Cd0.9Co0.1S Nanorods with an Internal Electric Field and Photothermal Effect Synergistically for Boosting Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
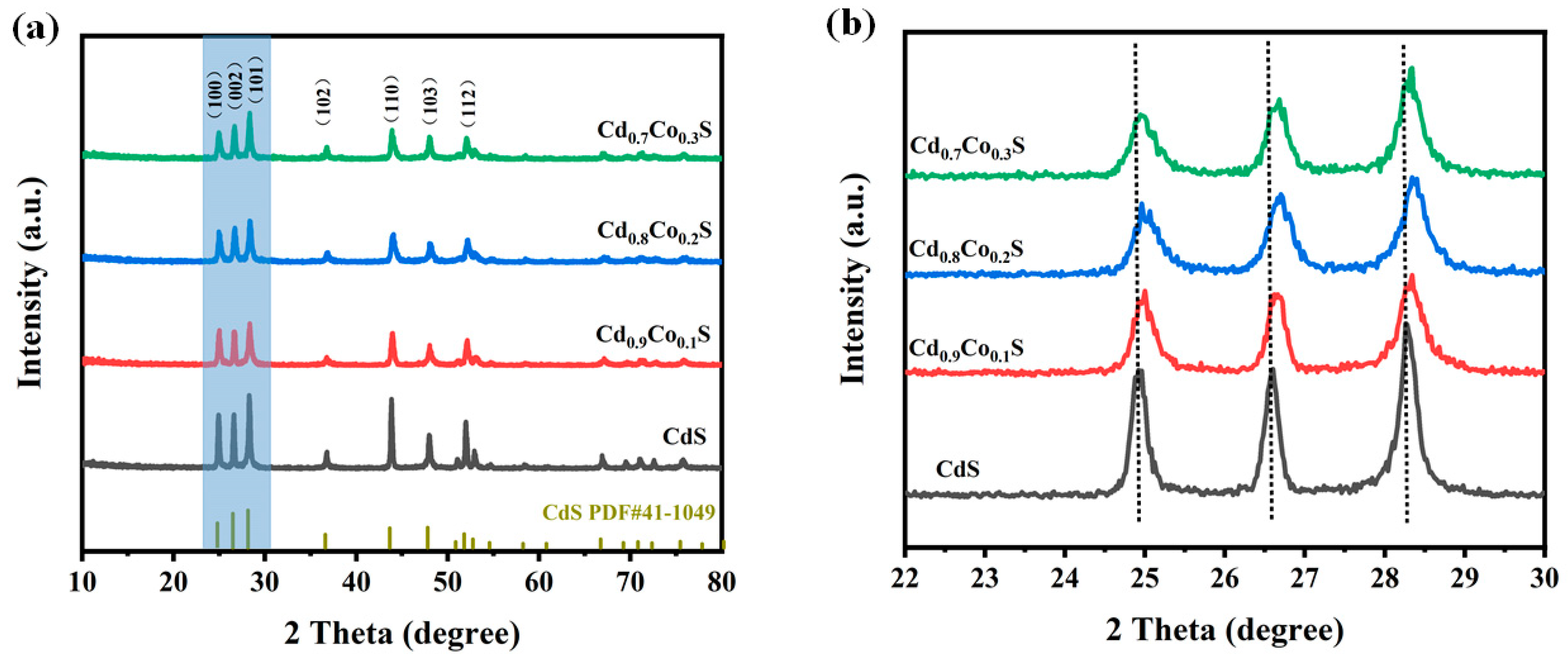
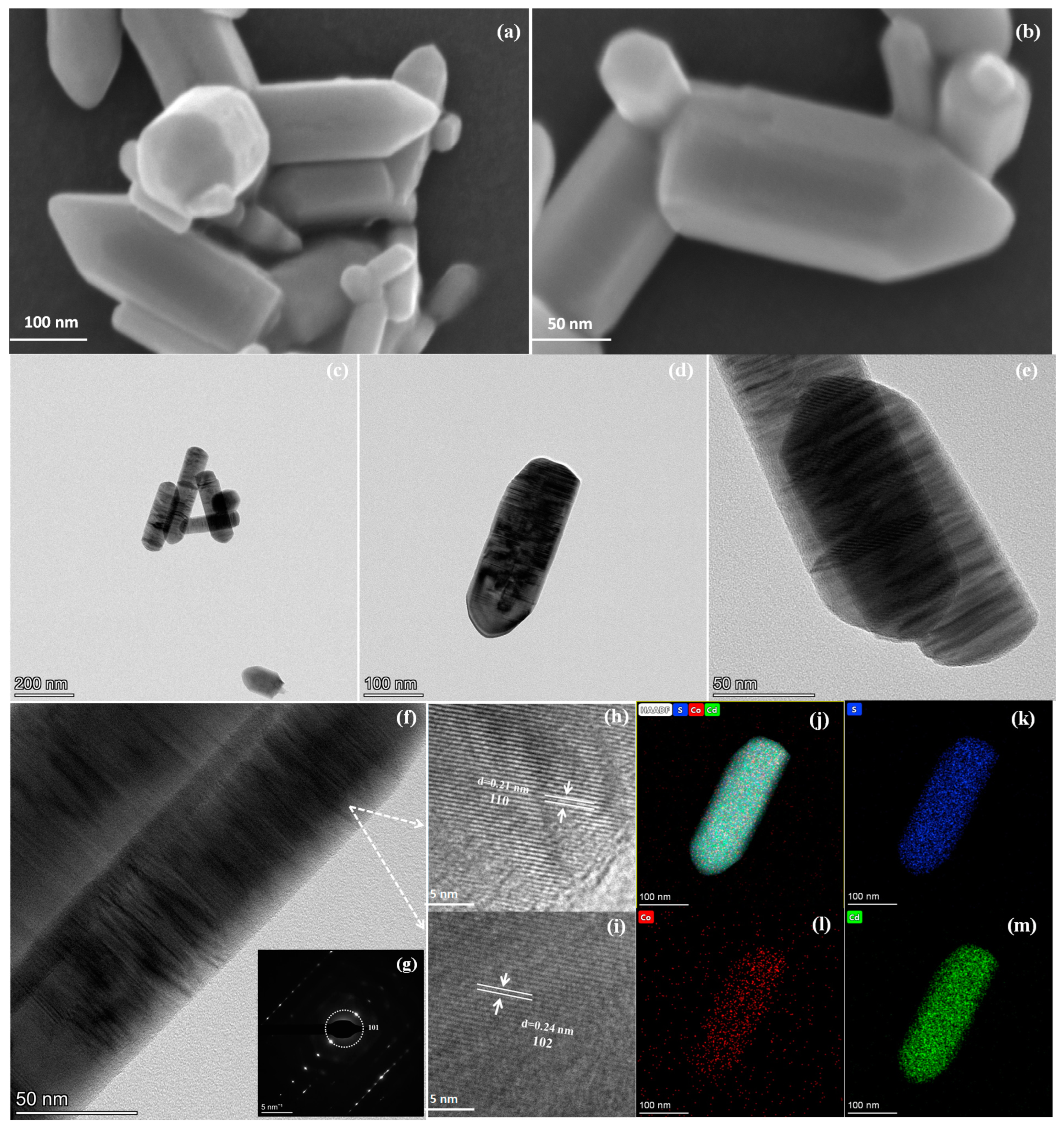
2.1. Structure and Morphology Analysis of Photocatalysts
2.2. Photocatalytic H2 Evolution Performance
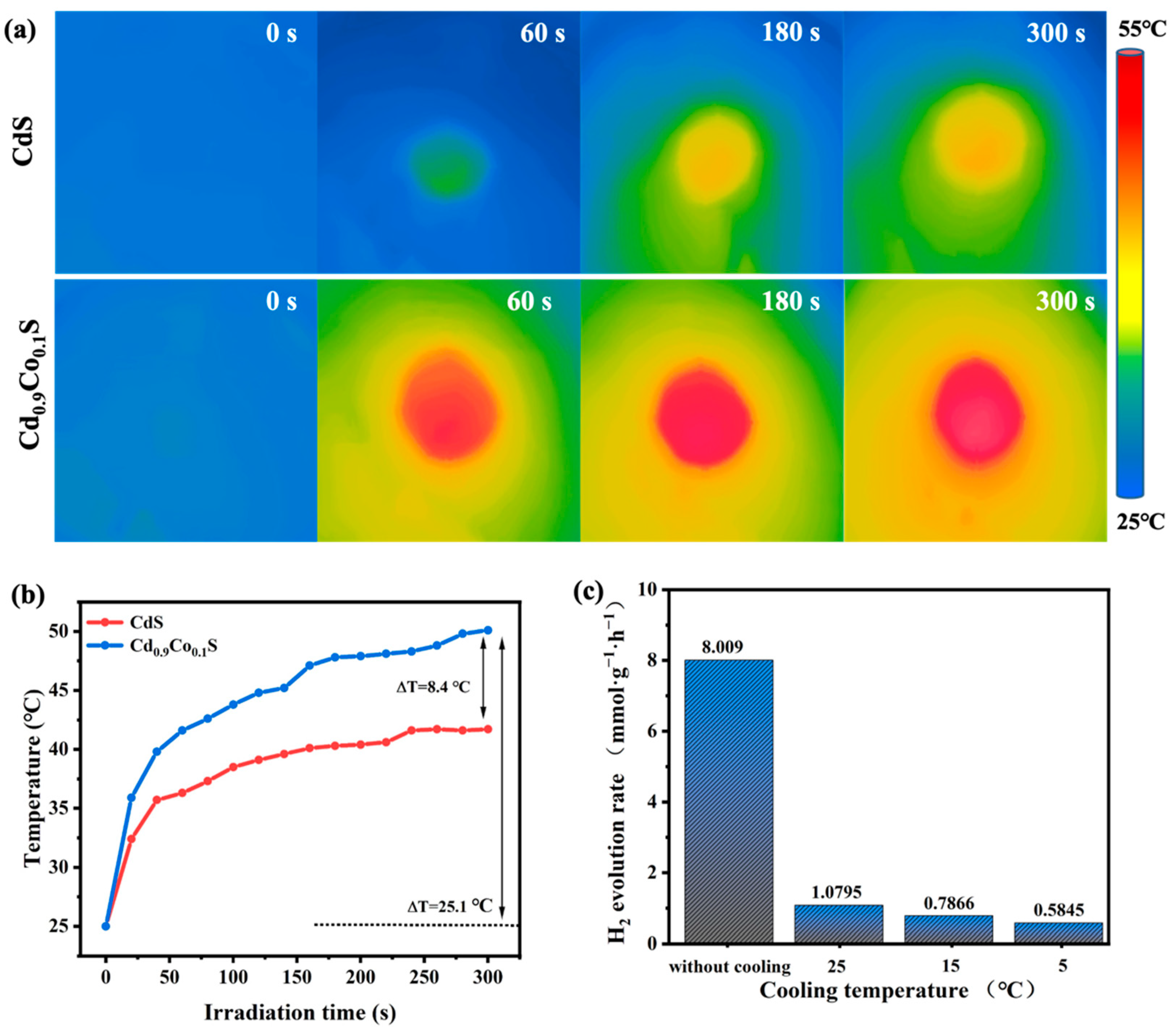
2.3. Photothermal Effect
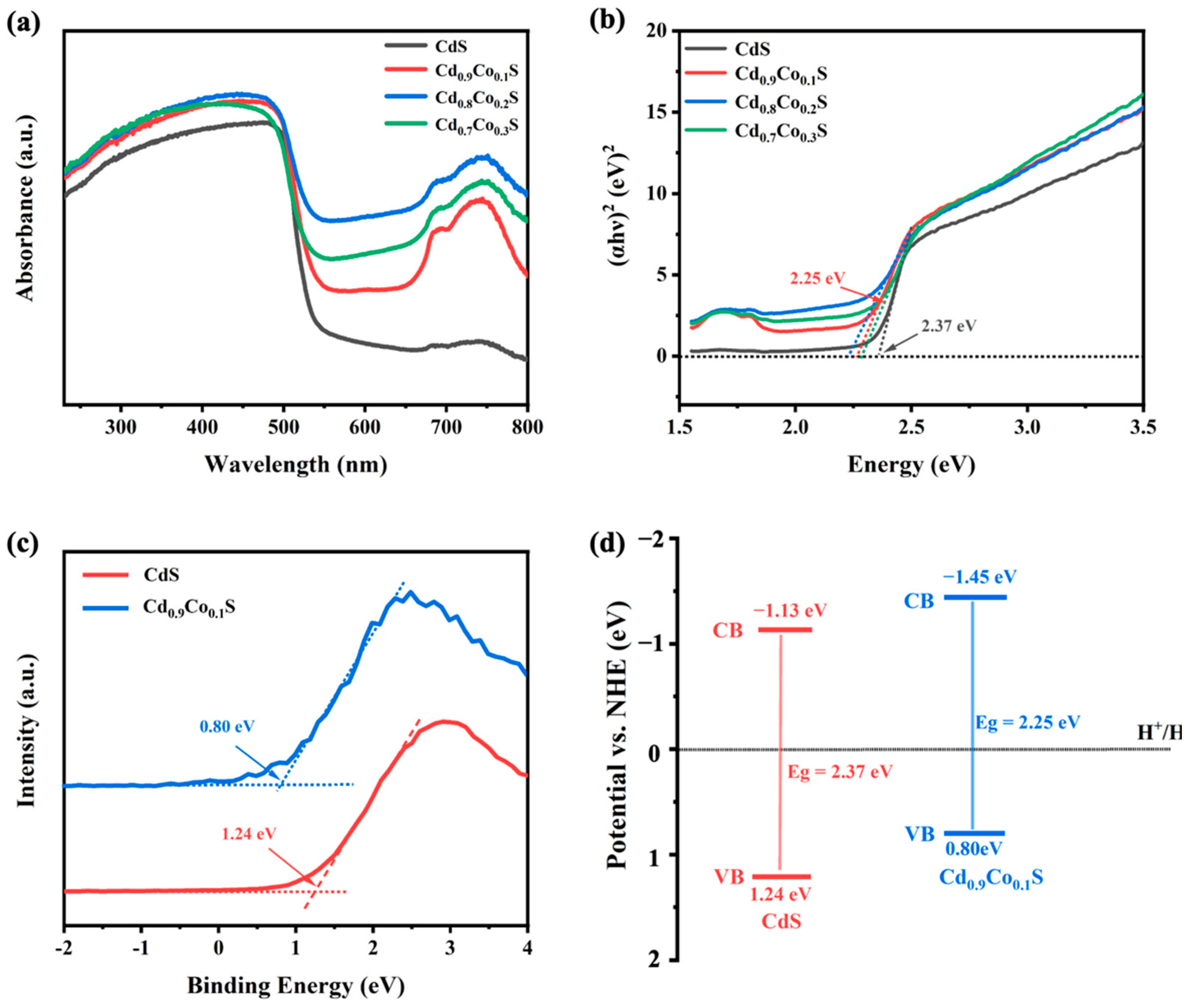
2.4. Optical Property and Band Structure
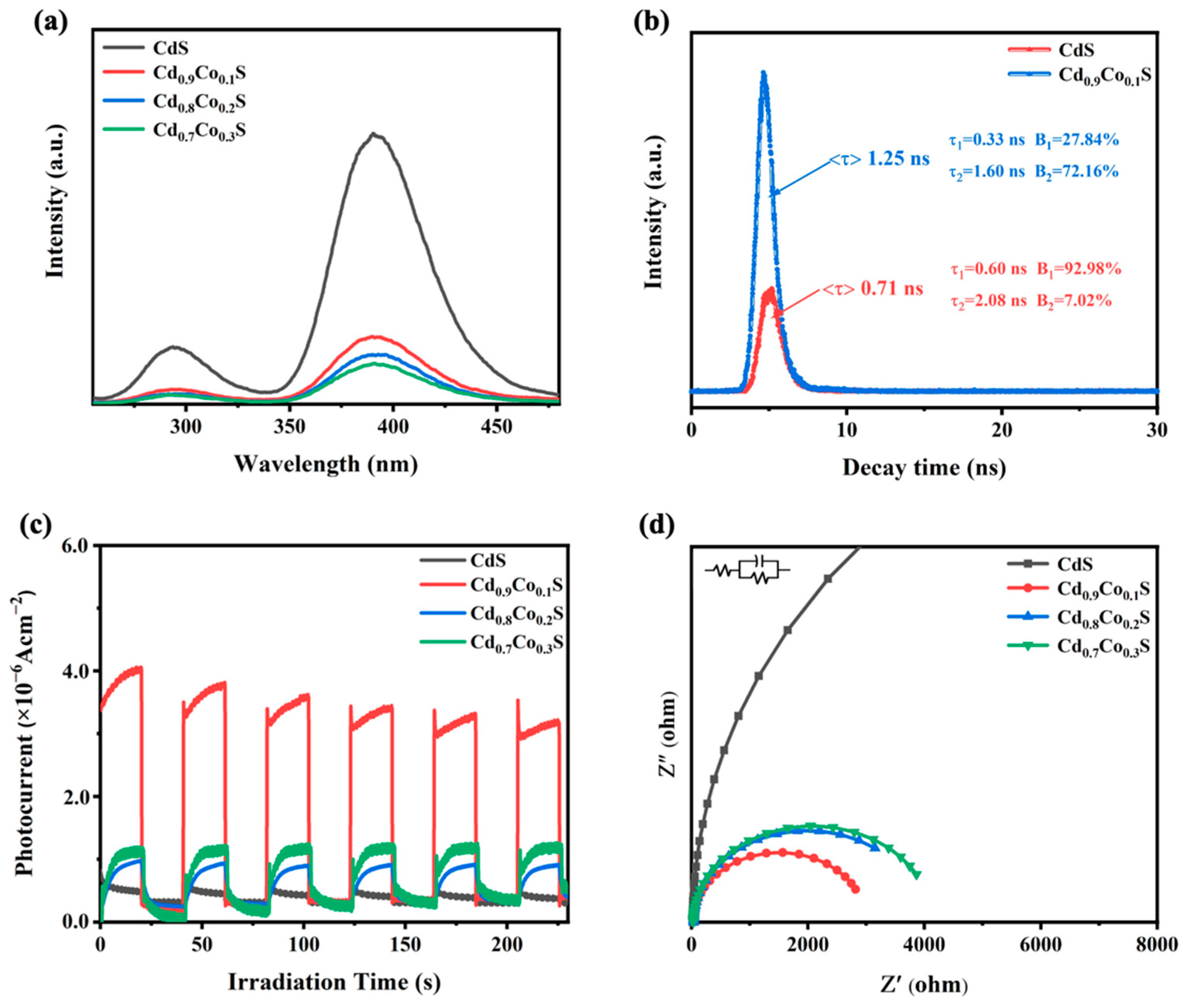
2.5. Separation and Transfer of Charge Carriers
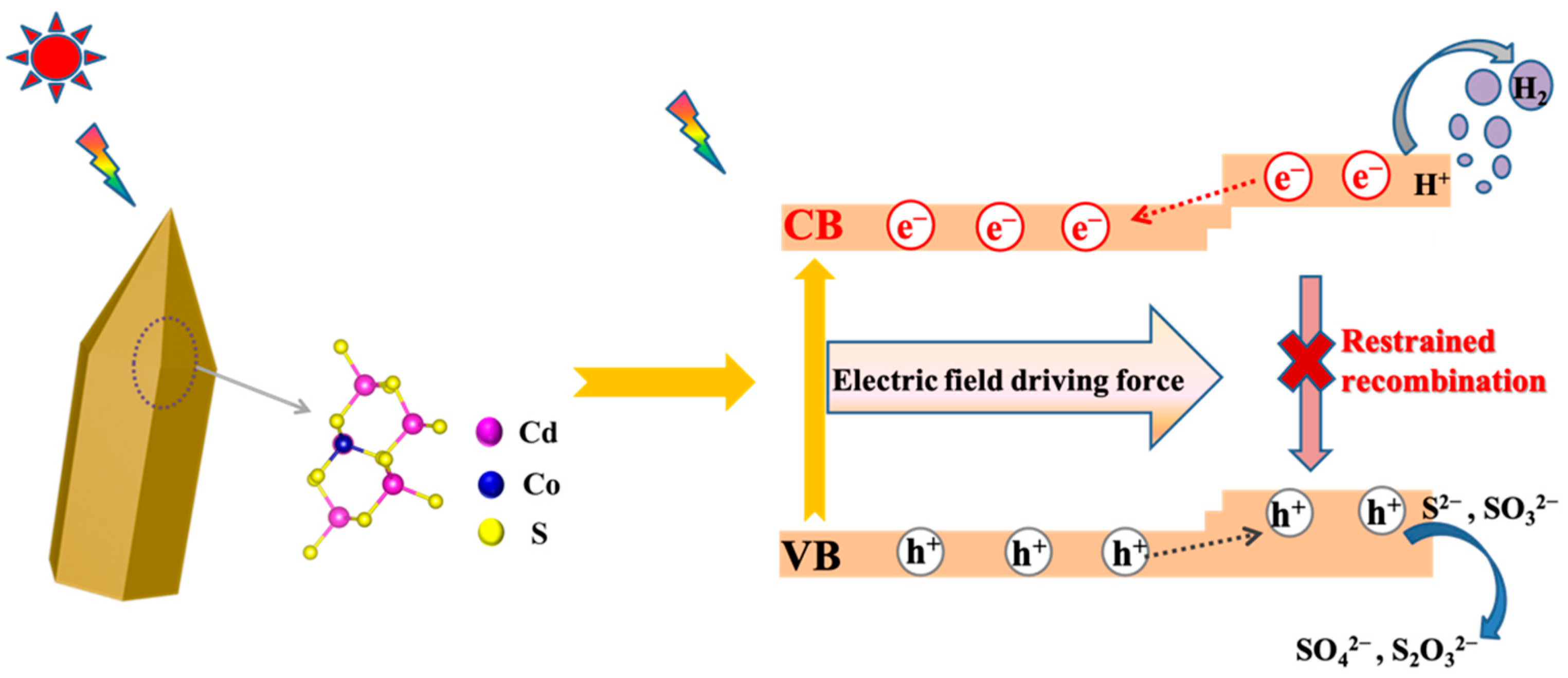
2.6. Photocatalytic Mechanism
3. Materials and Methods
3.1. Characterization
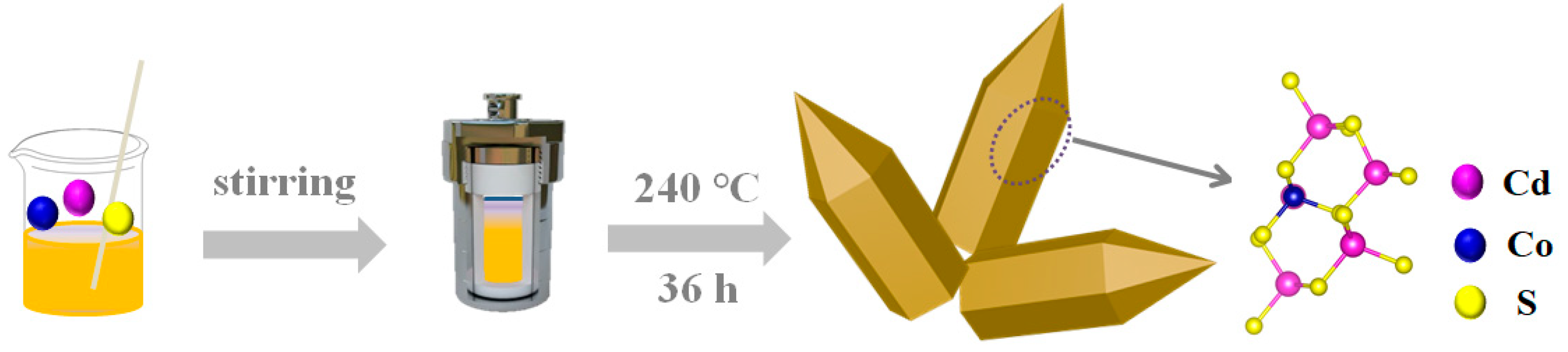
3.2. Synthesis of CdCoS NRs
3.3. Photocatalytic H2 Evolution
3.4. The Apparent Quantum Efficiency Analysis
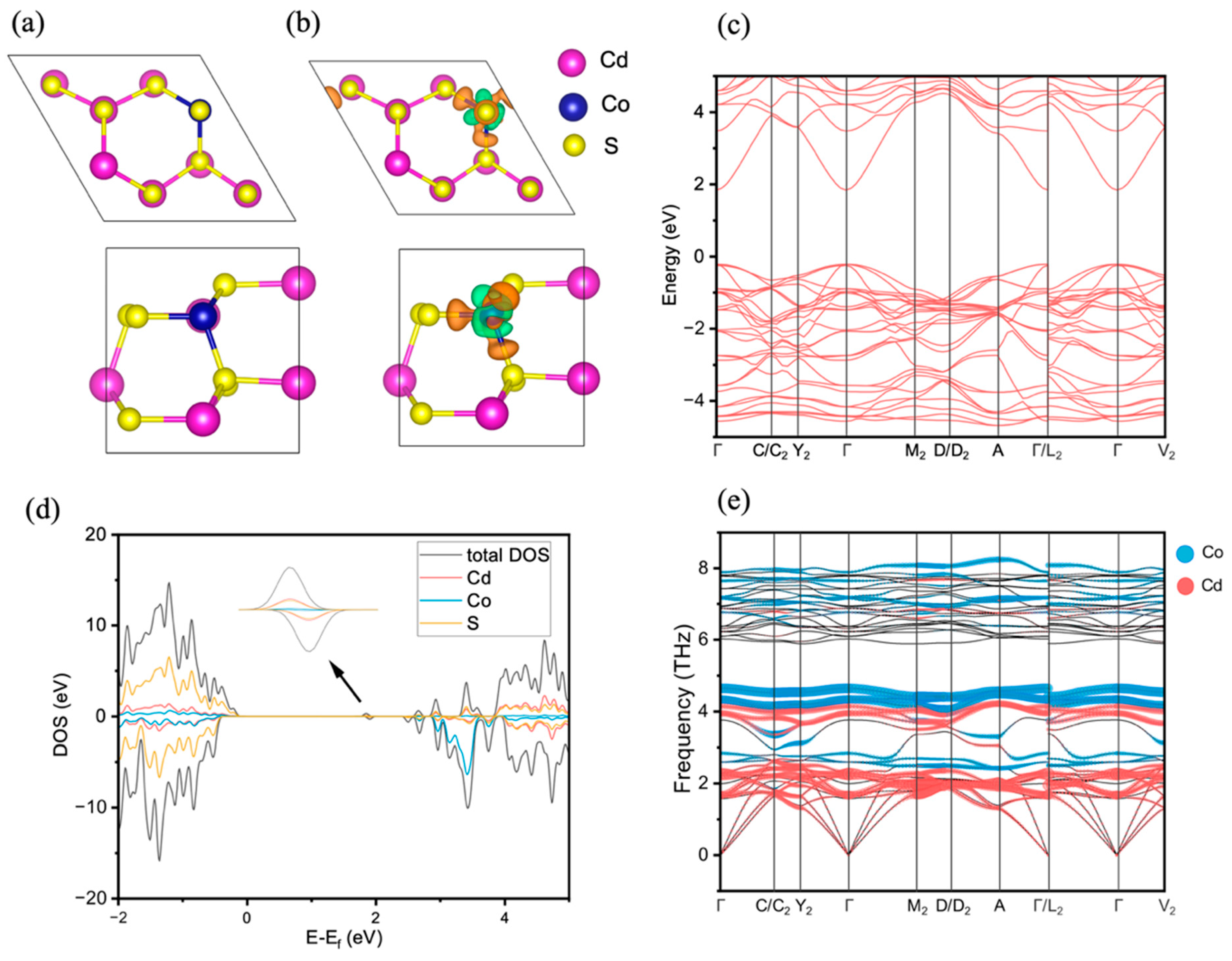
3.5. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Zhang, H.; Du, Y.; Jia, G.; Wang, S.; Yin, Z. Structural-Phase Catalytic Redox Reactions in Energy and Environmental Applications. Adv. Mater. 2020, 32, e1905739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Y.; Zhou, W.; Wang, X.; Chang, K.; Liu, G.; Liu, H.; Kako, T.; Ye, J. Superior Photocatalytic H2 Production with Cocatalytic Co/Ni Species Anchored on Sulfide Semiconductor. Adv. Mater. 2017, 29, 1703258. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.; Kang, M. Strategy for improving the visible photocatalytic H2 evolution activity of 2D graphitic carbon nitride nanosheets through the modification with metal and metal oxide nanocomponents. Appl. Catal. B-Environ. 2019, 248, 538–551. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.S.; Tong, Y.; Huang, Y. Oxygen vacancy–based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.-S.; Wang, P.; Tong, Y.; Huang, Y. Charge Relays via Dual Carbon-Actions on Nanostructured BiVO4 for High Performance Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B-Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Q.; Xu, Z.; Shang, Q.; Wang, L.; Chao, Y.; Li, Y.; Chen, H.; Lv, F.; Zhang, Q.; et al. Atomically Dispersed Co-P3 on CdS Nanorods with Electron-Rich Feature Boosts Photocatalysis. Adv. Mater. 2020, 32, e1904249. [Google Scholar] [CrossRef]
