Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223
Abstract
:1. Introduction
2. Results
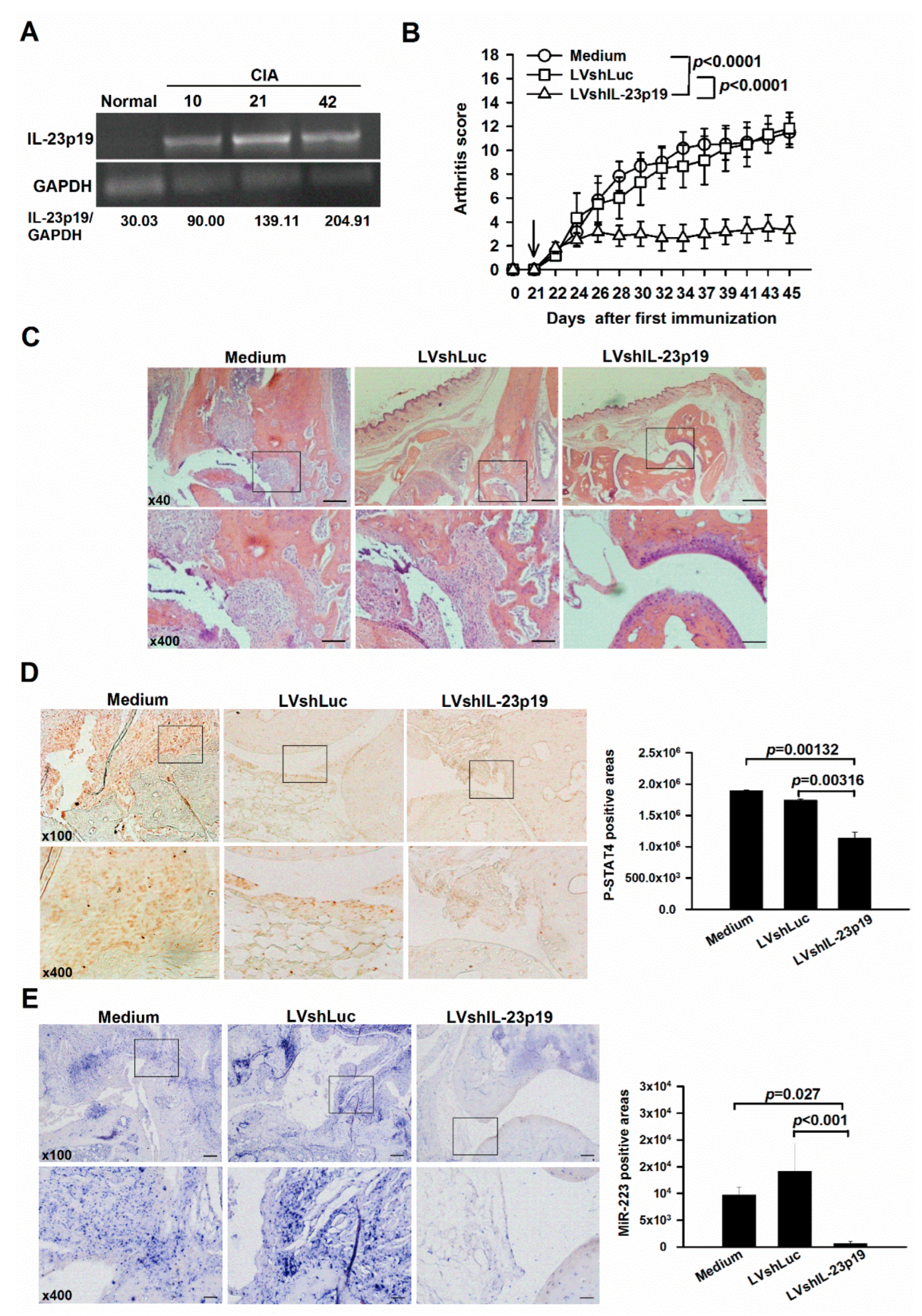
2.1. Amelioration of Arthritis by IL-23p19 Silencing Concomitantly with Down-Regulation of miR-223 in Mice with CIA
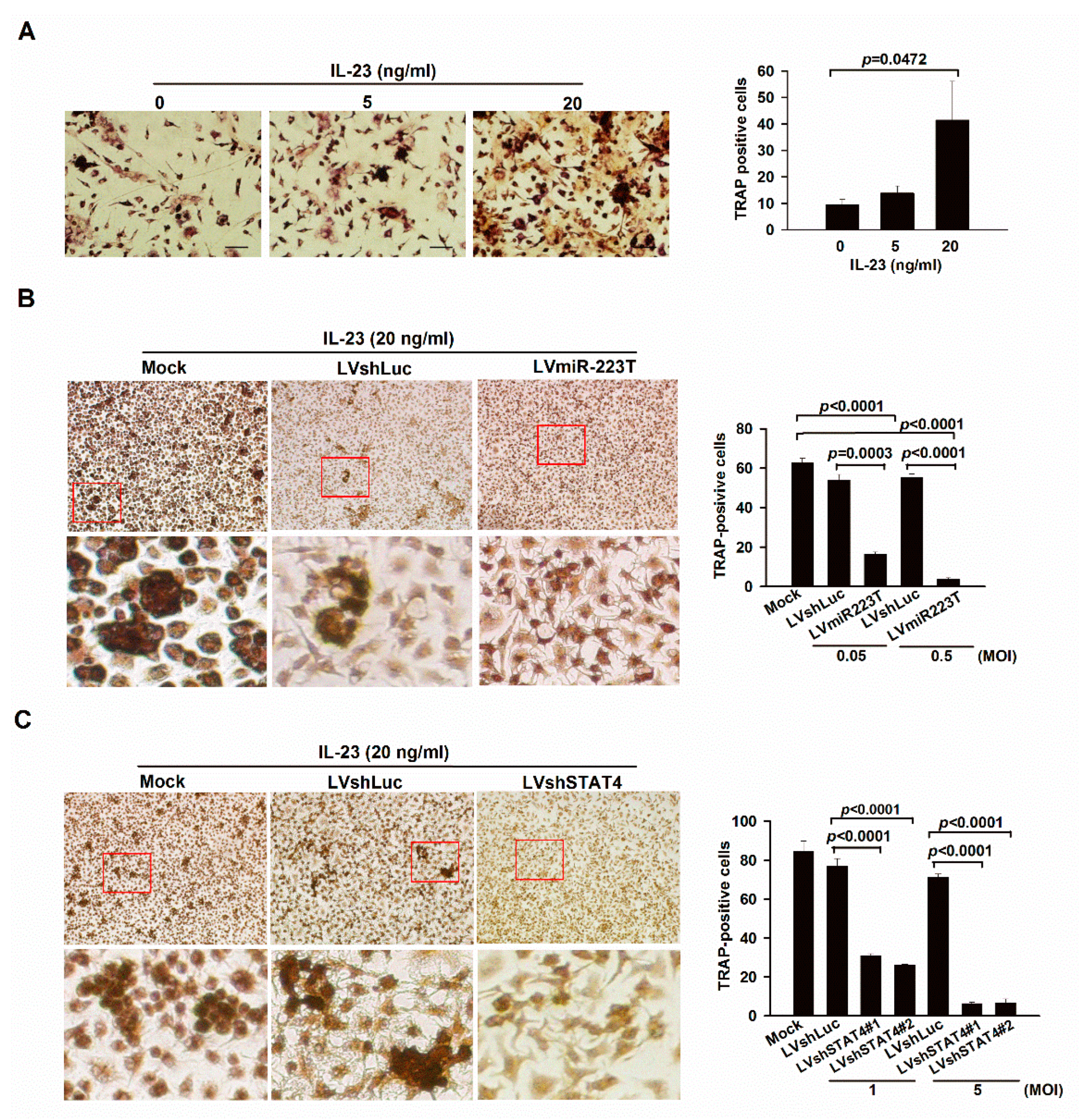
2.2. IL-23 Signaling Promotes Osteoclastogenesis in BMMs through miR-223
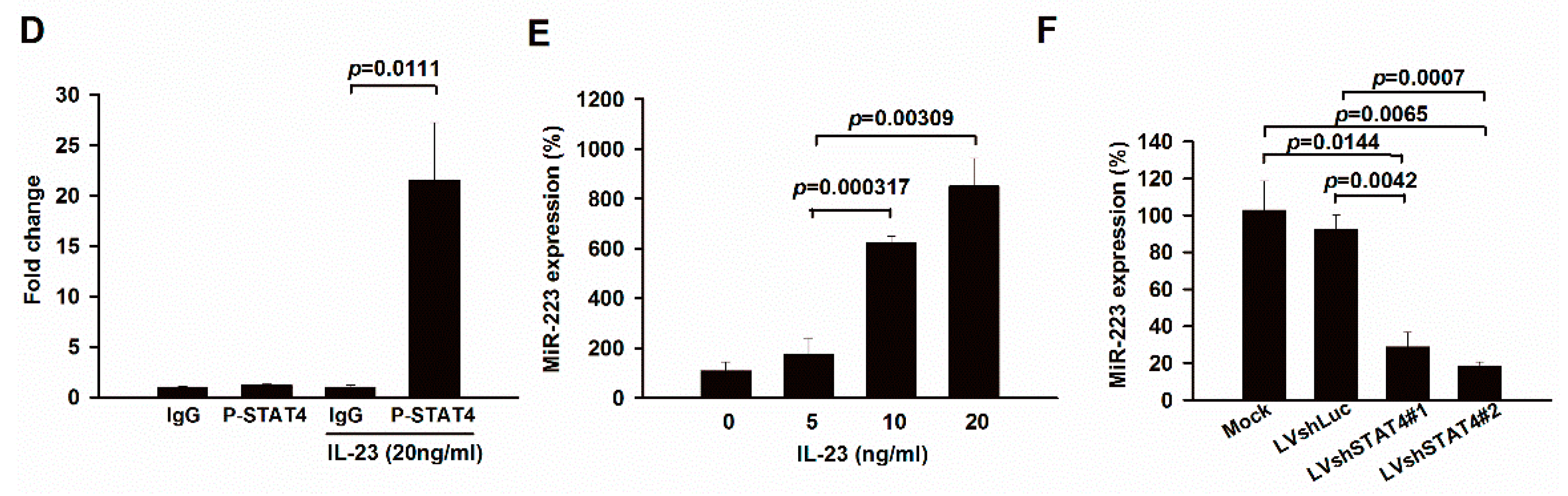
2.3. Up-Regulation of miR-223 by IL-23 Signaling in Murine Macrophages
2.4. LSF Treatment Ameliorates Arthritis through Down-Regulating miR-223-Associated Osteoclastogenesis in BMMs and Mice with CIA
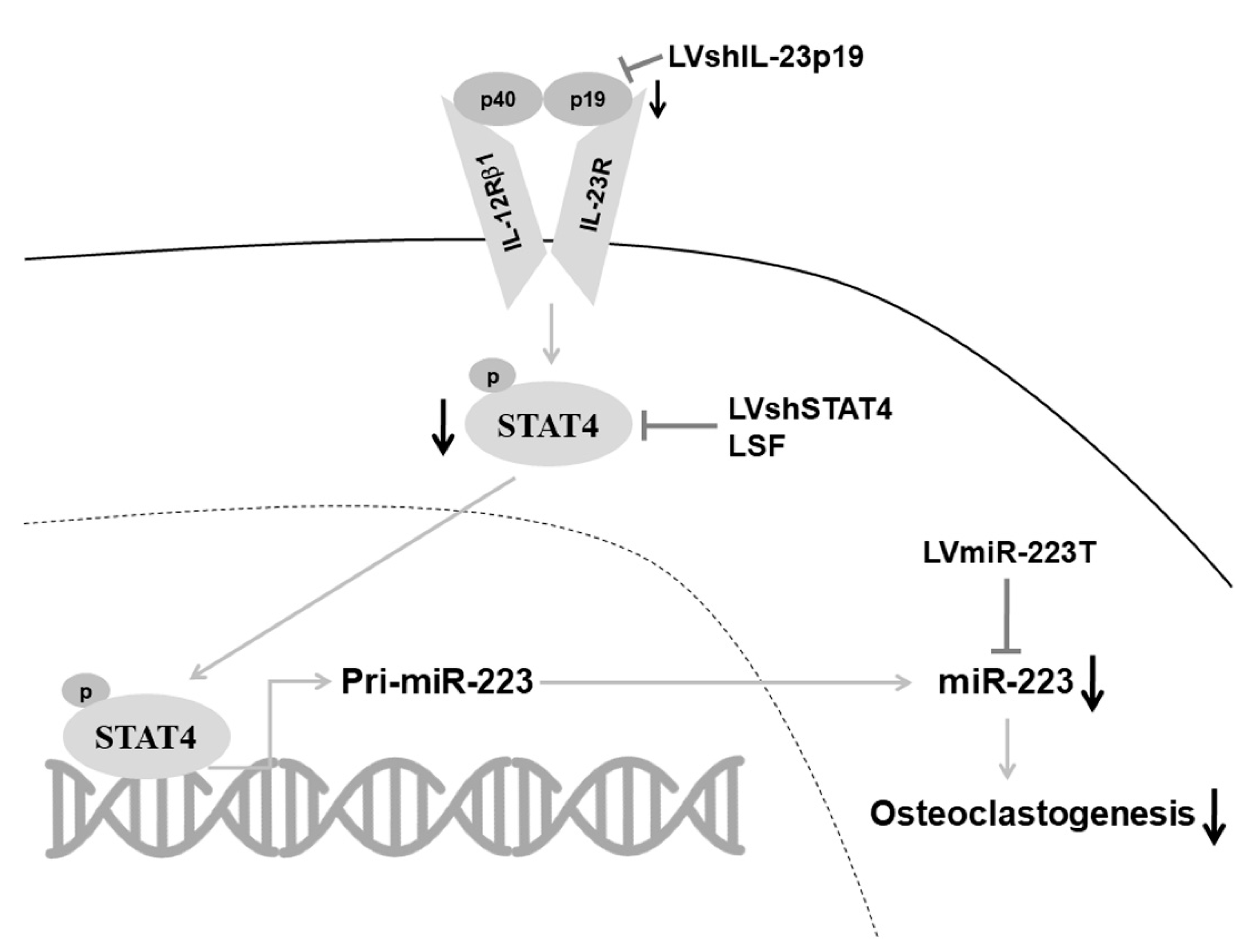
3. Discussion
4. Materials and Methods
4.1. Construction of Lentiviral Vectors
4.2. Animal Studies
4.3. RT-PCR, qRT-PCR, Immunohistochemistry, and In Situ Hybridization (ISH)
4.4. Quantitative Chromatin Immunoprecipitation (qChIP) and TRAP Staining
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ammari, M.; Jorgensen, C.; Apparailly, F. Impact of microRNAs on the Understanding and Treatment of Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2013, 25, 225–233. [Google Scholar] [CrossRef]
- Li, Y.-T.; Chen, S.-Y.; Wang, C.-R.; Liu, M.-F.; Lin, C.-C.; Jou, I.-M.; Shiau, A.-L.; Wu, C.-L. Brief Report: Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012, 64, 3240–3245. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Grzybowska-Kowalczyk, A.; Wojtecka-Lukasik, E.; Maslinski, S. IL-23 in the Pathogenesis of Rheumatoid Arthritis. Scand. J. Immunol. 2010, 71, 134–145. [Google Scholar] [CrossRef]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent pro-and Antiinflammatory Roles For IL-23 And IL-12 in Joint Autoimmune Inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Chen, L.; Wei, X.Q.; Evans, B.; Jiang, W.; Aeschlimann, D. IL-23 Promotes Osteoclast Formation by Up-Regulation of Receptor Activator Of NF-Κb (RANK) Expression in Myeloid Precursor Cells. Eur. J. Immunol. 2008, 38, 2845–2854. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune Axis: From Mechanisms to Therapeutic Testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Farré, D.; Roset, R.; Huerta, M.; Adsuara, J.E.; Roselló, L.; Albà, M.M.; Messeguer, X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003, 31, 3651–3653. [Google Scholar] [CrossRef]
- Fukao, T.; Fukuda, Y.; Kiga, K.; Sharif, J.; Hino, K.; Enomoto, Y.; Kawamura, A.; Nakamura, K.; Takeuchi, T.; Tanabe, M. An Evolutionarily Conserved Mechanism for MicroRNA-223 Expression Revealed by MicroRNA Gene Profiling. Cell 2007, 129, 617–631. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, M.; Nadler, J.L. Lisofylline: A Potential Lead for the Treatment of Diabetes. Biochem. Pharmacol. 2005, 69, 1–5. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Yamada, H.; Nakashima, Y.; Okazaki, K.; Mawatari, T.; Fukushi, J.-I.; Kaibara, N.; Hori, A.; Iwamoto, Y.; Yoshikai, Y. Th1 but Not Th17 Cells Predominate in the Joints of Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2007, 67, 1299–1304. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–Producing CD4+ Effector T Cells Develop Via a Lineage Distinct from the T Helper Type 1 And 2 Lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Leroy, H.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Hüffmeier, U.; Lascorz, J.; Böhm, B.; Lohmann, J.; Wendler, J.; Mössner, R.; Kristian, R.; Traupe, K.; Kurret, W.; Burkhardt, H.; et al. Genetic Variants of the IL-23R Pathway: Association with Psoriatic Arthritis and Psoriasis Vulgaris, But No Specific Risk Factor for Arthritis. J. Investig. Dermatol. 2009, 129, 355–358. [Google Scholar] [CrossRef]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.-J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of Osteoclastogenesis and Bone Loss by Human Autoantibodies Against Citrullinated Vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef]
- Kleyer, A.; Finzel, S.; Rech, J.; Manger, B.; Krieter, M.; Faustini, F.; Araujo, E.; Hueber, A.J.; Harre, U.; Engelke, K.; et al. Bone Loss Before the Clinical Onset of Rheumatoid Arthritis in Subjects with Anticitrullinated Protein Antibodies. Ann. Rheum. Dis. 2014, 73, 854–860. [Google Scholar] [CrossRef]
