Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning
Abstract
:1. Introduction
2. Results
2.1. Characterization of Human EPCs
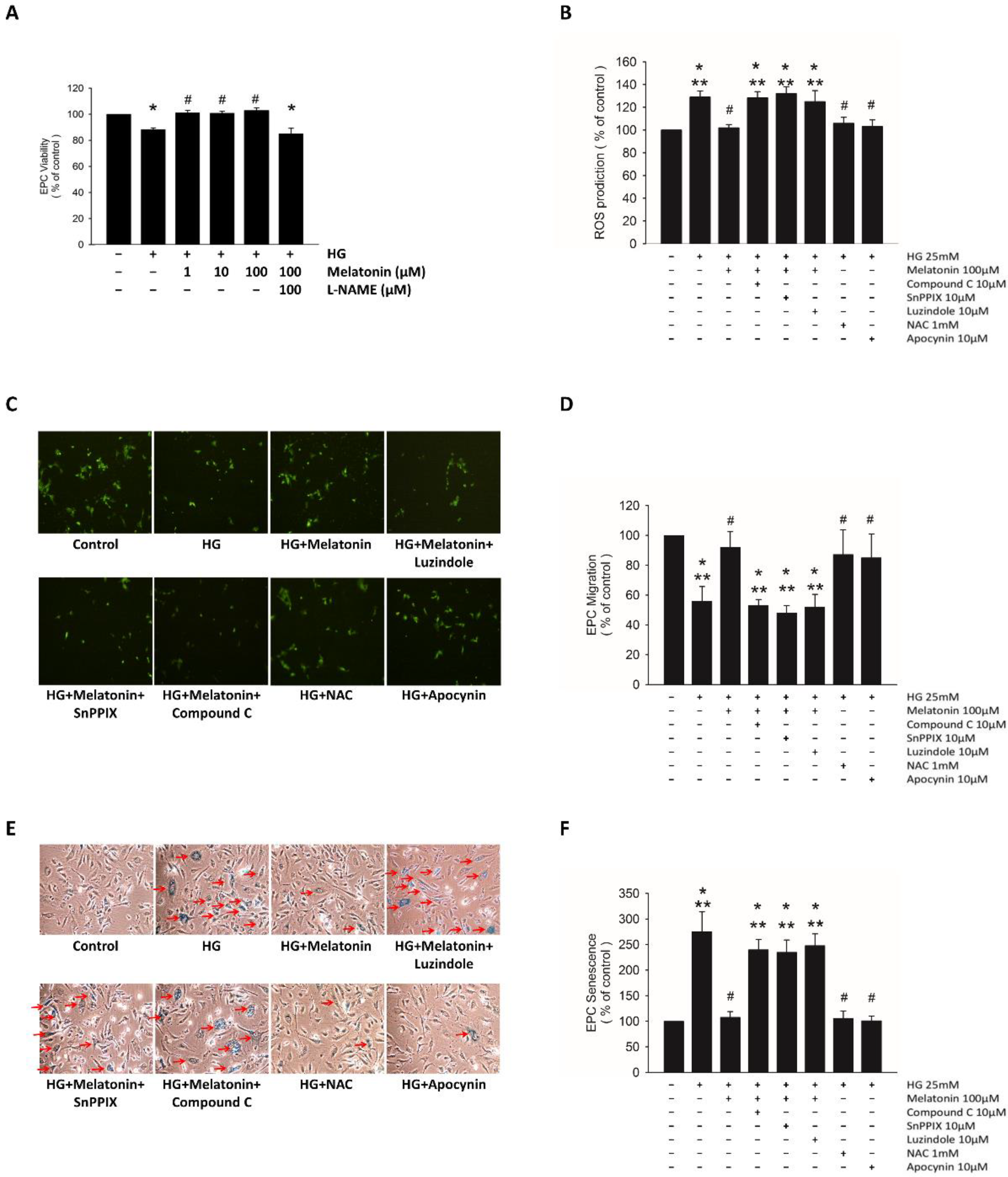
2.2. Effects of Melatonin on EPC Function under HG Conditions
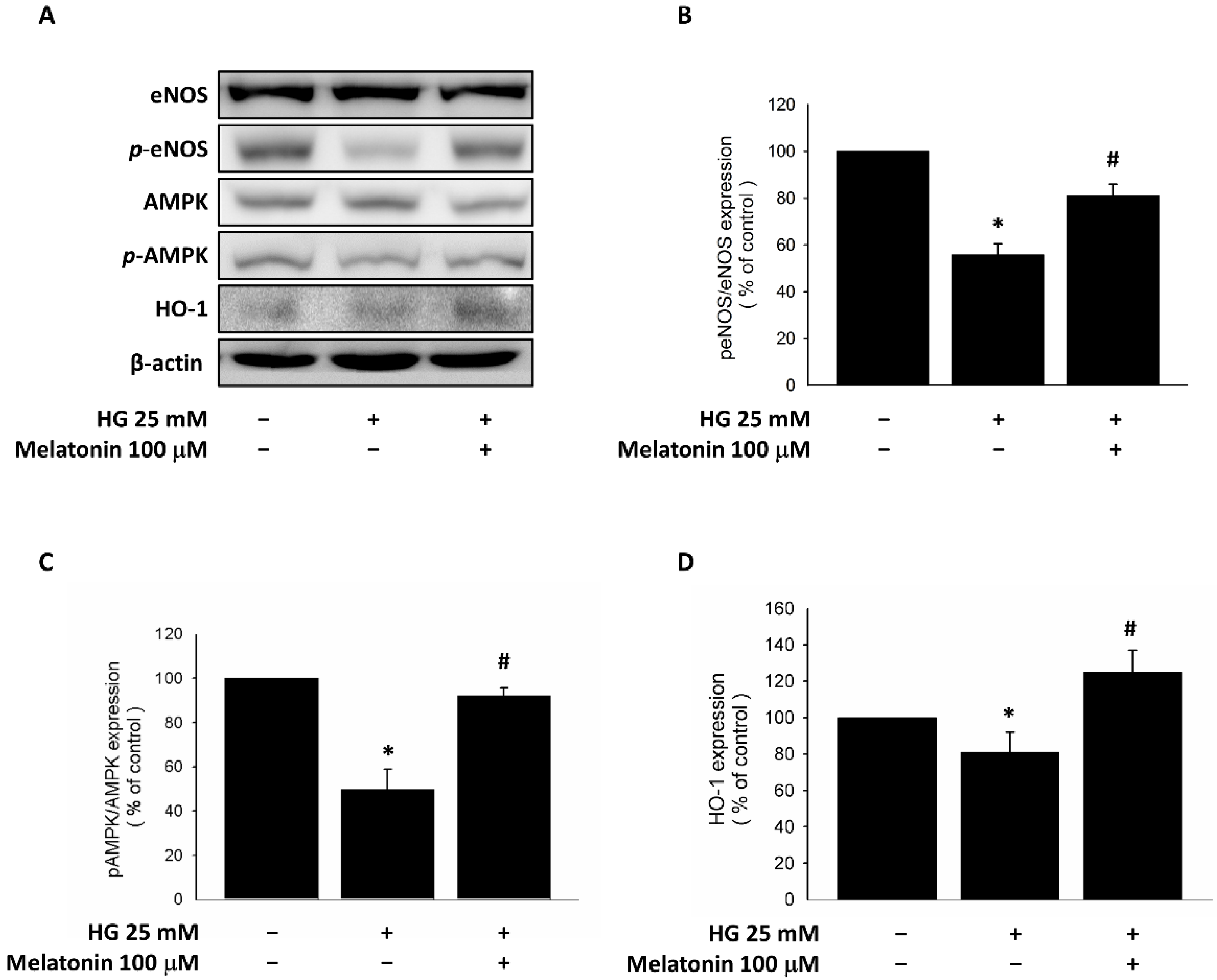
2.3. Expression of eNOS, HO-1, and AMPK in EPCs with Melatonin Treatment under HG Conditions
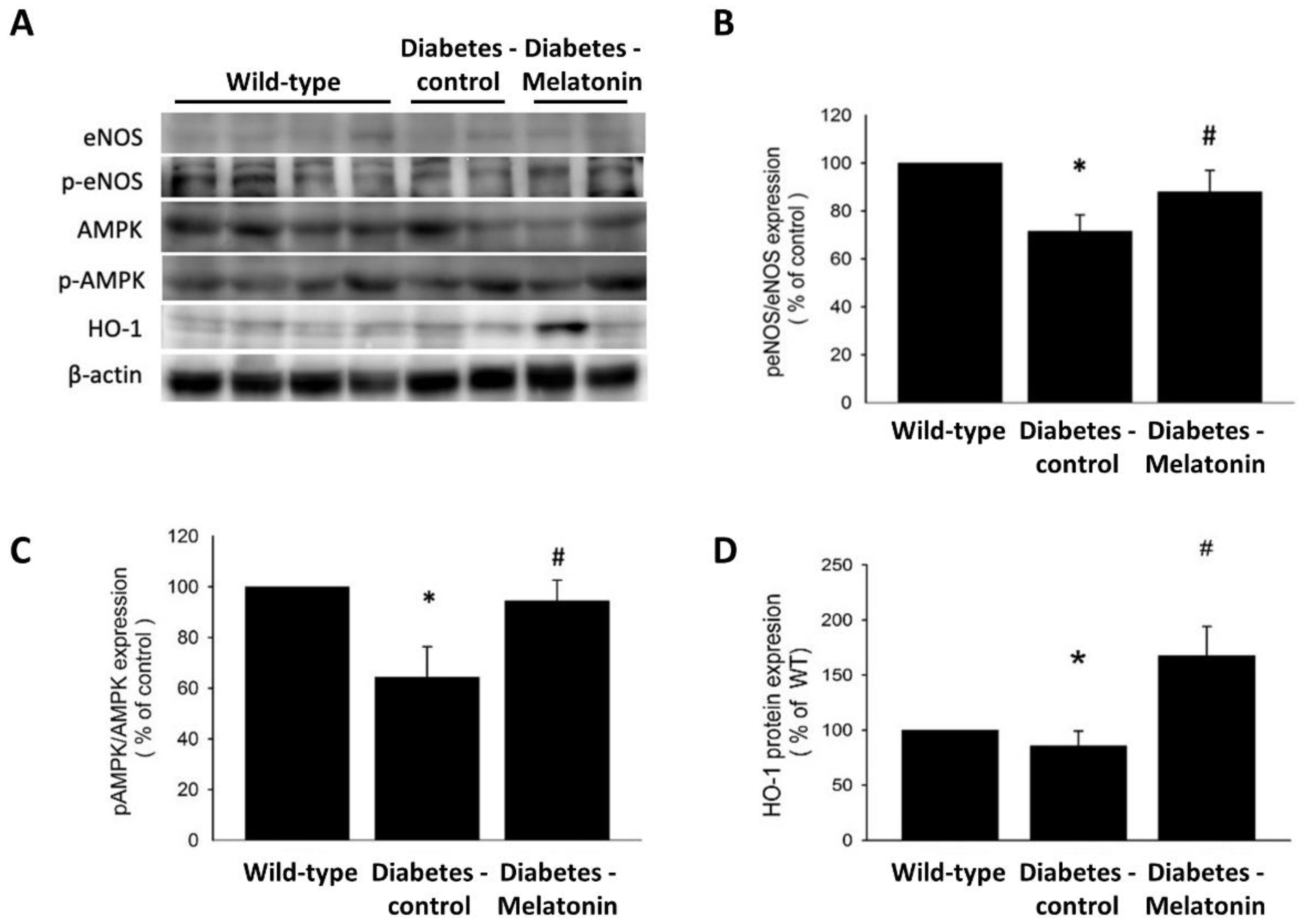
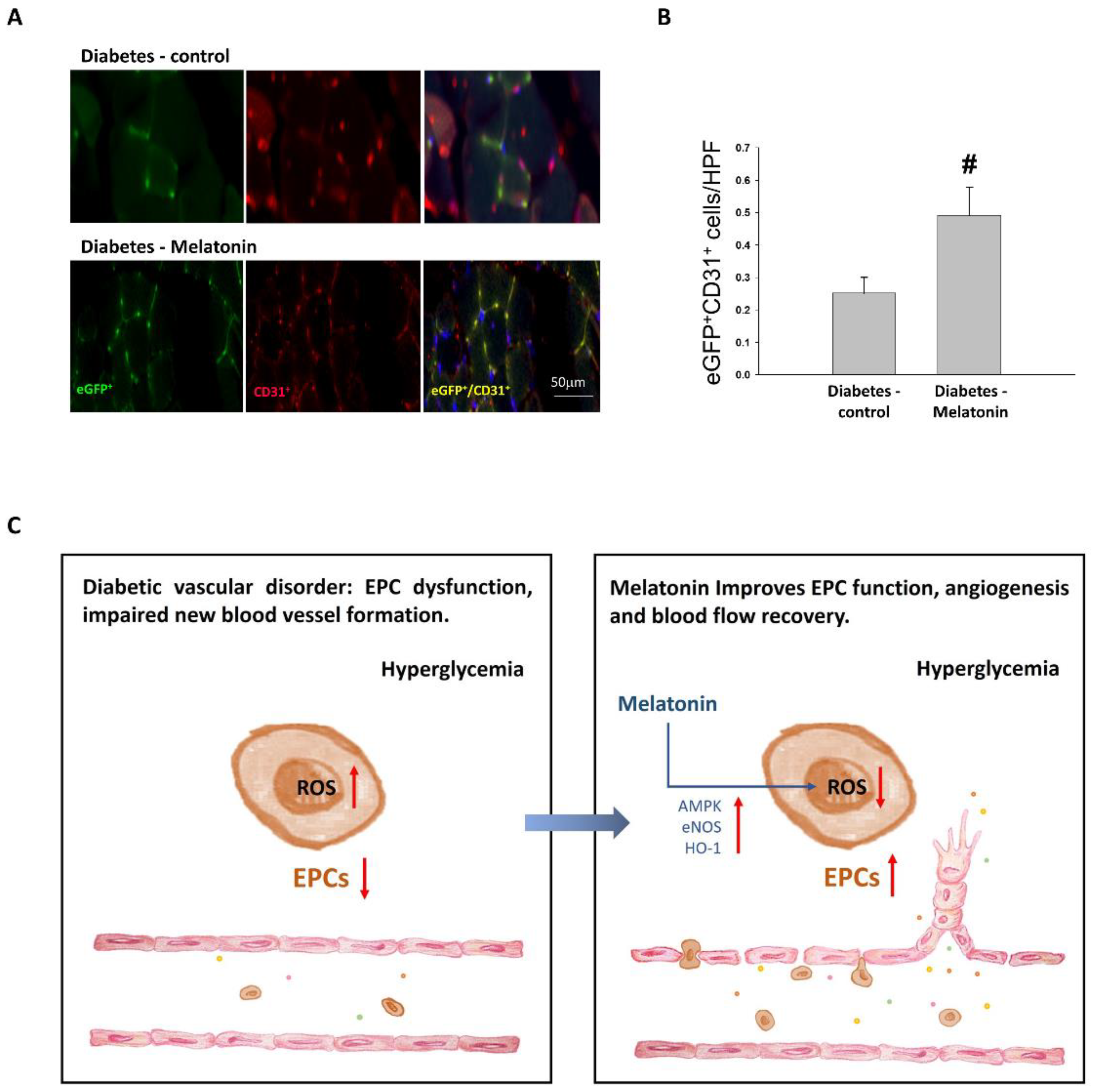
2.4. Effects of Melatonin on Ischemia-Induced Circulation Recovery and Circulating EPC Mobilization
2.5. EPC Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Characterization in EPC
4.2. Assessment of EPC Viability
4.3. Detection of Reactive Oxygen Species Production
4.4. Assessment of EPC Migration
4.5. Evaluation of Cellular Senescence in EPC
4.6. Animals
4.7. Hindlimb Ischemia Surgery
4.8. Flow Cytometry
4.9. Capillary Density Assessment
4.10. Western Blot Analysis
4.11. Bone Marrow Transplantation Model
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Meigs, J.B.; Singer, D.E.; Sullivan, L.M.; Dukes, K.A.; D’Agostino, R.B.; Nathan, D.M.; Wagner, E.H.; Kaplan, S.H.; Greenfield, S. Metabolic control and prevalent cardiovascular disease in non-insulin-dependent diabetes mellitus (NIDDM): The NIDDM Patient Outcome Research Team. Am. J. Med. 1997, 102, 38–47. [Google Scholar] [CrossRef]
- Abaci, A.; Oguzhan, A.; Kahraman, S.; Eryol, N.K.; Unal, S.; Arınç, H.; Ergin, A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 1999, 99, 2239–2242. [Google Scholar] [CrossRef]
- Sheetz, M.J.; King, G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. Jama 2002, 288, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar]
