Prophylactic Instillation of Hydrogen-Rich Water Decreases Corneal Inflammation and Promotes Wound Healing by Activating Antioxidant Activity in a Rat Alkali Burn Model
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity of Normal Cornea after Hydrogen Instillation
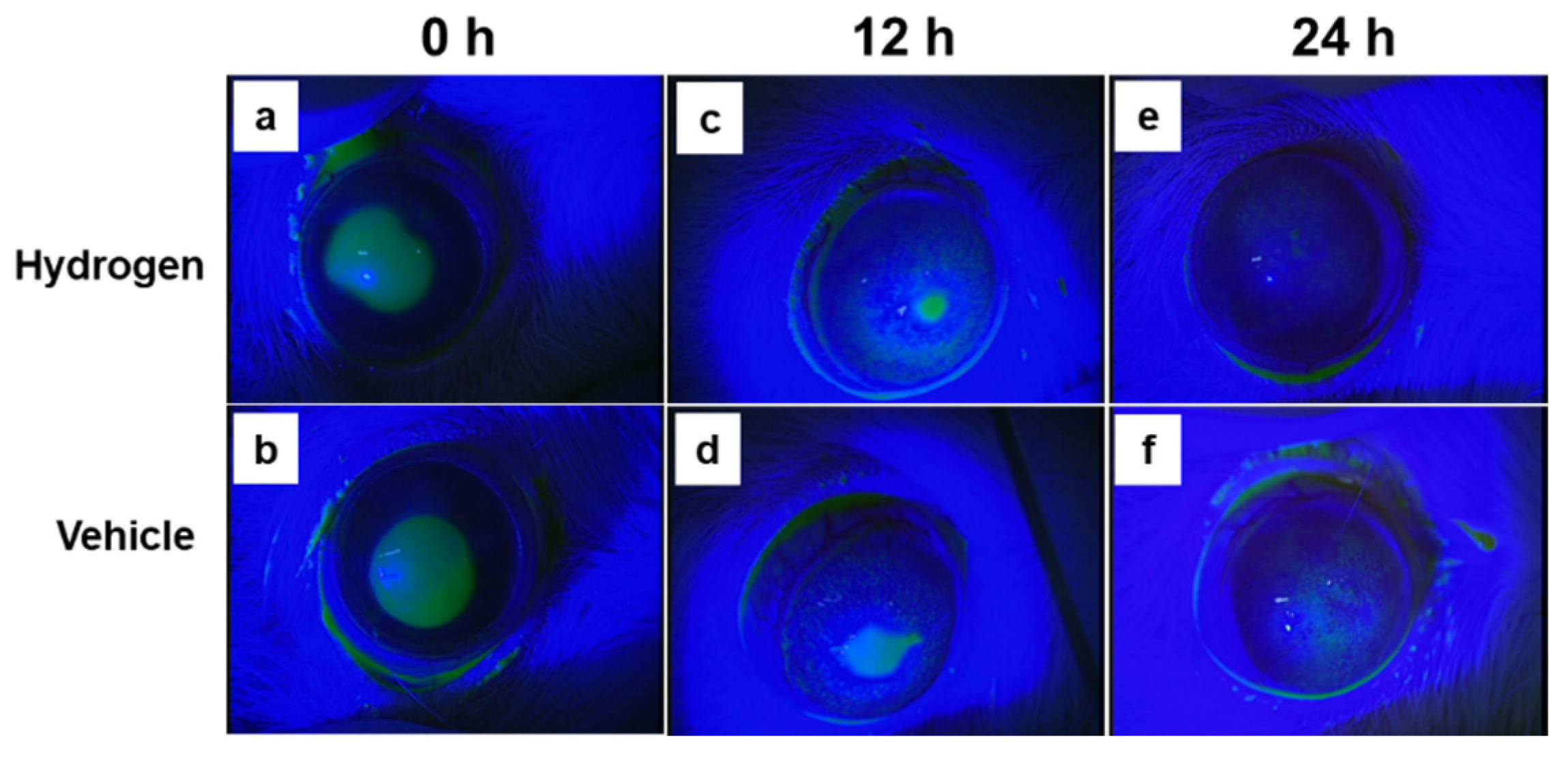
2.2. Corneal Wound Healing after Alkali Burn
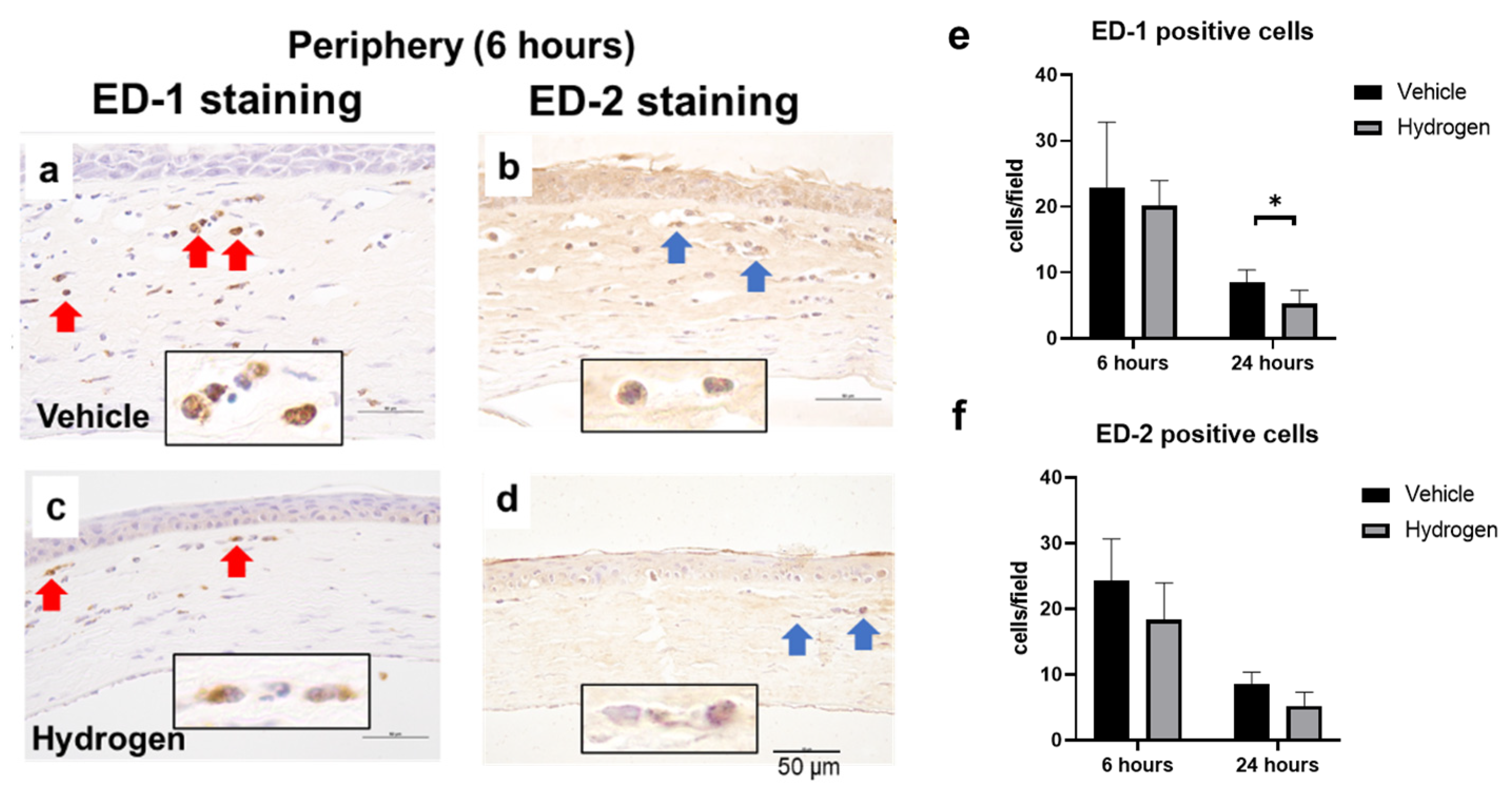
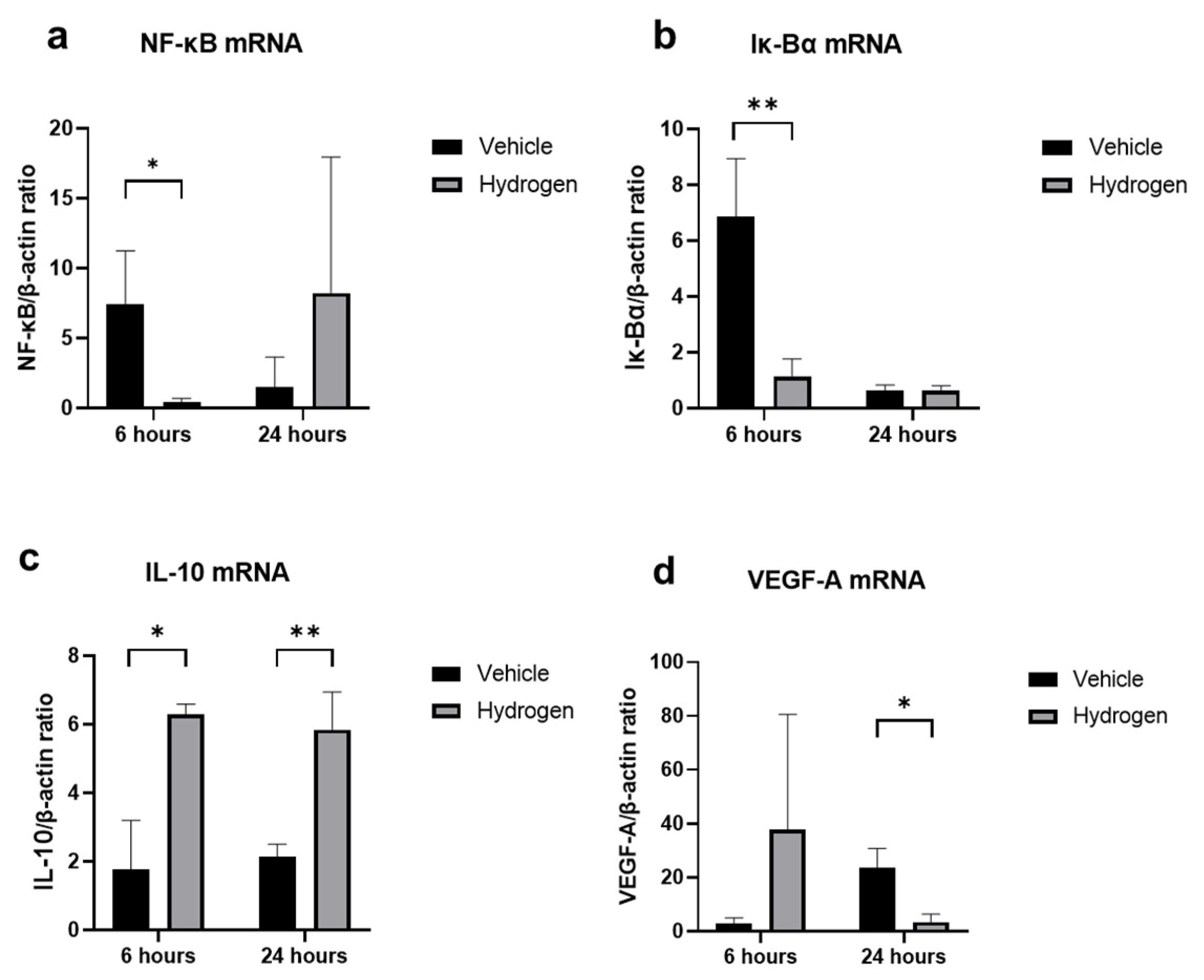
2.3. Inflammation and Oxidative Stress after Alkali Burn
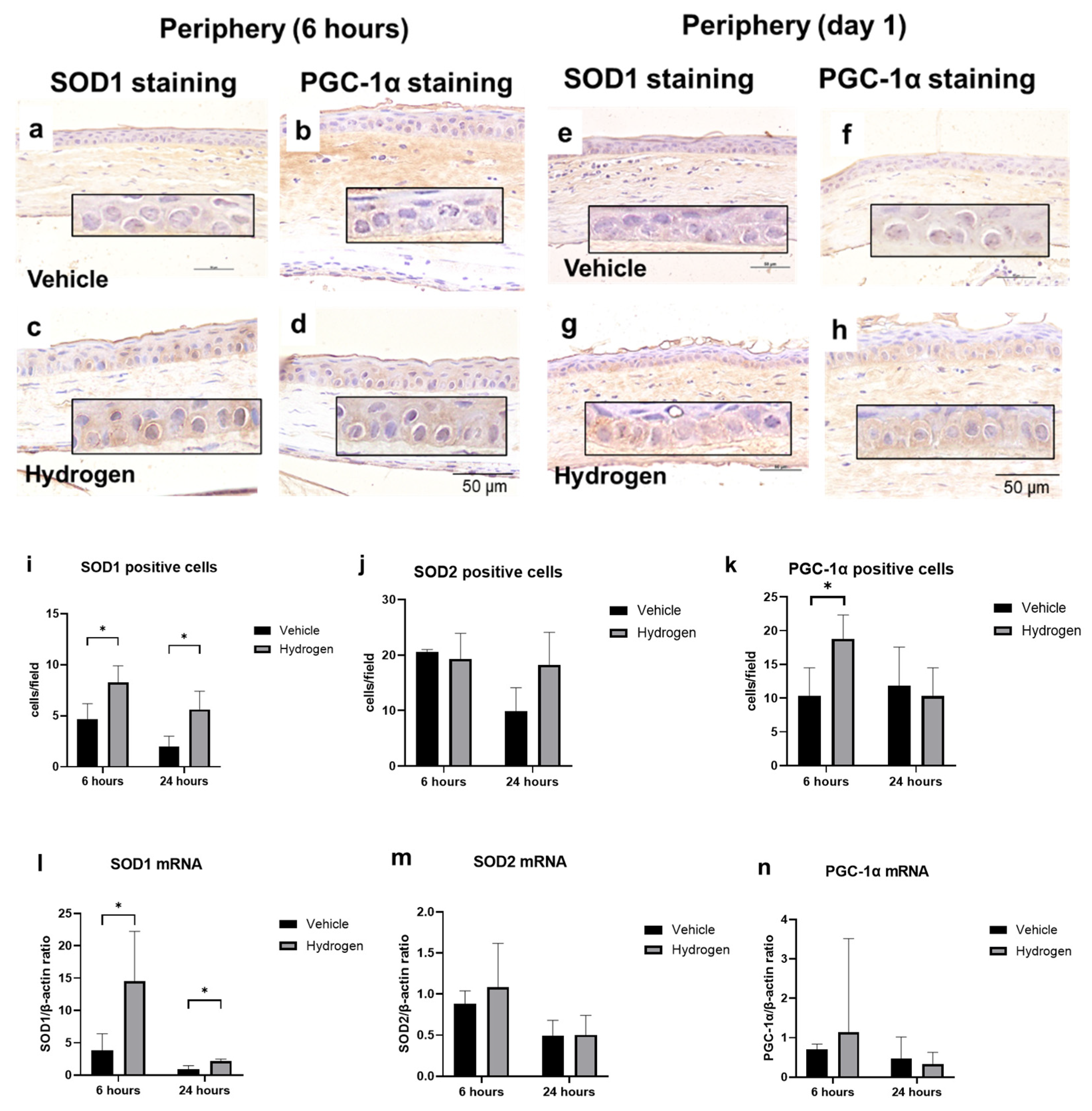
2.4. Antioxidant Activity after Alkali Burn
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Administration of Hydrogen to Normal Cornea
4.3. Prophylactic Administration of Hydrogen and Alkali Burn Model
4.4. Measurement Area of Corneal Epithelial Defect
4.5. Histological and Immunohistochemical Analyses
4.6. Real-Time RT-PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Maekawa, Y.; Kawamura, A.; Abe, T.; Ohta, S.; Fukuda, K.; et al. H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J. Am. Heart Assoc. 2012, 1, e003459. [Google Scholar] [CrossRef]
- Xie, K.; Wang, Y.; Yin, L.; Wang, Y.; Chen, H.; Mao, X.; Wang, G. Hydrogen gas alleviates sepsis-induced brain injury by improving mitochondrial biogenesis through the activation of PGC-α in mice. Shock 2021, 55, 100–109. [Google Scholar] [CrossRef]