- Wu, Y.H.; Liu, W.; Xue, B.; Zhang, L.; Liu, X.Y.; Liu, B.; Wang, Y.; Cai, Y.; Duan, R. Upregulated Expression of Microrna-16 Correlates with Th17/Treg Cell Imbalance in Patients with Rheumatoid Arthritis. DNA Cell Biol. 2016, 35, 853–860. [Google Scholar] [CrossRef]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated miR-146a Expression in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef]
- Niimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. Microrna-146a Expresses in Interleukin-17 Producing T Cells in Rheumatoid Arthritis Patients. BMC Musculoskelet. Disord. 2010, 11, 209. [Google Scholar] [CrossRef]
- Li, J.; Wan, Y.; Guo, Q.; Zou, L.; Zhang, J.; Fang, Y.; Zhang, J.; Zhang, J.; Fu, X.; Liu, H.; et al. Altered Microrna Expression Profile with Mir-146a Upregulation in CD4+ T Cells from Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2010, 12, R81. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.S.; Chen, S.Y.; Wu, C.L.; Chong, H.E.; Ding, Y.C.; Shiau, A.L.; Wang, C.R. Amelioration of Experimental Autoimmune Arthritis Through Targeting of Synovial Fibroblasts by Intraarticular Delivery of Micrornas 140-3p and 140-5p. Arthritis Rheumatol. 