Physiological and Molecular Response Modifications by Ultraviolet-C Radiation in Plutella xylostella and Its Compatibility with Cordyceps fumosorosea
Abstract
1. Introduction
2. Results
2.1. Effect of UV-C Radiation on the Physiology of Plutella xylostella
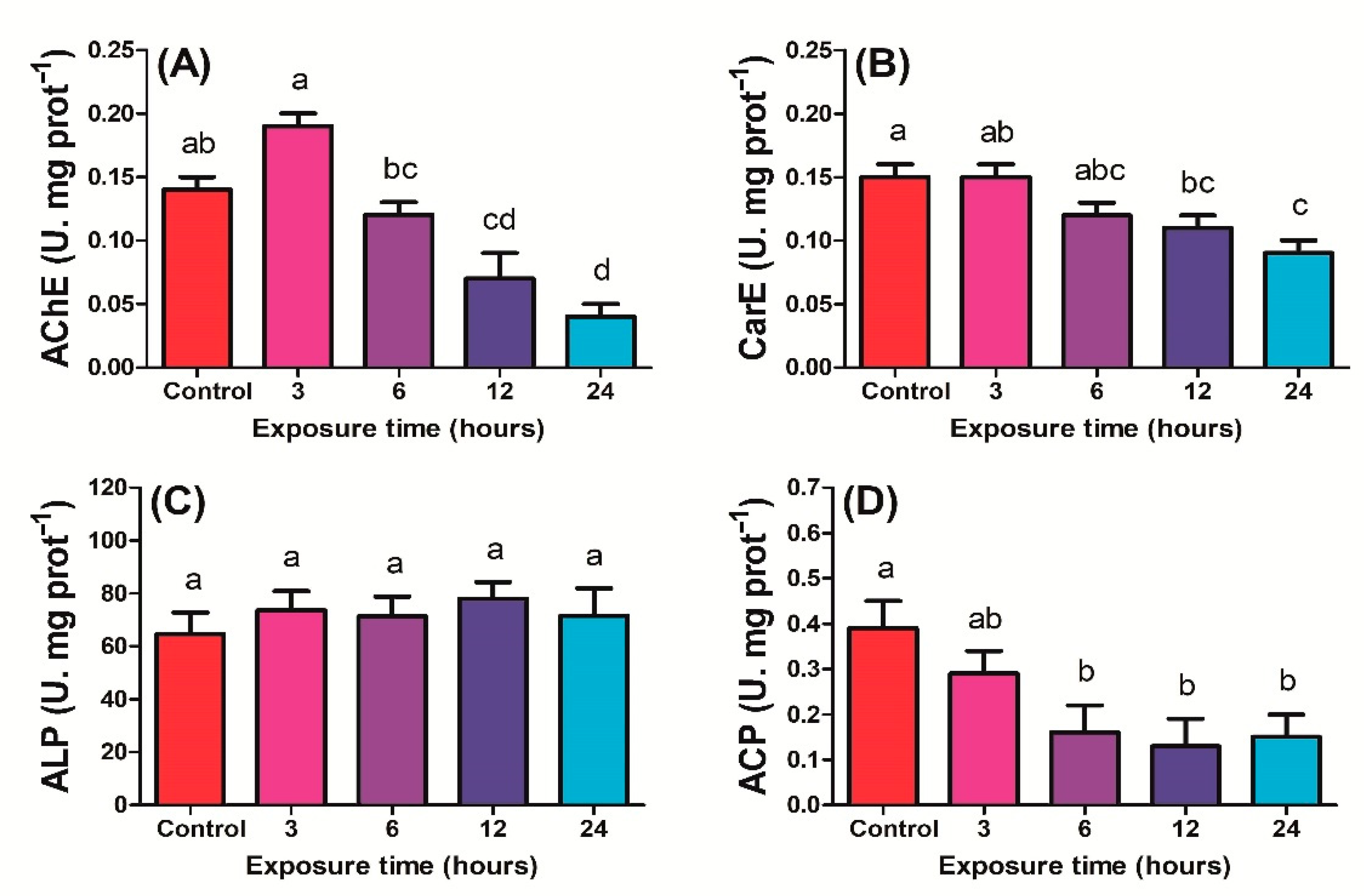
2.1.1. Antioxidant Enzyme Activities
2.1.2. Detoxification Enzyme Activity
2.1.3. Correlation of Enzyme Activity with UV Exposure Time
2.2. Effect of UV Radiation on Gene Expression of Plutella xylostella
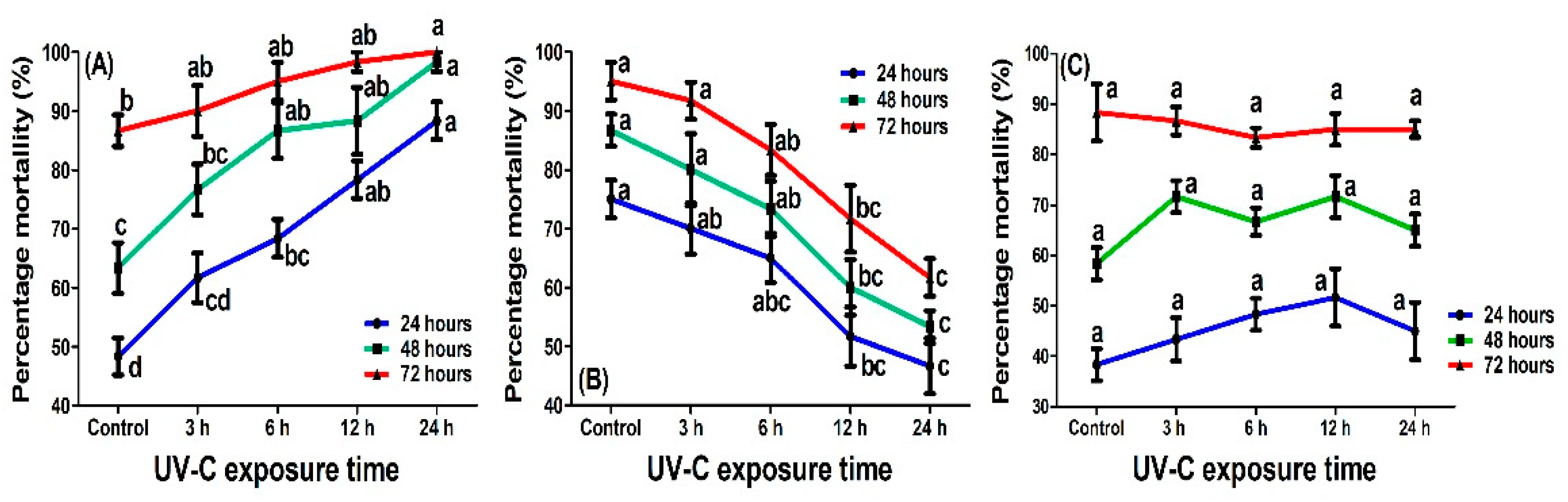
2.3. Effect of UV-C Radiation on the Virulence of Cordyceps fumosorosea
3. Discussion
4. Materials and Methods
4.1. Plutella xylostella Rearing
4.2. UV-Irradiation Exposure
4.3. Effect of UV-C Radiation on the Physiology of Plutella xylostella
4.3.1. Sample Preparation
4.3.2. Antioxidant Enzyme Activity Assay
4.3.3. Detoxifying Enzyme Activity Assay
4.4. Effect of UV-C Radiation on Gene Regulation of Plutella xylostella
4.4.1. RNA Extraction and cDNA Synthesis
4.4.2. Primer Design and Testing
4.4.3. RT-qPCR with SYBR Green
4.5. Preparation of the Conidial Suspension
4.6. Virulence Assessment Bioassay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer-Rochow, V.B. Risks, especially for the eye, emanating from the rise of solar UV-radiation in the Arctic and Antarctic regions. Int. J. Clin. Health 2000, 59, 38–51. [Google Scholar]
- Schauena, M.; Hornig-Doa, H.-T.; Schomberg, S.; Herrmann, G.; Wiesner, R.J. Mitochondrial electron transport chain activity is not involved in ultraviolet A (UVA)-induced cell death. Free Radic. Biol. Med. 2007, 42, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Antignus, Y. Manipulation of wavelength-dependent behaviour of insects: An IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res. 2000, 71, 213–220. [Google Scholar] [CrossRef]
- Kojima, Y.; Aoyagi, K.; Yasue, T. Effect of lithium ion addition on afterglow time of green-emitting Ce3+ and Pr3+ codoped CaS phosphor by black light irradiation. J. Lumin. 2005, 115, 13–18. [Google Scholar] [CrossRef]
- Mazza, C.A.; Izaguirre, M.M.; Zavala, J.; Scopel, A.L.; Ballaré, C.L. Insect perception of ambient ultraviolet-B radiation. Ecol. Lett. 2002, 5, 722–726. [Google Scholar] [CrossRef]
- Gunn, A. The determination of larval phase coloration in the African armyworm, Spodoptera exempta and its consequences for thermoregulation and protection from UV light. Entomol. Exp. Appl. 1998, 86, 125–133. [Google Scholar] [CrossRef]
