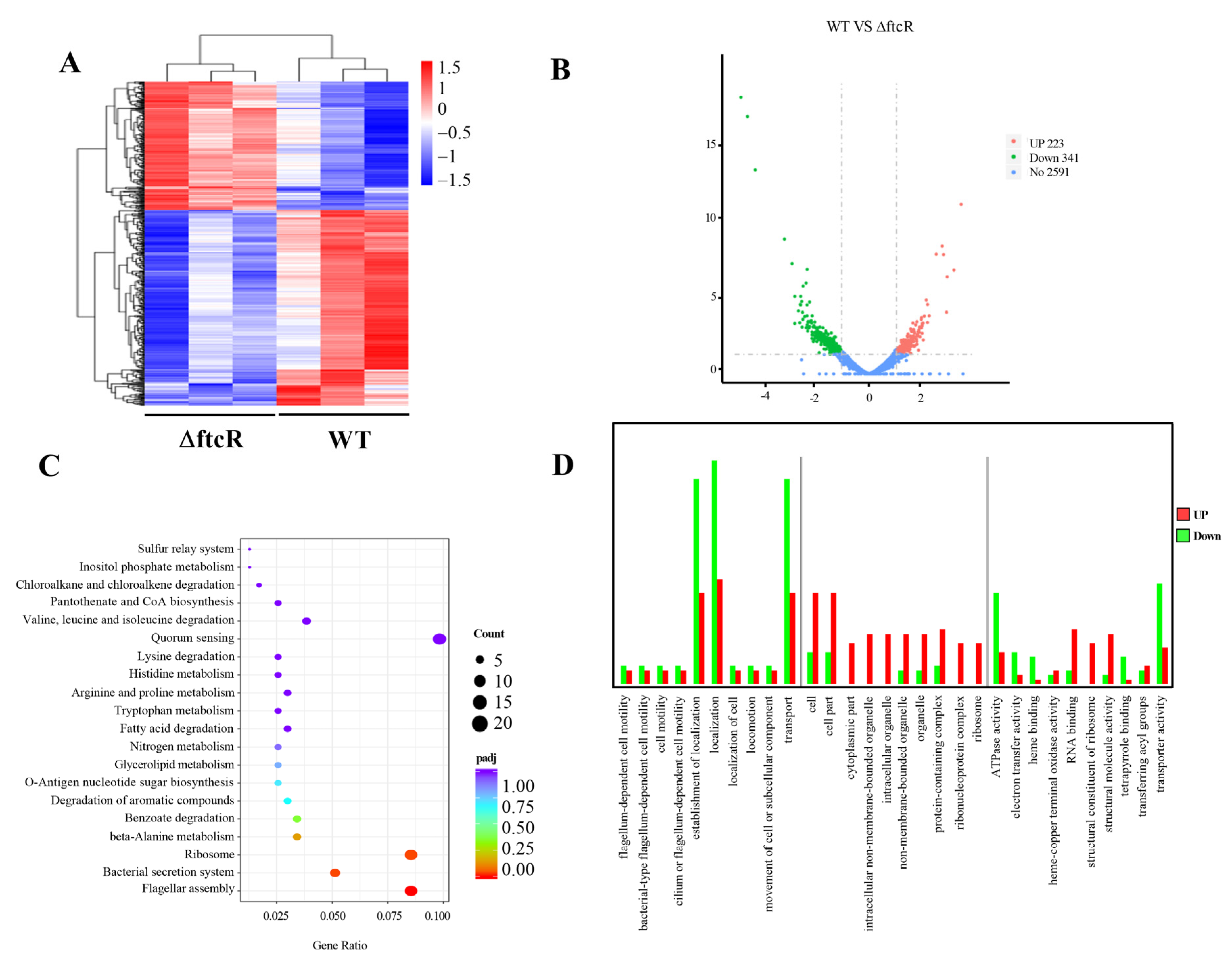
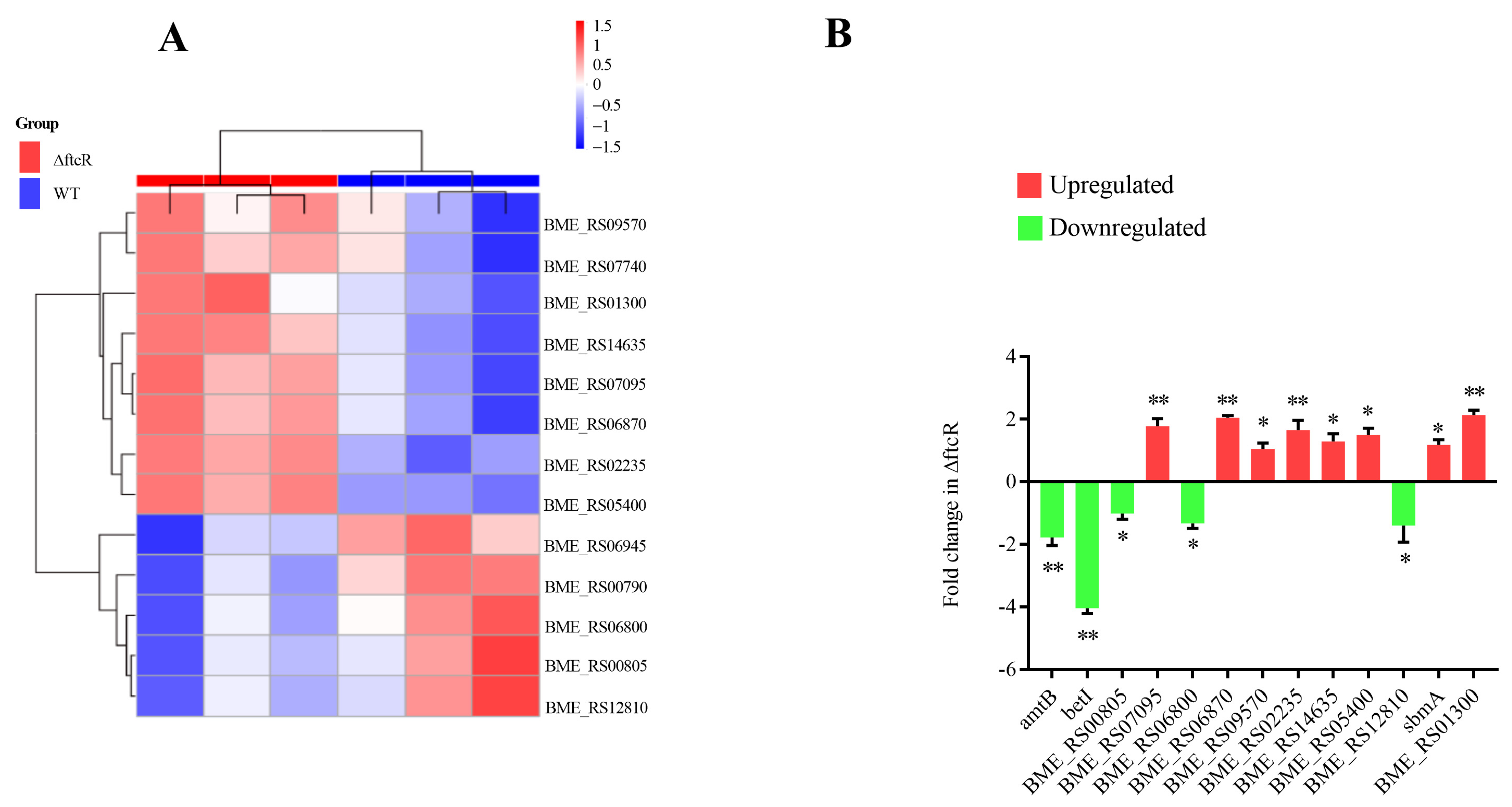
2.3. Identification of FtcR Target Genes Using RNA-Seq and ChIP-Seq Analyses
2.4. Transcriptional Autoregulatory Properties of FtcR and Independent of FliF
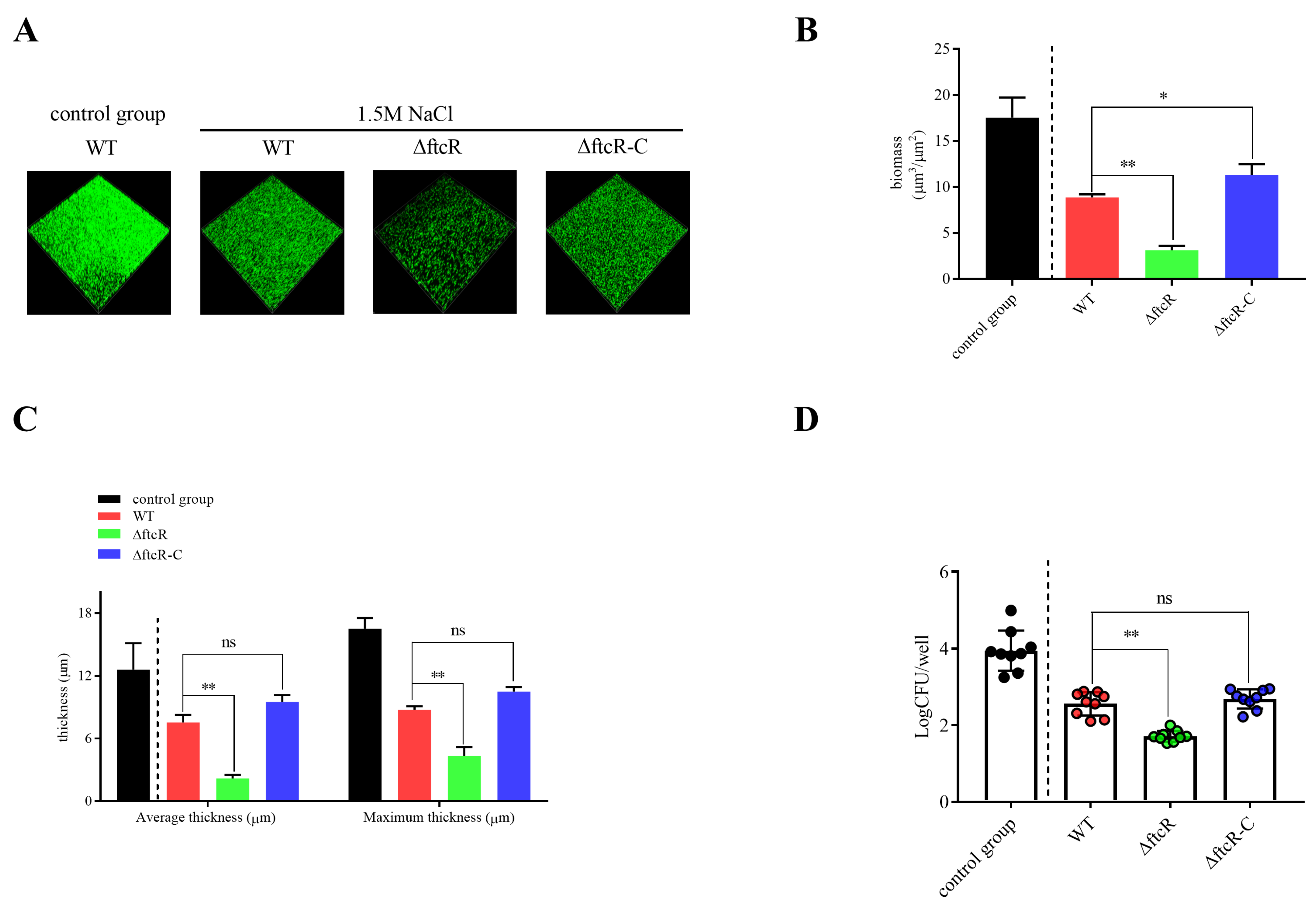
2.5. FtcR Mediates the Transport Pathway in B. melitensis 16M Biofilms under Hyperosmotic Stress
2.6. FtcR Binds to the Promoter of betI in Response to Hyperosmotic Stress Environments
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cell Lines
4.2. Infection Assay
4.3. Stress Tolerance in Planktonic Cells
4.4. Biofilm Culture and Observation
4.5. Biomass Assay
4.6. Biofilm Immunoblots
4.7. Chromatin Immunoprecipitation and High-Throughput Sequencing (ChIP-Seq)
4.8. RNA-Seq and Transcriptomic Analysis
4.9. Determination of Biofilm Bacteria’s Viability by Flow Cytometry
4.10. Quantitative Real-Time PCR
4.11. Protein Purification and EMSAs
4.12. β-Galactosidase Activity Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, M.T.; Campos, P.C.; de Almeida, L.A.; Oliveira, F.S.; Costa, M.M.; Marim, F.M.; Pereira, G.S.; Oliveira, S.C. The role of innate immune signals in immunity to Brucella abortus. Front. Cell. Infect. Microbiol. 2012, 2, 130. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Zhou, Z.; Li, B.; Xiao, Y.; Li, M.; Zeng, H.; Guo, X.; Gu, G. The mechanism of facultative intracellular parasitism of Brucella. Int. J. Mol. Sci. 2021, 22, 3673. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Panagopoulou, P.; Christou, L.; Akritidis, N. Brucella as a biological weapon. Cell. Mol. Life Sci. 2006, 63, 2229–2236. [Google Scholar] [CrossRef]
- Celli, J. The intracellular life cycle of Brucella spp. Microbiol. Spectr. 2019, 7, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, R.; Hu, R.; Deng, X.; Xu, Y.; Zheng, W.; Yi, J.; Wang, Y.; Chen, C. Brucella abortus BspJ is a nucleomodulin that inhibits macrophage apoptosis and promotes intracellular survival of Brucella. Front. Microbiol. 2020, 11, 599205. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neta, A.V.; Mol, J.P.; Xavier, M.N.; Paixão, T.A.; Lage, A.P.; Santos, R.L. Pathogenesis of bovine brucellosis. Vet. J. 2010, 184, 146–155. [Google Scholar] [CrossRef]
- Delrue, R.M.; Lestrate, P.; Tibor, A.; Letesson, J.J.; De Bolle, X. Brucella pathogenesis, genes identified from random large-scale screens. FEMS Microbiol. Lett. 2004, 231, 1–12. [Google Scholar] [CrossRef]