2016, 8, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Fulci, V.; Scappucci, G.; Sebastiani, G.D.; Giannitti, C.; Franceschini, D.; Meloni, F.; Colombo, T.; Citarella, F.; Barnaba, V.; Minisola, G.; et al. miR-223 is Overexpressed In T-Lymphocytes of Patients Affected by Rheumatoid Arthritis. Hum. Immunol. 2010, 71, 206–211. [Google Scholar] [CrossRef]
- Sugatani, T.; Hruska, K.A. Impaired Micro-RNA Pathways Diminish Osteoclast Differentiation and Function. J. Biol. Chem. 2009, 284, 4667–4678. [Google Scholar] [CrossRef]
- Hildner, K.M.; Schirmacher, P.; Atreya, I.; Dittmayer, M.; Bartsch, B.; Galle, P.R.; Wirtz, S.; Neurath, M.F. Targeting of the Transcription Factor STAT4 by Antisense Phosphorothioate Oligonucleotides Suppresses Collagen-Induced Arthritis. J. Immunol. 2007, 178, 3427–3436. [Google Scholar] [CrossRef]
- Du, C.; Cooper, J.C.; Klaus, S.J.; Sriram, S. Amelioration of CR-EAE with lisofylline: Effects on mRNA levels of IL-12 and IFN-γ in the CNS. J. Neuroimmunol. 2000, 110, 13–19. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Chen, M.; Wu, R.; McDuffie, M.; Nadler, J.-L. The Anti-Inflammatory Compound Lisofylline Prevents Type I Diabetes in Non-Obese Diabetic Mice. Diabetologia 2002, 45, 1307–1314. [Google Scholar] [CrossRef]