- Khan, M.M.; Fan, Z.; Rothenberg, D.O.N.; Peng, J.; Hafeez, M.; Chen, X.; Pan, H.; Wu, J.; Qiu, B. Phototoxicity of ultraviolet-A against the whitefly Bemisia tabaci and its compatibility with an entomopathogenic fungus and whitefly parasitoid. Oxidative Med. Cell. Longev. 2021, 2021, 2060288. [Google Scholar] [CrossRef]
- Mackerness, A.H.S.; Surplus, S.L.; Blake, P.; John, C.F.; Buchanan-Wollaston, V.; Jordan, B.R.; Thomas, B. Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: Role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ. 1999, 22, 1413–1423. [Google Scholar] [CrossRef]
- Beard, R.L. Lethal action of UV irradiation on insects. J. Econ. Entomol. 1972, 65, 650–654. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2000, 115, 581. [Google Scholar] [CrossRef]
- Hori, M.; Shibuya, K.; Sato, M.; Saito, Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 2014, 4, 7383. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yoshida, H. Studies on ultraviolet sensitivity in the silkworm, with Special Reference to the Effect of UV-irradiation on melanin formation in the cuticle of the striped Silkworm. Jpn. J. Appl. Entomol. Zool. 1971, 15, 51–55. [Google Scholar] [CrossRef]
- Wharton, D.R.A. Ultraviolet repellent and lethal action on the American Cockroach. J. Econ. Entomol. 1971, 64, 252–255. [Google Scholar] [CrossRef]
- Lah, E.F.C.; Musa, R.N.A.R.; Ming, H.T. Effect of germicidal UV-C light (254 nm) on eggs and adult of house dustmites, Dermatophagoides pteronyssinus and Dermatophagoides farinae (Astigmata: Pyroglyhidae). Asian Pac. J. Trop. Biomed. 2012, 2, 679–683. [Google Scholar] [CrossRef]
- Cagan, L.; Svercel, M. The Influence of ultraviolet light on pathogenicity of entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin to the European corn borer, Ostrinia Nubilalis Hbn. (Lepidoptera: Crambidae). J. Cent. Eur. Agric. 2002, 2, 227–234. [Google Scholar]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef]
- Dahms, H.U.; Lee, J.S. UV radiation in marine ectotherms: Molecular effects and responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defence system of two lepidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Lei, C.L. Influence of UV-A radiation on oxidative stress and antioxidant enzymes in Mythimna separata (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2017, 24, 8392–8398. [Google Scholar] [CrossRef]
- Cui, H.; Zeng, Y.; Singh, G.V.P.; Gao, F.; Li, Z.; Zhao, Z. UV radiation increases mortality and decreases the antioxidant activity in a tephritid fly. Food Energy Secur. 2021, 10, e297. [Google Scholar] [CrossRef]