- Fadini, G.P.; De Kreutzenberg, S.; Albiero, M.; Coracina, A.; Pagnin, E.; Baesso, I.; Cignarella, A.; Bolego, C.; Plebani, M.; Nardelli, G.B.; et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: The role of female estrogens. Arter. Thromb. Vasc. Biol. 2008, 28, 997–1004. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef]
- Campos, L.A.; Cipolla-Neto, J.; Amaral, F.G.; Michelini, L.C.; Bader, M.; Baltatu, O.C. The Angiotensin-melatonin axis. Int. J. Hypertens. 2013, 2013, 521783. [Google Scholar] [CrossRef]
- Peschke, E. Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 2008, 44, 26–40. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef]
- Kilic, U.; Yilmaz, B.; Ugur, M.; Yüksel, A.; Reiter, R.J.; Hermann, D.M.; Kilic, E. Evidence that membrane-bound G protein-coupled melatonin receptors MT1 and MT2 are not involved in the neuroprotective effects of melatonin in focal cerebral ischemia. J. Pineal Res. 2012, 52, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef]
- Tengattini, S.; Reiter, R.J.; Tan, D.X.; Terron, M.P.; Rodella, L.F.; Rezzani, R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 2013, 55, 275–286. [Google Scholar] [CrossRef]
- Kang, J.W.; Koh, E.J.; Lee, S.M. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J. Pineal Res. 2011, 50, 403–411. [Google Scholar] [CrossRef]
- Mias, C.; Trouche, E.; Seguelas, M.-H.; Calcagno, F.; Dignat-George, F.; Sabatier, F.; Piercecchi-Marti, M.-D.; Daniel, L.; Bianchi, P.; Calise, D.; et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 2008, 26, 1749–1757. [Google Scholar] [CrossRef]
- Yip, H.-K.; Chang, Y.-C.; Wallace, C.G.; Chang, L.-T.; Tsai, T.-H.; Chen, Y.-L.; Chang, H.-W.; Leu, S.; Zhen, Y.-Y.; Tsai, C.-Y.; et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res. 2013, 54, 207–221. [Google Scholar] [CrossRef]