- Kubota, M.; Shimmura, S.; Kubota, S.; Miyashita, H.; Kato, N.; Noda, K.; Ozawa, Y.; Usui, T.; Ishida, S.; Umezawa, K.; et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 427–433. [Google Scholar] [CrossRef]
- Igarashi, T.; Ohsawa, I.; Kobayashi, M.; Igarashi, T.; Suzuki, H.; Iketani, M.; Takahashi, H. Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci. Rep. 2016, 6, 31190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective effect of hydrogen on sodium iodate-induced age-related macular degeneration in mice. Front. Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef]
- Hirano, S.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean Technol. 2020, 2, 529–541. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.-S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [Green Version]
- Arima, T.; Igarashi, T.; Uchiyama, M.; Kobayashi, M.; Ohsawa, I.; Shimizu, A.; Takahashi, H. Hydrogen promotes the activation of Cu, Zn superoxide dismutase in a rat corneal alkali-burn model. Int. Ophthalmol. 2020, 13, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F.S. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, Z.; Zhou, T.; Zong, R.; He, H.; Liu, Z.; Ma, J.-X.; Liu, Z.; Zhou, Y. Anti-angiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS ONE 2011, 6, e16712. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.-K.; Won, J.-K.; Fulcher, S. Comprehensive modeling of corneal alkali injury in the rat eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, H.J.; Ko, A.