- Yudoh, K.; Matsuno, H.; Nakazawa, F.; Yonezawa, T.; Kimura, T. Reduced Expression of the Regulatory CD4+ T Cell Subset Is Related to Th1/Th2 Balance and Disease Severity in Rheumatoid Arthritis. Arthritis Rheumatol 2000, 43, 617–627. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Shiau, A.-L.; Wu, C.-L.; Wang, C.-R. Intraarticular Overexpression of Smad7 Ameliorates Experimental Arthritis. Sci. Rep. 2016, 6, 35163. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Tsai, T.-C.; Li, Y.-T.; Ding, Y.-C.; Wang, C.-T.; Hsieh, J.-L.; Wu, C.-L.; Wu, P.-T.; Shiau, A.-L. Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223. Int. J. Mol. Sci. 2022, 23, 9718. https://doi.org/10.3390/ijms23179718
Chen S-Y, Tsai T-C, Li Y-T, Ding Y-C, Wang C-T, Hsieh J-L, Wu C-L, Wu P-T, Shiau A-L. Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223. International Journal of Molecular Sciences. 2022; 23(17):9718. https://doi.org/10.3390/ijms23179718
Chicago/Turabian StyleChen, Shih-Yao, Ting-Chien Tsai, Yuan-Tsung Li, Yun-Chiao Ding, Chung-Teng Wang, Jeng-Long Hsieh, Chao-Liang Wu, Po-Ting Wu, and Ai-Li Shiau. 2022. "Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223" International Journal of Molecular Sciences 23, no. 17: 9718. https://doi.org/10.3390/ijms23179718
APA StyleChen, S.-Y., Tsai, T.-C., Li, Y.-T., Ding, Y.-C., Wang, C.-T., Hsieh, J.-L., Wu, C.-L., Wu, P.-T., & Shiau, A.-L. (2022). Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223. International Journal of Molecular Sciences, 23(17), 9718. https://doi.org/10.3390/ijms23179718