- Patschan, D.; Hildebrandt, A.; Rinneburger, J.; Wessels, J.T.; Patschan, S.; Becker, J.U.; Henze, E.; Kruger, A.; Muller, G.A. The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F1305–F1312. [Google Scholar] [CrossRef]
- Aksoy, N.; Vural, H.; Sabuncu, T.; Aksoy, S. Effects of melatonin on oxidative-antioxidative status of tissues in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2003, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, K.; Fraczyk, T.; Malinska, D.; Drozak, J.; Bryla, J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J. Pineal Res. 2006, 40, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.-Y.; Sun, C.-K.; Sung, P.-H.; Chen, K.-H.; Chua, S.; Sheu, J.-J.; Chung, S.-Y.; Chai, H.-T.; Chen, Y.-L.; Huang, T.-H.; et al. Daily melatonin protects the endothelial lineage and functional integrity against the aging process, oxidative stress, and toxic environment and restores blood flow in critical limb ischemia area in mice. J. Pineal Res. 2018, 65, e12489. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, Z.; Wang, C.; Tang, Q.; Wang, J.; Bai, X.; Wang, Q.; Nisar, M.; Tian, N.; Wang, Q.; et al. Melatonin protects endothelial progenitor cells against AGE-induced apoptosis via autophagy flux stimulation and promotes wound healing in diabetic mice. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Qiu, X.-F.; Li, X.-X.; Chen, Y.; Lin, H.-C.; Yu, W.; Wang, R.; Dai, Y.-T. Mobilisation of endothelial progenitor cells: One of the possible mechanisms involved in the chronic administration of melatonin preventing erectile dysfunction in diabetic rats. Asian J. Androl. 