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R. Analysis of macrophage phenotype in rejected corneal allografts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7779–7784. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef]
- Nakano, Y.; Arima, T.; Tobita, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Combination of peroxisome proliferator-activated receptor (PPAR) alpha and gamma agonists prevents corneal inflammation and neovascularization in a rat alkali burn model. Int. J. Mol. Sci. 2020, 21, 5093. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, M.; Chen, Z.; Wu, G.; Fujino, M.; Zhang, C.; Zhou, W.; Zhao, M.; Hirano, S.-I.; Li, X.-K.; et al. Hydrogen gas attenuates hypoxic-ischemic brain injury via regulation of the MAPK/HO-1/PGC-1a pathway in neonatal rats. Oxid. Med. Cell. Longev. 2020, 2020, 6978784. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.C.; Jiang, A.; Arany, Z. PGC-1 coactivators in cardiac development and disease. Circ. Res. 2010, 107, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jiang, W.; Liu, H.; Wang, J.; Zheng, K.; Cui, P.; Feng, Y.; Dang, C.; Bu, Y.; Wang, Q.M.; et al. Upregulation of neuronal PGC-1α ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Theranostics 2020, 10, 2832–2848. [Google Scholar] [CrossRef] [PubMed]
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M.; Takahashi, H.; Ohta, S.; Ohsawa, I. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2010, 51, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Szaflik, J.P.; Szaflik, M.; Ulinska, M.; Szaflik, J.; Majsterek, I. Analysis of antioxidative factors related to AMD risk development in the polish patients. Acta Ophthalmol. 2017, 95, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.A.; Lin, C.M.; Rickman, C.B.; Yang, D. Lack of the P2X 7 receptor protects against AMD-like defects and microparticle accumulation in a chronic oxidative stress-induced mouse model of AMD. Biochem. Biophys. Res. Commun. 2017, 482, 81–86. [Google Scholar] [CrossRef]
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 11282–11287. [Google Scholar] [CrossRef] [Green Version]




| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| β-actin | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
| SOD1 | AATGTGTCCATTGAAGATCGTGTGA | GCTTCCAGCATTTCCAGTCTTTGTA |
| SOD2 | AGGGCCTGTCCCATGATGTC | AGAAACCCGTTTGCCTCTACTGAA |
| PGC-1α | GTGCAGCCAAGACTCTGTATGG | GTCCAGGTCATTCACATCAAGTT |
| NF-κB | TGGACGATCTGTTTCCCCTC | TCGCACTTGTAACGGAAACG |
| Iκ-Bα | TGACCATGGAAGTGATTGGTCAG | GATCACAGCCAAGTGGAGTGGA |
| IL-10 | GCAGGACTTTAAGGGTTACTTGG | GGGGAGAAATCGATGACAGC |
| VEGF-A | GCAGCGACAAGGCAGACTAT | GCAACCTCTCCAAACCGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasamatsu, M.; Arima, T.; Ikebukuro, T.; Nakano, Y.; Tobita, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Prophylactic