- Galińska, E.M.; Zagórski, J. Brucellosis in humans—Etiology, diagnostics, clinical forms. Ann. Agric. Environ. Med. 2013, 20, 233–238. [Google Scholar]
- Dean, A.S.; Schelling, E.; Bonfoh, B.; Kulo, A.E.; Boukaya, G.A.; Pilo, P. Deletion in the gene BruAb2_0168 of Brucella abortus strains: Diagnostic challenges. Clin. Microbiol. Infect. 2014, 20, O550–O553. [Google Scholar] [CrossRef][Green Version]
- D’Anastasio, R.; Staniscia, T.; Milia, M.L.; Manzoli, L.; Capasso, L. Origin, evolution and paleoepidemiology of brucellosis. Epidemiol. Infect. 2011, 139, 149–156. [Google Scholar] [CrossRef]
- Almirón, M.A.; Roset, M.S.; Sanjuan, N. The aggregation of Brucella abortus occurs under microaerobic conditions and promotes desiccation tolerance and biofilm formation. Open Microbiol. J. 2013, 7, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godefroid, M.; Svensson, M.V.; Cambier, P.; Uzureau, S.; Mirabella, A.; De Bolle, X.; Van Cutsem, P.; Widmalm, G.; Letesson, J.J. Brucella melitensis 16M produces a mannan and other extracellular matrix components typical of a biofilm. FEMS Immunol. Med. Microbiol. 2010, 59, 364–377. [Google Scholar] [CrossRef]
- Pu, M.; Rowe-Magnus, D.A. A Tad pilus promotes the establishment and resistance of Vibrio vulnificus biofilms to mechanical clearance. NPJ Biofilms Microbiomes 2018, 4, 10. [Google Scholar] [CrossRef]
- Tang, T.; Xu, Y.; Wang, J.; Tan, X.; Zhao, X.; Zhou, P.; Kong, F.; Zhu, C.; Lu, C.; Lin, H. Evaluation of the differences between biofilm and planktonic Brucella abortus via metabolomics and proteomics. Funct. Integr. Genomics 2021, 21, 421–433. [Google Scholar] [CrossRef]
- Tang, T.; Chen, G.; Guo, A.; Xu, Y.; Zhao, L.; Wang, M.; Lu, C.; Jiang, Y.; Zhang, C. Comparative proteomic and genomic analyses of Brucella abortus biofilm and planktonic cells. Mol. Med. Rep. 2020, 21, 731–743. [Google Scholar] [CrossRef]
- Soares, A.; Caron, F.; Etienne, M. Commentary: Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 2164. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; Ruoff, K.L.; O’Toole, G.A. Pseudomonas aeruginosa Increases the sensitivity of biofilm-grown Staphylococcus aureus to membrane-targeting antiseptics and antibiotics. mBio 2019, 10, e01501-19. [Google Scholar] [CrossRef] [PubMed]