- Wang, W.; Gao, C.; Ren, L.; Luo, Y. The effect of longwave ultraviolet light radiation on Dendrolimus tabulaeformis antioxidant and detoxifying enzymes. Insects 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Du, Y.; Yang, J.; Zhang, L.; Zhao, H.; Hu, Z.; Hu, X. Effect of UV-B radiation in successive generation on the activity of protective enzymes in the grain aphid, Sitobion avenae (Hemiptera: Aphididae). Acta Entomol. Sin. 2014, 57, 762–768. [Google Scholar]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef]
- Mao, T.; Li, F.; Fang, Y.; Wang, H.; Chen, J.; Li, M.; Lu, Z.; Qu, J.; Li, J.; Hu, J.; et al. Effects of chlorantraniliprole exposure on detoxification enzyme activities and detoxification-related gene expression in the fat body of the silkworm, Bombyx mori. Ecotoxicol. Environ. Saf. 2019, 176, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, R.; Pongjaroenkit, S.; Wongsantichon, J.; Oakley, A.J.; Prapanthadara, L.A.; Wilce, M.C.J.; Ketterman, A.J. Identification, characterization and structure of a new Delta class glutathione transferase isoenzyme. Biochem. J. 2005, 388, 763–771. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Gu, Z.; Zhang, Y.; Wang, Z. Activity and transcriptional responses of hepatopancreatic biotransformation and antioxidant enzymes in the oriental river prawn Macrobrachium nipponense exposed to microcystin-LR. Toxins 2015, 7, 4006–4022. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Yang, J.; Tian, L.; Xu, B.; Xie, W.; Wang, S.; Zhang, Y.; Wang, X.; Wu, Q. Insight into the migration routes of Plutella xylostella in China using mtCOI and ISSR markers. PLoS ONE 2015, 10, e0130905. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, M.; Shi, H.; Gao, X.; Liang, P. Genome-wide identification of lncRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). BMC Genom. 2017, 18, 380. [Google Scholar] [CrossRef]
- Sarfraz, M.; Keddie, B.A. Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae). J. Appl. Entomol. 2005, 129, 149–157. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Li, Y.-X.; Collins, H.L.; Gusukuma-Minuto, L.; Mau, R.F.L.; Thompson, G.D.; Shelton, A.M. Monitoring and characterization of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad. J. Econ. Entomol. 2009, 95, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Yang, Y.; Wu, S.; Wu, Y. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag. Sci. 2010, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.H.; Wright, D.J. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae). Pest Manag. Sci. 2006, 62, 1045–1051. [Google Scholar] [CrossRef]