2012, 14, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Lin, C.-P.; Huang, P.-H.; Li, S.-Y.; Chen, J.-S.; Lin, F.-Y.; Chen, J.-W.; Lin, S.-J. Coenzyme Q10 Attenuates High Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathways. J. Diabetes Res. 2016, 2016, 6384759. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.P.; Yin, W.H.; Chen, J.S.; Huang, P.H.; Chen, J.W.; Lin, S.J. Fenofibrate Reverses Dysfunction of EPCs Caused by Chronic Heart Failure. J. Cardiovasc. Transl. Res. 2020, 13, 158–170. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chen, J.-S.; Wang, C.-H.; Huang, P.-H.; Lin, F.-Y.; Lin, L.-Y.; Lin, S.-J.; Chen, J.-W. Activation of heme oxygenase-1 by Ginkgo biloba extract differentially modulates endothelial and smooth muscle-like progenitor cells for vascular repair. Sci. Rep. 2019, 9, 17316. [Google Scholar] [CrossRef]
- Morrow, V.A.; Foufelle, F.; Connell, J.M.; Petrie, J.R.; Gould, G.W.; Salt, I.P. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J. Biol. Chem. 2003, 278, 31629–31639. [Google Scholar] [CrossRef]
- Gaskin, F.S.; Kamada, K.; Yusof, M.; Korthuis, R.J. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H326–H332. [Google Scholar] [CrossRef]
- Li, F.Y.; Lam, K.S.; Tse, H.-F.; Chen, C.; Wang, Y.; Vanhoutte, P.M.; Xu, A. Endothelium-selective activation of AMP-activated protein kinase prevents diabetes mellitus-induced impairment in vascular function and reendothelialization via induction of heme oxygenase-1 in mice. Circulation 2012, 126, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Lerner, A.B. The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 1977, 45, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 1999, 50, 271–279. [Google Scholar] [CrossRef]