- Flury, D.; Behrend, H.; Sendi, P.; von Kietzell, M.; Strahm, C. Brucella melitensis prosthetic joint infection. J. Bone Jt. Infect. 2017, 2, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Valentin, J.; Straub, H.; Pietsch, F.; Lemare, M.; Ahrens, C.H.; Schreiber, F.; Webb, J.S.; van der Mei, H.C.; Ren, Q. Role of the flagellar hook in the structural development and antibiotic tolerance of Pseudomonas aeruginosa biofilms. ISME J. 2022, 16, 1176–1186. [Google Scholar] [CrossRef]
- Coloma-Rivero, R.F.; Gómez, L.; Alvarez, F.; Saitz, W.; Del Canto, F.; Céspedes, S.; Vidal, R.; Oñate, A.A. The role of the flagellar protein FlgJ in the virulence of Brucella abortus. Front. Cell. Infect. Microbiol. 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Léonard, S.; Ferooz, J.; Haine, V.; Danese, I.; Fretin, D.; Tibor, A.; de Walque, S.; De Bolle, X.; Letesson, J.J. FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae. J. Bacteriol. 2007, 189, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P. Peculiarities of the regulation of the Brucella flagellum. Microbiology 2011, 157, 1251–1252. [Google Scholar] [CrossRef][Green Version]
- Ferooz, J.; Lemaire, J.; Delory, M.; De Bolle, X.; Letesson, J.J. RpoE1, an extracytoplasmic function sigma factor, is a repressor of the flagellar system in Brucella melitensis. Microbiology 2011, 157, 1263–1268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soler-Lloréns, P.F.; Quance, C.R.; Lawhon, S.D.; Stuber, T.P.; Edwards, J.F.; Ficht, T.A.; Robbe-Austerman, S.; O’Callaghan, D.; Keriel, A. A Brucella spp. isolate from a Pac-Man frog (Ceratophrys ornata) reveals characteristics departing from classical brucellae. Front. Cell. Infect. Microbiol. 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Melzer, F.; Neubauer, H. In Brucella: Selective pressure may turn some genes on instead of default off position. Med. Hypotheses 2017, 103, 29–31. [Google Scholar] [CrossRef] [PubMed]
- DelVecchio, V.G.; Kapatral, V.; Redkar, R.J.; Patra, G.; Mujer, C.; Los, T.; Ivanova, N.; Anderson, I.; Bhattacharyya, A.; Lykidis, A.; et al. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 2002, 99, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, A.; Terwagne, M.; Zygmunt, M.S.; Cloeckaert, A.; De Bolle, X.; Letesson, J.J. Brucella melitensis MucR, an orthologue of Sinorhizobium meliloti MucR, is involved in resistance to oxidative, detergent, and saline stresses and cell envelope modifications. J. Bacteriol. 2013, 195, 453–465. [Google Scholar] [CrossRef]