- Tian, L.; Min, S.; Wang, F. Integrating noble-metal-free metallic vanadium carbide cocatalyst with CdS for efficient visible-light-driven photocatalytic H2 evolution. Appl. Catal. B-Environ. 2019, 259, 118029. [Google Scholar] [CrossRef]
- Zhang, P.; Luan, D.; Lou, X.W.D. Fabrication of CdS Frame-in-Cage Particles for Efficient Photocatalytic Hydrogen Generation under Visible-Light Irradiation. Adv. Mater. 2020, 32, e2004561. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Unraveling Photoexcited Charge Transfer Pathway and Process of CdS/Graphene Nanoribbon Composites toward Visible-Light Photocatalytic Hydrogen Evolution. Small 2019, 15, e1902459. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, S.; Ng, Y.H.; Gao, Q.; Yang, S.; Zhang, S.; Peng, F.; Fang, Y.; Zhang, S. ZnO/CdS/PbS nanotube arrays with multi-heterojunctions for efficient visible-light-driven photoelectrochemical hydrogen evolution. Chem. Eng. J. 2019, 362, 658–666. [Google Scholar] [CrossRef]
- Feng, R.; Wan, K.; Sui, X.; Zhao, N.; Li, H.; Lei, W.; Yu, J.; Liu, X.; Shi, X.; Zhai, M.; et al. Anchoring single Pt atoms and black phosphorene dual co-catalysts on CdS nanospheres to boost visible-light photocatalytic H2 evolution. Nano Today 2021, 37, 101080. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Jiang, Y.; Ni, P.; Zhang, C.; Yang, Y.; Lu, Y.; Liu, P. Rational designing 0D/1D Z-scheme heterojunction on CdS nanorods for efficient visible-light-driven photocatalytic H2 evolution. Chem. Eng. J. 2021, 412, 128690. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Q.; Ma, K.; Ding, Y.; Li, C. Pyroelectric effect in CdS nanorods decorated with a molecular Co-catalyst for hydrogen evolution. Nano Energy 2020, 73, 104801. [Google Scholar] [CrossRef]
- Lu, X.; Chen, W.; Yao, Y.; Wen, X.; Hart, J.N.; Tsounis, C.; Ying Toe, C.; Scott, J.; Ng, Y.H. Photogenerated charge dynamics of CdS nanorods with spatially distributed MoS2 for photocatalytic hydrogen generation. Chem. Eng. J. 2021, 420, 127709. [Google Scholar] [CrossRef]
- Ai, Z.; Zhang, K.; Chang, B.; Shao, Y.; Zhang, L.; Wu, Y.; Hao, X. Construction of CdS@Ti3C2@CoO hierarchical tandem p-n heterojunction for boosting photocatalytic hydrogen production in pure water. Chem. Eng. J. 2020, 383, 123130. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Liu, M.-L.; Li, D.-C.; Shang, L.; Dou, J.-M. MoSx/CdS nano-heterostructures accurately constructed on the defects of CdS for efficient photocatalytic H2 evolution under visible light irradiation. Chem. Eng. J. 2019, 370, 305–313. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, F.; Cheng, Y.; Dewangan, N.; Ho, G.W.; Kawi, S. Z-scheme transition metal bridge of Co9S8/Cd/CdS tubular heterostructure for enhanced photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2021, 286, 119853. [Google Scholar] [CrossRef]