Instillation of Hydrogen-Rich Water Decreases Corneal Inflammation and Promotes Wound Healing by Activating Antioxidant Activity in a Rat Alkali Burn Model. Int. J. Mol. Sci. 2022, 23, 9774. https://doi.org/10.3390/ijms23179774
Kasamatsu M, Arima T, Ikebukuro T, Nakano Y, Tobita Y, Uchiyama M, Shimizu A, Takahashi H. Prophylactic Instillation of Hydrogen-Rich Water Decreases Corneal Inflammation and Promotes Wound Healing by Activating Antioxidant Activity in a Rat Alkali Burn Model. International Journal of Molecular Sciences. 2022; 23(17):9774. https://doi.org/10.3390/ijms23179774
Chicago/Turabian StyleKasamatsu, Momoko, Takeshi Arima, Toyo Ikebukuro, Yuji Nakano, Yutaro Tobita, Masaaki Uchiyama, Akira Shimizu, and Hiroshi Takahashi. 2022. "Prophylactic Instillation of Hydrogen-Rich Water Decreases Corneal Inflammation and Promotes Wound Healing by Activating Antioxidant Activity in a Rat Alkali Burn Model" International Journal of Molecular Sciences 23, no. 17: 9774. https://doi.org/10.3390/ijms23179774
APA StyleKasamatsu, M., Arima, T., Ikebukuro, T., Nakano, Y., Tobita, Y., Uchiyama, M., Shimizu, A., & Takahashi, H. (2022). Prophylactic Instillation of Hydrogen-Rich Water Decreases Corneal Inflammation and Promotes Wound Healing by Activating Antioxidant Activity in a Rat Alkali Burn Model. International Journal of Molecular Sciences, 23(17), 9774. https://doi.org/10.3390/ijms23179774