- Petersen, E.; Rajashekara, G.; Sanakkayala, N.; Eskra, L.; Harms, J.; Splitter, G. Erythritol triggers expression of virulence traits in Brucella melitensis. Microbes Infect. 2013, 15, 440–449. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Yin, Z.; Zhang, C.; Bible, A.; Xie, Z. The FtcR-like protein ActR in Azorhizobium caulinodans ORS571 is involved in bacterial motility and symbiosis with the host plant. Front. Microbiol. 2021, 12, 744268. [Google Scholar] [CrossRef]
- Coloma-Rivero, R.F.; Flores-Concha, M.; Molina, R.E.; Soto-Shara, R.; Cartes, Á.; Oñate, Á.A. Brucella and its hidden flagellar system. Microorganisms 2021, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Roop, R.M.; Gee, J.M., 2nd; Robertson, G.T.; Richardson, J.M.; Ng, W.L.; Winkler, M.E. Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 2003, 57, 57–76. [Google Scholar] [CrossRef]
- Jiang, X.; Leonard, B.; Benson, R.; Baldwin, C.L. Macrophage control of Brucella abortus: Role of reactive oxygen intermediates and nitric oxide. Cell. Immunol. 1993, 151, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Warmbold, B.; Ronzheimer, S.; Freibert, S.A.; Seubert, A.; Hoffmann, T.; Bremer, E. Two MarR-type repressors balance precursor uptake and glycine betaine synthesis in Bacillus subtilis to provide cytoprotection against sustained osmotic stress. Front. Microbiol. 2020, 11, 1700. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.; Saier, M.H., Jr. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 1993, 57, 320–346. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, B.; Surendran, S.; Lim, B.R.; Yim, J.S.; Kim, D.H.; Woo, K.; Kim, H.J.; Oh, M.H.; Choi, C.H. The osmotic stress response operon betIBA is under the functional regulation of BetI and the quorum-sensing regulator AnoR in Acinetobacter nosocomialis. J. Microbiol. 2020, 58, 519–529. [Google Scholar] [CrossRef]
- van Kessel, J.C.; Rutherford, S.T.; Cong, J.P.; Quinodoz, S.; Healy, J.; Bassler, B.L. Quorum sensing regulates the osmotic stress response in Vibrio harveyi. J. Bacteriol. 2015, 197, 73–80. [Google Scholar] [CrossRef]
- Lamark, T.; Kaasen, I.; Eshoo, M.W.; Falkenberg, P.; McDougall, J.; Strøm, A.R. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol. Microbiol. 1991, 5, 1049–1064. [Google Scholar] [CrossRef]