- Shi, J.-W.; Sun, D.; Zou, Y.; Ma, D.; He, C.; Ji, X.; Niu, C. Trap-level-tunable Se doped CdS quantum dots with excellent hydrogen evolution performance without co-catalyst. Chem. Eng. J. 2019, 364, 11–19. [Google Scholar] [CrossRef]
- Huang, H.; Dai, B.; Wang, W.; Lu, C.; Kou, J.; Ni, Y.; Wang, L.; Xu, Z. Oriented Built-in Electric Field Introduced by Surface Gradient Diffusion Doping for Enhanced Photocatalytic H2 Evolution in CdS Nanorods. Nano Lett. 2017, 17, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, L.; Chen, F.; Ning, J.; Zhong, Y.; Hu, Y. One-step phosphorization preparation of gradient-P-doped CdS/CoP hybrid nanorods having multiple channel charge separation for photocatalytic reduction of water. J. Colloid Interface Sci. 2021, 596, 431–441. [Google Scholar] [CrossRef]
- Yan, J.; Wei, Z.; Xu, M.; Jiang, Z.; Shangguan, W. Polyoxometalate Template-Based Synthetic Strategy to Prepare Ni, Mo Co-Doped CdS for Efficient Photocatalytic Hydrogen Evolution from Water Splitting. Catalysts 2020, 10, 1478. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Jiang, H.; Xing, Z.; Zhao, T.; Yang, Z.; Wang, K.; Li, Z.; Yang, S.; Xie, L.; Zhou, W. Plasmon Ag nanoparticle/Bi2S3 ultrathin nanobelt/oxygen-doped flower-like MoS2 nanosphere ternary heterojunctions for promoting charge separation and enhancing solar-driven photothermal and photocatalytic performances. Appl. Catal. B-Environ. 2020, 274, 118947. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Shen, Q.; Jia, S.; Li, Q.; Li, Y.; Liu, X.; Jia, H. Construction of a ternary spatial junction in yolk–shell nanoreactor for efficient photo-thermal catalytic hydrogen generation. Chem. Eng. J. 2021, 423, 130188. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, T.; Xing, Z.; Qiu, Y.; Pan, K.; Li, Z.; Yang, S.; Zhou, W. Hollow Octahedral Cu2-xS/CdS/Bi2S3 p-n-p Type Tandem Heterojunctions for Efficient Photothermal Effect and Robust Visible-Light-Driven Photocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 40328–40338. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Lin, Y.; Mao, Y.; Ouyang, G.; Liu, H.; Zhang, S.; Tong, Y. Cerium-based hybrid nanorods for synergetic photo-thermocatalytic degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 24740–24747. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Yang, C.; Ding, X.; Zhang, Y.; Zheng, Y.-Z.; Li, S.; Sun, X.; Tao, X. Large-Size, Porous, Ultrathin NiCoP Nanosheets for Efficient Electro/Photocatalytic Water Splitting. Adv. Funct. Mater. 2020, 30, 1910830. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energ. Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Hu, J.; Liu, H.; Li, J. One-pot synthesis of CdS and Ni-doped CdS hollow spheres with enhanced photocatalytic activity and durability. ACS Appl. Mater. Interfaces 2012, 4, 1813–1821. [Google Scholar] [PubMed]
- Deka, K.; Kalita, M.P.C. Structural phase controlled transition metal (Fe, Co, Ni, Mn) doping in CdS nanocrystals and their optical, magnetic and photocatalytic properties. J. Alloys Comp. 2018, 757, 209–220. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, C.; Liu, J.; Qiao, W.; Zhao, J.; Yang, J.; Yu, Y.; Chen, S.; Qin, Y. Simultaneous Ni nanoparticles decoration and Ni doping of CdS nanorods for synergistically promoting photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 508, 144869. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, S.L.; Zang, S.Q.; Lou, X.W.D. Supporting Ultrathin ZnIn2S4 Nanosheets on Co/N-Doped Graphitic Carbon Nanocages for Efficient Photocatalytic H2 Generation. Adv. Mater. 2019, 31, e1903404. [Google Scholar] [CrossRef]
- Ning, X.; Wu, Y.; Ma, X.; Zhang, Z.; Gao, R.; Chen, J.; Shan, D.; Lu, X. A Novel Charge Transfer Channel to Simultaneously Enhance Photocatalytic Water Splitting Activity and Stability of CdS. Adv. Func. Mater. 2019, 29, 1902992. [Google Scholar] [CrossRef]
- Li, N.; Ding, Y.; Wu, J.; Zhao, Z.; Li, X.; Zheng, Y.Z.; Huang, M.; Tao, X. Efficient, Full Spectrum-Driven H2 Evolution Z-Scheme Co2P/CdS Photocatalysts with Co-S Bonds. ACS Appl. Mater. Interfaces 2019, 11, 22297–22306. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, Y.; Zhang, L.; Wang, A.; Xiao, N.; Gao, Y.; Li, N.; Song, W.; Ge, L.; et al. Novel photocatalyst incorporating Ni-Co layered double hydroxides with P-doped CdS for enhancing photocatalytic activity towards hydrogen evolution. Appl. Catal. B-Environ. 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Ren, X.; Ji, X.; Liu, Y.; Guo, X.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X. Co(OH)2 Nanoparticle-Encapsulating Conductive Nanowires Array: Room-Temperature Electrochemical Preparation for High-Performance Water Oxidation Electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef]