- Garfinkel, D.; Zorin, M.; Wainstein, J.; Matas, Z.; Laudon, M.; Zisapel, N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: A randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. 2011, 4, 307–313. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P.; Rubio-Sastre, P.; Madrid, J.A.; Saxena, R.; Scheer, F.A. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 2015, 64, 1650–1657. [Google Scholar] [CrossRef]
- Lauritzen, E.S.; Støy, J.; Bæch-Laursen, C.; Grarup, N.; Jessen, N.; Hansen, T.; Møller, N.; Hartmann, B.; Holst, J.J.; Kampmann, U. The Effect of Melatonin on Incretin Hormones: Results From Experimental and Randomized Clinical Studies. J. Clin. Endocrinol. Metab. 2021, 106, e5109–e5123. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and humans. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Lv, T.; Yan, J.; Lou, Y.; Zhang, Z.; Ye, M.; Zhou, J.; Luo, F.; Bi, C.; Lin, H.; Zhang, J.; et al. Evaluation of Melatonin Therapy in Patients with Myocardial Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis. Oxidative Med. Cell Longev. 2022, 2022, 4610522. [Google Scholar] [CrossRef]
- Andersen, L.P.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, S.-J.; Lin, F.-Y.; Wu, T.-C.; Tsao, C.-R.; Huang, P.-H.; Liu, P.-L.; Chen, Y.-L.; Chen, J.-W. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes 2007, 56, 1559–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.-H.; Chen, C.-Y.; Lin, C.-P.; Wang, C.-H.; Tsai, H.-Y.; Lo, W.-Y.; Leu, H.-B.; Chen, J.-W.; Lin, S.-J.; Chu, P.-H. Deletion of FHL2 gene impaired ischemia-induced blood flow recovery by modulating circulating proangiogenic cells. Arter. Thromb. Vasc. Biol. 2013, 33, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-H.; Lin, C.