- Sand, M.; Stahl, J.; Waclawska, I.; Ziegler, C.; Averhoff, B. Identification of an osmo-dependent and an osmo-independent choline transporter in Acinetobacter baylyi: Implications in osmostress protection and metabolic adaptation. Environ. Microbiol. 2014, 16, 1490–1502. [Google Scholar] [CrossRef]
- Fretin, D.; Fauconnier, A.; Köhler, S.; Halling, S.; Léonard, S.; Nijskens, C.; Ferooz, J.; Lestrate, P.; Delrue, R.M.; Danese, I.; et al. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell. Microbiol. 2005, 7, 687–698. [Google Scholar] [CrossRef]
- Cullen, L.; McClean, S. Bacterial adaptation during chronic respiratory infections. Pathogens 2015, 4, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Bernier, S.P.; Workentine, M.L.; Surette, M.G. Genetic signature of bacterial pathogen adaptation during chronic pulmonary infections. Nat. Genet. 2014, 46, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Winardhi, R.S.; Morgan, L.K.; Yan, J.; Kenney, L.J. Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat. Commun. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Dragoš, A.; Kovács, Á.T. The peculiar functions of the bacterial extracellular matrix. Trends. Microbiol. 2017, 25, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, M.L.; Fontaine, A.; Sansonetti, P.J. The two-component regulatory system ompR-envZ controls the virulence of Shigella flexneri. J. Bacteriol. 1990, 172, 6274–6281. [Google Scholar] [CrossRef]
- Bang, I.S.; Audia, J.P.; Park, Y.K.; Foster, J.W. Autoinduction of the ompR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol. Microbiol. 2002, 44, 1235–1250. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nagura, R.; Tanabe, H.; Fujita, N.; Ishihama, A.; Utsumi, R. Negative regulation of the bolA1p of Escherichia coli K-12 by the transcription factor OmpR for osmolarity response genes. FEMS Microbiol. Lett. 2000, 186, 257–262. [Google Scholar] [CrossRef][Green Version]
- Dorrell, N.; Li, S.R.; Everest, P.H.; Dougan, G.; Wren, B.W. Construction and characterisation of a Yersinia enterocolitica O:8 ompR mutant. FEMS Microbiol. Lett. 1998, 165, 145–151. [Google Scholar] [CrossRef][Green Version]
- Nieckarz, M.; Raczkowska, A.; Dębski, J.; Kistowski, M.; Dadlez, M.; Heesemann, J.; Rossier, O.; Brzostek, K. Impact of OmpR on the membrane proteome of Yersinia enterocolitica in different environments: Repression of major adhesin YadA and heme receptor HemR. Environ. Microbiol. 2016, 18, 997–1021. [Google Scholar] [CrossRef]