- Qiu, B.; Zhu, Q.; Du, M.; Fan, L.; Xing, M.; Zhang, J. Efficient Solar Light Harvesting CdS/Co9 S8 Hollow Cubes for Z-Scheme Photocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2017, 56, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Liu, M.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W. In situ photodeposition of cobalt on CdS nanorod for promoting photocatalytic hydrogen production under visible light irradiation. Appl. Surf. Sci. 2018, 444, 485–490. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, Y.; Duan, X.; Xu, X.; Liu, S.; Sun, H.; Wang, S. Synergy of NiO quantum dots and temperature on enhanced photocatalytic and thermophoto hydrogen evolution. Chem. Eng. J. 2020, 390, 124634. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Li, J.; Zhang, H.; Duan, X.; Sun, H.; Huang, Y.; Fang, Y.; Wang, S. Temperature-Induced Variations in Photocatalyst Properties and Photocatalytic Hydrogen Evolution: Differences in UV, Visible, and Infrared Radiation. ACS Sustain. Chem. Eng. 2021, 9, 7277–7285. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Wang, J.; Meng, F.; Cao, S.; He, B.; Jia, S.; Wang, Y.; Li, Z.; Liu, X. Promoting Oxygen Evolution Reaction of Co-Based Catalysts (Co3O4, CoS, CoP, and CoN) through Photothermal Effect. Small 2019, 15, 1903847. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Q.; Guo, W.; Liu, H.; Ma, T.; Qu, F. Co2.67S4-Based Photothermal Membrane with High Mechanical Properties for Efficient Solar Water Evaporation and Photothermal Antibacterial Applications. ACS Appl. Mater. Interfaces 2019, 11, 20820–20827. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Guan, Z.; Yang, J.; Li, Q. Spatially Separating Redox Centers and Photothermal Effect Synergistically Boosting the Photocatalytic Hydrogen Evolution of ZnIn2 S4 Nanosheets. Small 2021, 17, e2006952. [Google Scholar] [CrossRef]