- Liao, J.; Xue, Y.; Xiao, G.; Xie, M.; Huang, S.; You, S.; Wyckhuys, K.A.G.; You, M. Inheritance and fitness costs of resistance to Bacillus thuringiensis toxin Cry2Ad in laboratory strains of the diamondback moth, Plutella xylostella (L.). Sci. Rep. 2019, 9, 6113. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.H.; Omar, D.; Wright, D.J. Genetics of spinosad resistance in a multi-resistant field-selected population of Plutella xylostella. Pest Manag. Sci. 2004, 60, 827–832. [Google Scholar] [CrossRef]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Diamondback moth-host plant interactions: Implications for pest management. Crop Prot. 2006, 25, 625–639. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Collins, H.L.; Li, Y.-X.; Mau, R.F.L.; Thompson, G.D.; Hertlein, M.; Andaloro, J.T.; Boykin, R.; Shelton, A.M. Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. J. Econ. Entomol. 2006, 99, 176–181. [Google Scholar] [CrossRef]
- Nian, X.G.; He, Y.R.; Lu, L.H.; Zhao, R. Evaluation of the time-concentration-mortality responses of Plutella xylostella larvae to the interaction of Isaria fumosorosea with the insecticides beta-cypermethrin and Bacillus thuringiensis. Pest Manag. Sci. 2015, 71, 216–224. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Ashraf, U.; Qiu, B.; Ali, S. Transfer of lead (Pb) in the soil-plant-mealybug-ladybird beetle food chain, a comparison between two host plants. Ecotoxicol. Environ. Saf. 2017, 143, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zhang, C.; Wang, Z.; Wang, X.M.; Wu, J.H.; Cuthbertson, A.G.S.; Shao, Z.; Qiu, B.L. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 2017, 7, 46558. [Google Scholar] [CrossRef] [PubMed]
- Altre, J.A.; Vandenberg, J.D. Factors influencing the infectivity of isolates of Paecilomyces fumosoroseus against diamondback moth, Plutella xylostella. J. Invertebr. Pathol. 2001, 78, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Altre, J.A.; Vandenberg, J.D.; Cantone, F.A. Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback Moth, Plutella xylostella: Correlation with spore size, germination speed, and Attachment to Cuticle. J. Invertebr. Pathol. 1999, 73, 332–338. [Google Scholar] [CrossRef]
- Li, H.U.; Yu, R.H.; Ya, J.W.; Xia, F.; Huan, Y.C. The time-dose-mortality model of a Paecilomyces fumosoroseus isolate on the diamondback moth, Plutella xylostella. Acta Entomol. Sin. 2007, 50, 567–573. [Google Scholar]