-P.; Wang, C.-H.; Chiang, C.-H.; Tsai, H.-Y.; Chen, J.-S.; Lin, F.-Y.; Leu, H.-B.; Wu, T.-C.; Chen, J.-W.; et al. Niacin improves ischemia-induced neovascularization in diabetic mice by enhancement of endothelial progenitor cell functions independent of changes in plasma lipids. Angiogenesis 2012, 15, 377–389. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Huang, P.-H.; Chen, Y.-H.; Wang, C.-H.; Chen, J.-S.; Tsai, H.-Y.; Lin, F.-Y.; Lo, W.-Y.; Wu, T.-C.; Sata, M.; Chen, J.-W.; et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arter. Thromb. Vasc. Biol. 2009, 29, 1179–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-S.; Chen, C.-Y.; Huang, H.-L.; Tsai, H.-Y.; Chou, R.-H.; Wei, J.-H.; Huang, P.-H.; Lin, S.-J. Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning. Int. J. Mol. Sci. 2022, 23, 9839. https://doi.org/10.3390/ijms23179839
Kuo C-S, Chen C-Y, Huang H-L, Tsai H-Y, Chou R-H, Wei J-H, Huang P-H, Lin S-J. Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning. International Journal of Molecular Sciences. 2022; 23(17):9839. https://doi.org/10.3390/ijms23179839
Chicago/Turabian StyleKuo, Chin-Sung, Chi-Yu Chen, Hsin-Lei Huang, Hsiao-Ya Tsai, Ruey-Hsing Chou, Jih-Hua Wei, Po-Hsun Huang, and Shing-Jong Lin. 2022. "Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning" International Journal of Molecular Sciences 23, no. 17: 9839. https://doi.org/10.3390/ijms23179839
APA StyleKuo, C.-S., Chen, C.-Y., Huang, H.-L., Tsai, H.-Y., Chou, R.-H., Wei, J.-H., Huang, P.-H., & Lin, S.-J. (2022). Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning. International Journal of Molecular Sciences, 23(17), 9839. https://doi.org/10.3390/ijms23179839