- Lu, L.; Xu, X.; An, K.; Wang, Y.; Shi, F.-N. Coordination Polymer Derived NiS@g-C3N4 Composite Photocatalyst for Sulfur Vacancy and Photothermal Effect Synergistic Enhanced H2 Production. ACS Sustain. Chem. Eng. 2018, 6, 11869–11876. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Z.; Meng, D.; Zhang, S.; Li, Z.; Pan, K.; Zhou, W. Hollow MoSe2@Bi2S3/CdS Core-Shell Nanostructure as Dual Z-Scheme Heterojunctions with Enhanced Full Spectrum Photocatalytic-Photothermal Performance. Appl. Catal. B-Environ. 2021, 281, 119482. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.; Yu, W.; Si, R.; Liu, Y.; Zhao, Z. Modulating Location of Single Copper Atoms in Polymeric Carbon Nitride for Enhanced Photoredox Catalysis. ACS Catalysis 2020, 10, 5715–5722. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2020, 268, 118382. [Google Scholar] [CrossRef]
- Yang, X.; Qian, F.; Wang, Y.; Li, M.; Lu, J.; Li, Y.; Bao, M. Constructing a novel ternary composite (C16H33(CH3)3N)4W10O32/g-C3N4/rGO with enhanced visible-light-driven photocatalytic activity for degradation of dyes and phenol. Appl. Catal. B-Environ. 2017, 200, 283–296. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Han, M.; Lu, S.; Qi, F.; Zhu, S.; Sun, H.; Yang, B. Carbon Dots–Implanted Graphitic Carbon Nitride Nanosheets for Photocatalysis: Simultaneously Manipulating Carrier Transport in Inter- and Intralayers. Solar RRL 2020, 4, 2070041. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Tong, T.; Chen, H.-C.; Yu, A.; Yu, J.; Chen, H.M. Single-Atom Engineering of Directional Charge Transfer Channels and Active Sites for Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28, 1870224. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Xie, J.; Zhang, X.; Liu, Q.; Yao, T.; Wei, S.; Zhang, Q.; Xie, Y. Enhanced Photoexcited Carrier Separation in Oxygen-Doped ZnIn2 S4 Nanosheets for Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2016, 55, 6716–6720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, J.; Zhang, B.; Cao, S.; Wu, Y.; Gao, Z.; Nie, X.; Sun, L. Two-dimensional Janus heterostructures for superior Z-scheme photocatalytic water splitting. Nano Energy 2019, 59, 537–544. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Q.; Shi, L.; Yao, Q.; Wang, X.; Yu, A.; Chen, Z.; Fu, Y. Merging Single-Atom-Dispersed Iron and Graphitic Carbon Nitride to a Joint Electronic System for High-Efficiency Photocatalytic Hydrogen Evolution. Small 2019, 15, e1905166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Xia, Y.; Xun, M.; Chen, H.; Liu, X.; Yin, X. Metal-Free Carbon Quantum Dots Implant Graphitic Carbon Nitride: Enhanced Photocatalytic Dye Wastewater Purification with Simultaneous Hydrogen Production. Int. J. Mol. Sci. 2020, 21, 1052. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Qi, M.; Guo, X.; Feng, X. First-Principles Investigation of Single-Atom Ni–g-C3N4 as an Efficient Catalyst for Direct Reduction of NO with CO. Energ. Fuel. 2020, 34, 12792–12799. [Google Scholar] [CrossRef]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Xu, J.; Ju, Z.; Zhang, W.; Pan, Y.; Zhu, J.; Mao, J.; Zheng, X.; Fu, H.; Yuan, M.; Chen, H.; et al. Efficient Infrared-Light-Driven CO2 Reduction Over Ultrathin Metallic Ni-doped CoS2 Nanosheets. Angew. Chem. Int. Ed. Engl. 2021, 60, 8705–8709. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Li, N.; Wu, J.; Zheng, Y.-Z.; Tao, X. One-step hydrothermal synthesis of high-percentage 1T-phase MoS2 quantum dots for remarkably enhanced visible-light-driven photocatalytic H2 evolution. Appl. Catal. B-Environ. 2019, 243, 76–85. [Google Scholar] [CrossRef]
- Zhao, Q.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Effective and Durable Co Single Atomic Cocatalysts for Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 42734–42741. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, C.; Ai, L.; Jiang, J. Boosting charge transfer and hydrogen evolution performance of CdS nanocrystals hybridized with MoS2 nanosheets under visible light irradiation. Appl. Surf. Sci. 2019, 484, 692–700. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Gu, W.; Cai, X.; Mao, B.; Li, D.; Shi, W. In-situ construction of hierarchical CdS/MoS2 microboxes for enhanced visible-light photocatalytic H2 production. Chem. Eng. J. 2018, 339, 117–124. [Google Scholar] [CrossRef]
- Li, H.; Pan, J.; Zhao, W.; Li, C. The 2D nickel-molybdenum bimetals sulfide synergistic modified hollow cubic CdS towards enhanced photocatalytic water splitting hydrogen production. Appl. Surf. Sci. 2019, 497, 143769. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Liu, L.; Hu, J.; Wang, H.; An, W.; Cui, W. Core-shell structure of sulphur vacancies-CdS@CuS: Enhanced photocatalytic hydrogen generation activity based on photoinduced interfacial charge transfer. J. Colloid Interface Sci. 2021, 600, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Fujitsuka, M.; Majima, T. Exfoliated Mo2C nanosheets hybridized on CdS with fast electron transfer for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B-Environ. 