- Boopathi, T.; Karuppuchamy, P.; Singh, S.B.; Kalyanasundaram, M.; Mohankumar, S.; Ravi, M. Microbial control of the invasive spiraling whitefly on cassava with entomopathogenic fungi. Braz. J. Microbiol. 2015, 46, 1077–1085. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Huang, Y.; Keyhani, N.O.; Huang, Z. Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 2017, 7, 42727. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, D.; Peng, F.; Li, Z. Wettable powder development of Isaria javanica for control of the lesser green leafhopper, Empoasca vitis. Chin. J. Biol. Control 2014, 30, 51–57. [Google Scholar]
- Jandricic, S.E.; Filotas, M.; Sanderson, J.P.; Wraight, S.P. Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol. 2014, 118, 34–46. [Google Scholar] [CrossRef]
- Sevim, A.; Demir, I.; Sönmez, E.; Kocaçevik, S.; Demirbaǧ, Z. Evaluation of entomopathogenic fungi against the sycamore lace bug, Corythucha ciliata (Say) (Hemiptera: Tingidae). Turk. J. Agric. For. 2013, 37, 595–603. [Google Scholar] [CrossRef]
- Bugti, G.A.; Na, C.; Bin, W.; Lin, H.F. Control of plant sap-sucking insects using entomopathogenic fungi Isaria fumosorosea strain (Ifu13a). Plant Prot. Sci. 2018, 54, 258–264. [Google Scholar]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Ahmad, S. Oxidative stress from environmental pollutants. Arch. Insect Biochem. Physiol. 1995, 29, 135–157. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [PubMed]
- Polte, T.; Tyrrell, R.M. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic. Biol. Med. 2004, 36, 1566–1574. [Google Scholar] [CrossRef]
- Tabatabaie, T.; Floyd, R.A. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch. Biochem. Biophys. 1994, 314, 112–119. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Borowiak-Sobkowiak, B.; Chrzanowski, G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Biochem. 2017, 118, 529–540. [Google Scholar] [CrossRef]
- Haddouche, L.; Phalak, A.; Tikekar, R.V. Inactivation of polyphenol oxidase using 254nm ultraviolet light in a model system. LWT Food Sci. Technol. 2015, 62, 97–103. [Google Scholar] [CrossRef]
- Ahmad, S.; Duval, D.L.; Weinhold, L.C.; Pardini, R.S. Cabbage looper antioxidant enzymes: Tissue specificity. Insect Biochem. 1991, 21, 563–572. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Miao, L.; Dong, F.; Wang, J. Advances in the research on invertebrate acetylcholinesterase. J. Environ. Entomol. 2019, 41, 508–519. [Google Scholar]
- Kim, Y.H.; Lee, S.H. Which acetylcholinesterase functions as the main catalytic enzyme in the Class Insecta? Insect Biochem. Mol. Biol. 2013, 43, 47–53. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, G.P.; Rajam, M.V. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 2009, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B.; Crowder, M.W.; Averill, B.A. Hydrolysis of phosphate monoesters: A biological problem with multiple chemical solutions. Trends Biochem. Sci. 1992, 17, 105–110. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, S.; Yin, F.; Cai, S.; Zhong, G.; Ren, S. Diversity and virulence of soil-dwelling fungi Isaria spp. and Paecilomyces spp. against Solenopsis invicta (Hymenoptera: Formicidae). Biocontrol Sci. Technol. 2011, 21, 225–234. [Google Scholar] [CrossRef]
- da Silva Lopes, R.; de Lima, G.; dos Santos Correia, M.T.; da Costa, A.F.; de Luna Alves Lima, E.Á.; de Menezes Lima, V.L. The potential of Isaria spp. as a bioinsecticide for the biological control of Nasutitermes corniger. Biocontrol Sci. Technol. 2017, 27, 1038–1048. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Audsley, N. Further screening of entomopathogenic fungi and nematodes as control agents for Drosophila suzukii. Insects 2016, 7, 24. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Huang, Z.; Sahar, F.; Ren, S.; Ali, S. Effect of Isaria fumosoroseus on eretmocerus sp. nr. furuhashii (hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (hemiptera: Aleyrodidae). Pak. J. Zool. 2010, 42, 121–127. [Google Scholar]
- Xu, J.; Xu, X.; Li, S.; Wang, S.; Xu, X.; Zhou, X.; Yu, J.; Yu, X.; Shakeel, M.; Jin, F. Genome-wide profiling of Plutella xylostella immunity-related miRNAs after Isaria fumosorosea infection. Front. Physiol. 2017, 8, 1054. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; St. Leger, R.J. Enhanced UV resistance and improved killing of malaria mosquitoes by photolyase transgenic entomopathogenic fungi. PLoS ONE 2012, 7, e43069. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, É.K.K.; Rangel, D.E.N.; Braga, G.U.L.; Roberts, D.W. Tolerance of entomopathogenic fungi to ultraviolet radiation: A review on screening of strains and their formulation. Curr. Genet. 2015, 61, 427–440. [Google Scholar] [CrossRef]
- Ignoffo, C.; Garcia, C. Influence of conidial colour on inactivation of several entomogenous fungi (hyphomycetes) by simulated sunlight. Environ. Entomol. 1992, 21, 913–917. [Google Scholar] [CrossRef]
- Moore, D.; Bridge, P.; Higgins, P.; Bateman, R.; Prior, C. Ultraviolet radiation damage to Metarhizium flavoviride conidia and the protection given by vegetable and mineral oils and chemical sunscreens. Ann. Appl. Biol. 1993, 122, 605–616. [Google Scholar] [CrossRef]