- Schwan, W.R.; Lee, J.L.; Lenard, F.A.; Matthews, B.T.; Beck, M.T. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect. Immun. 2002, 70, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, A.E.; Lovrich, S.D.; Fitton, R.; Enos-Berlage, J.; Schwan, W.R. OmpR regulation of the uropathogenic Escherichia coli fimB gene in an acidic/high osmolality environment. Microbiology 2013, 159, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Richter, A.M.; Klauck, G.; Mika, F.; Hengge, R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. mBio 2013, 4, e00103-13. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Zhou, Y.; Pinkner, J.S.; Dodson, K.W.; Crowley, J.R.; Heuser, J.; Chapman, M.R.; Hadjifrangiskou, M.; Henderson, J.P.; Hultgren, S.J. Escherichia coli biofilms have an organized and complex extracellular matrix structure. mBio 2013, 4, e00645-13. [Google Scholar] [CrossRef]
- Lestrate, P.; Delrue, R.M.; Danese, I.; Didembourg, C.; Taminiau, B.; Mertens, P.; De Bolle, X.; Tibor, A.; Tang, C.M.; Letesson, J.J. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol. Microbiol. 2000, 38, 543–551. [Google Scholar] [CrossRef]
- Oshima, T.; Aiba, H.; Masuda, Y.; Kanaya, S.; Sugiura, M.; Wanner, B.L.; Mori, H.; Mizuno, T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 2002, 46, 281–291. [Google Scholar] [CrossRef]
- Paul, D. Osmotic stress adaptations in rhizobacteria. J. Basic Microbiol. 2013, 53, 101–110. [Google Scholar] [CrossRef]
- Fraser, K.R.; Harvie, D.; Coote, P.J.; O’Byrne, C.P. Identification and characterization of an ATP binding cassette L-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 2000, 66, 4696–4704. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Zhang, H.; Zhang, J.; Xi, L.; Zhang, J.; Chen, C. Transcriptional regulator GntR of Brucella abortus regulates cytotoxicity, induces the secretion of inflammatory cytokines and affects expression of the type IV secretion system and quorum sensing system in macrophages. World J. Microbiol. Biotechnol. 2017, 33, 60. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Cotto-Rosario, A.; Borghesan, E.; Held, K.; Miller, C.N.; Celli, J. Epistatic interplay between type IV secretion effectors engages the small GTPase Rab2 in the Brucella intracellular cycle. mBio 2020, 11, e03350-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Wu, Q. Cold shock protein A plays an important role in the stress adaptation and virulence of Brucella melitensis. FEMS Microbiol. Lett. 2014, 354, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, B.; Sauer, K. The ABC of biofilm drug tolerance: The MerR-Like regulator BrlR is an activator of ABC transport systems, with PA1874-77 contributing to the tolerance of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother. 2018, 62, e01981-17. [Google Scholar] [CrossRef] [PubMed]
- Okaro, U.; Green, R.; Mohapatra, S.; Anderson, B. The trimeric autotransporter adhesin BadA is required for in vitro biofilm formation by Bartonella henselae. NPJ Biofilms Microbiomes 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Zhang, H.; Wang, S.; Xi, L.; Zhang, X.; Yi, J.; Zhang, H. ChIP-seq analysis of Brucella reveals transcriptional regulation of GntR. J. Basic Microbiol. 2020, 60, 149–157. [Google Scholar] [CrossRef]
- Zhi, F.; Zhou, D.; Chen, J.; Fang, J.; Zheng, W.; Li, J.; Hao, M.; Shi, Y.; Jin, Y.; Wang, A. An ArsR transcriptional regulator facilitates Brucella sp. survival via regulating self and outer membrane protein. Int. J. Mol. Sci. 2021, 22, 10860. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Deng, X.; Zhang, Y.; Song, S.; Zhao, T.; Zhu, D.; Cao, S.; Baryshnikov, P.I.; Cao, G.; Blair, H.T.; et al. The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress. Int. J. Mol. Sci. 2022, 23, 9905. https://doi.org/10.3390/ijms23179905
Guo J, Deng X, Zhang Y, Song S, Zhao T, Zhu D, Cao S, Baryshnikov PI, Cao G, Blair HT, et al. The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress. International Journal of Molecular Sciences. 2022; 23(17):9905. https://doi.org/10.3390/ijms23179905
Chicago/Turabian StyleGuo, Jia, Xingmei Deng, Yu Zhang, Shengnan Song, Tianyi Zhao, Dexin Zhu, Shuzhu Cao, Peter Ivanovic Baryshnikov, Gang Cao, Hugh T. Blair, and et al. 2022. "The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress" International Journal of Molecular Sciences 23, no. 17: 9905. https://doi.org/10.3390/ijms23179905
APA StyleGuo, J., Deng, X., Zhang, Y., Song, S., Zhao, T., Zhu, D., Cao, S., Baryshnikov, P. I., Cao, G., Blair, H. T., Chen, C., Gu, X., Liu, L., & Zhang, H. (2022). The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress. International Journal of Molecular Sciences, 23(17), 9905. https://doi.org/10.3390/ijms23179905