2020, 264, 118541. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Nitrogen-doped hydrogenated TiO2 modified with CdS nanorods with enhanced optical absorption, charge separation and photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 384, 123275. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Shao, L.; Xia, X.; Liu, Y.; Wang, L. Oriented facet heterojunctions on CdS nanowires with high photoactivity and photostability for water splitting. Appl. Catal. B-Environ. 2020, 268, 118744. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.L.; Li, D.C.; Wei, D.H.; Hu, C.C.; Dou, J.M. Room temperature synthesis of CdS/SrTiO3 nanodots-on-nanocubes for efficient photocatalytic H2 evolution from water. J. Colloid Interface Sci. 2019, 536, 694–700. [Google Scholar] [CrossRef]
- Luo, X.; Ke, Y.; Yu, L.; Wang, Y.; Homewood, K.P.; Chen, X.; Gao, Y. Tandem CdS/TiO2(B) nanosheet photocatalysts for enhanced H2 evolution. Appl. Surf. Sci. 2020, 515, 145970. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Hong, M.; Zhang, K.; Li, B.; Fang, H.; Feng, X.; Xiao, X. Cd0.9Co0.1S Nanorods with an Internal Electric Field and Photothermal Effect Synergistically for Boosting Photocatalytic H2 Evolution. Int. J. Mol. Sci. 2022, 23, 9756. https://doi.org/10.3390/ijms23179756
Zhang L, Hong M, Zhang K, Li B, Fang H, Feng X, Xiao X. Cd0.9Co0.1S Nanorods with an Internal Electric Field and Photothermal Effect Synergistically for Boosting Photocatalytic H2 Evolution. International Journal of Molecular Sciences. 2022; 23(17):9756. https://doi.org/10.3390/ijms23179756
Chicago/Turabian StyleZhang, Lilei, Manzhou Hong, Ka Zhang, Botan Li, Haipeng Fang, Xun Feng, and Xiuchan Xiao. 2022. "Cd0.9Co0.1S Nanorods with an Internal Electric Field and Photothermal Effect Synergistically for Boosting Photocatalytic H2 Evolution" International Journal of Molecular Sciences 23, no. 17: 9756. https://doi.org/10.3390/ijms23179756
APA StyleZhang, L., Hong, M., Zhang, K., Li, B., Fang, H., Feng, X., & Xiao, X. (2022). Cd0.9Co0.1S Nanorods with an Internal Electric Field and Photothermal Effect Synergistically for Boosting Photocatalytic H2 Evolution. International Journal of Molecular Sciences, 23(17), 9756. https://doi.org/10.3390/ijms23179756