- Fernandes, É.; Rangel, D.; Moraes, A.; Bittencourt, V.; Roberts, D. Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J. Invertebr. Pathol. 2007, 96, 237–243. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Vidal, C.; Lacey, L.A.; Lomer, C.J.; Rougier, M. Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic Hyphomycetes. Mycopathologia 1996, 135, 171–181. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Flint, S.D.; Messias, C.L.; Anderson, A.J.; Roberts, D.W. Effects of UVB irradiance on conidia and germinants of the entomopathogenic hyphomycete Metarhizium anisopliae: A Study of reciprocity and recovery. Photochem. Photobiol. 2001, 73, 140. [Google Scholar] [CrossRef]
- Nascimento, É.; da Silva, S.; Marques, E.; Roberts, D.; Braga, G. Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii. Photochem. Photobiol. 2010, 86, 1256–1266. [Google Scholar] [CrossRef]
- Fargues, J.; Rougier, M.; Goujet, R.; Smits, N.; Coustere, C.; Itier, B. Inactivation of conidia of Paecilomyces fumosoroseus by near-ultraviolet (UVB and UVA) and visible radiation. J. Invertebr. Pathol. 1997, 69, 70–78. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Both Solar UVA and UVB Radiation impair conidial culturability and delay germination in the entomopathogenic fungus Metarhizium anisopliae. Photochem. Photobiol. 2001, 74, 734. [Google Scholar] [CrossRef]
- Zimmermann, G. Effect of high temperatures and artificial sunlight on the viability of conidia of Metarhizium anisopliae. J. Invertebr. Pathol. 1982, 40, 36–40. [Google Scholar] [CrossRef]
- Roberts, D.W.; Campbell, A.S. Stability of entomopathogenic fungi. Entomol. Soc. Am. 1977, 10, 19–76. [Google Scholar]
- Guo, C.F.; Pan, H.P.; Zhang, L.H.; Ou, D.; Lu, Z.T.; Khan, M.M.; Qiu, B.L. Comprehensive assessment of candidate reference genes for gene expression studies using RT-qPCR in Tamarixia radiata, a predominant parasitoid of Diaphorina citri. Genes 2020, 11, 1178. [Google Scholar] [CrossRef]
- Pinheiro, D.H.; Siegfried, B.D. Selection of reference genes for normalization of RT-qPCR data in gene expression studies in Anthonomus eugenii Cano (Coleoptera: Curculionidae). Sci. Rep. 2020, 10, 5070. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tian, J.; Diao, H.; Liang, L.; Hao, C.; Arthurs, S.; Ma, R. Pathogenicity of Isaria fumosorosea to Bemisia tabaci, with some observations on the fungal infection process and host immune response. J. Invertebr. Pathol. 2015, 130, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, A.; Er, M.K. Pathogenicity of Paecilomyces spp. to the glasshouse whitefly, Trialeurodes vaporariorum, with some observations on the fungal infection process. Turk. J. Agric. For. 2005, 29, 331–339. [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]


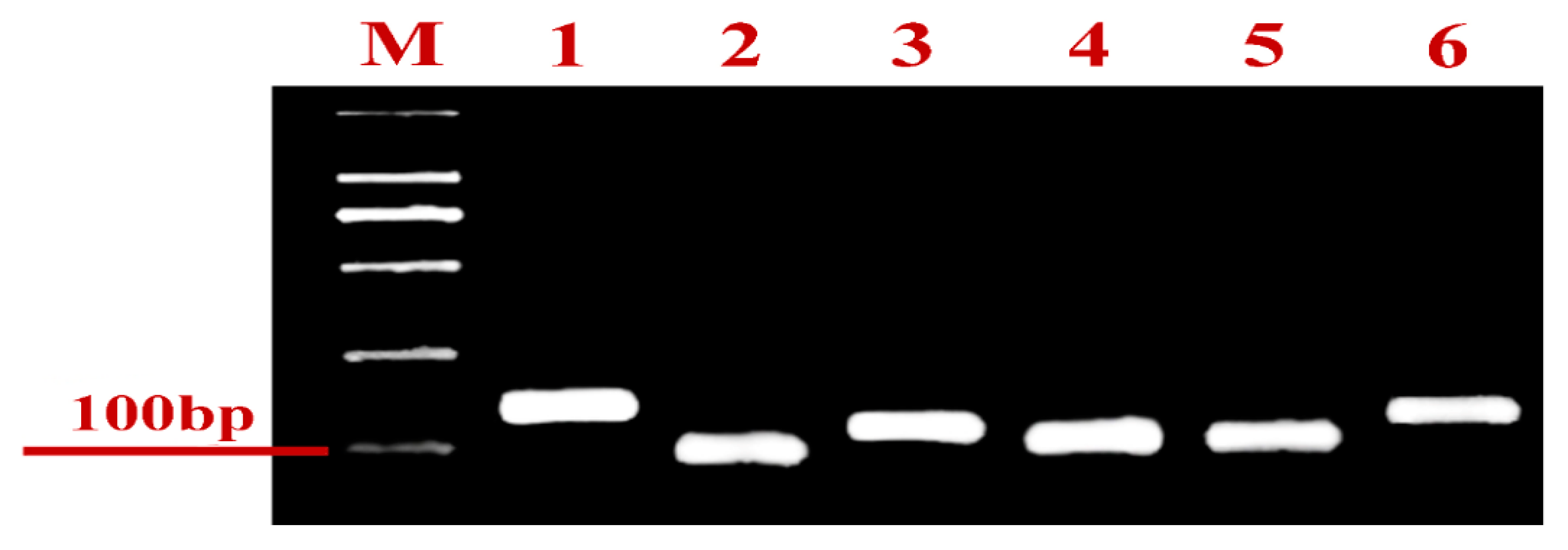

| Gene Name | NCBI Reference Sequence Number | Gene Name | Sequence (5′ to 3′) | Sequence |
|---|---|---|---|---|
| CAT1 | XM_011560295.1 | PREDICTED: Plutella xylostella catalase-like (LOC105389213), mRNA | F | ggctcaacgacaacctcatccg |
| R | cgtgcgtgacctcgaagtagc | |||
| CAT2 | XM_011561829.1 | PREDICTED: Plutella xylostella catalase-like (LOC105390515), mRNA | F | caccaagtattccgccgccaag |
| R | tccgcccaccgtcgagaatc | |||
| CarE1 | XM_011552809.1 | PREDICTED: Plutella xylostella carboxylesterase 1C-like (LOC105382842), mRNA | F | catgggaaagtatccgggaacacg |
| R | tggttgtggtggcagaaatctcag | |||
| CarE2 | XM_011558702.1 | PREDICTED: Plutella xylostella carboxylesterase 1E-like (LOC105387900), mRNA | F | actgcccatgccaagaccaaac |
| R | agacgctcgccttagctccag | |||
| PPO1 | NW_011952494.1 | Plutella xylostella strain DBM-FZ-S unplaced genomic scaffold, DBM_FJ_V1.1 scaffold_467, whole genome shotgun sequence | F | agccataggaagcctgacctcatc |
| R | gctgacgacaccgaccacaat | |||
| PPO2 | XM_011565232.1 | PREDICTED: Plutella xylostella uncharacterized LOC105393465 (LOC105393465), mRNA | F | ggagtgaagccgccgaaagc |
| R | tgttgccaccgataatccgatcag | |||
| RPS13 | NM_001017.3 | Ribosomal protein S13 | F | tcaggcttattctcgtcg |
| R | gctgtgctggattcgtac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Fan, Z.-Y.; Sabir, I.A.; Hafeez, M.; Wen, S.; Wu, J.-H.; Qiu, B.-L. Physiological and Molecular Response Modifications by Ultraviolet-C Radiation in Plutella xylostella and Its Compatibility with Cordyceps fumosorosea. Int. J. Mol. Sci. 2022, 23, 9800. https://doi.org/10.3390/ijms23179800
Khan MM, Fan Z-Y, Sabir IA, Hafeez M, Wen S, Wu J-H, Qiu B-L. Physiological and Molecular Response Modifications by Ultraviolet-C Radiation in Plutella xylostella and Its Compatibility with Cordyceps fumosorosea. International Journal of Molecular Sciences. 2022; 23(17):9800. https://doi.org/10.3390/ijms23179800
Chicago/Turabian StyleKhan, Muhammad Musa, Ze-Yun Fan, Irfan Ali Sabir, Muhammad Hafeez, Sang Wen, Jian-Hui Wu, and Bao-Li Qiu. 2022. "Physiological and Molecular Response Modifications by Ultraviolet-C Radiation in Plutella xylostella and Its Compatibility with Cordyceps fumosorosea" International Journal of Molecular Sciences 23, no. 17: 9800. https://doi.org/10.3390/ijms23179800
APA StyleKhan, M. M., Fan, Z.-Y., Sabir, I. A., Hafeez, M., Wen, S., Wu, J.-H., & Qiu, B.-L. (2022). Physiological and Molecular Response Modifications by Ultraviolet-C Radiation in Plutella xylostella and Its Compatibility with Cordyceps fumosorosea. International Journal of Molecular Sciences, 23(17), 9800. https://doi.org/10.3390/ijms23179800








