Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model
Abstract
:1. Introduction
2. Results
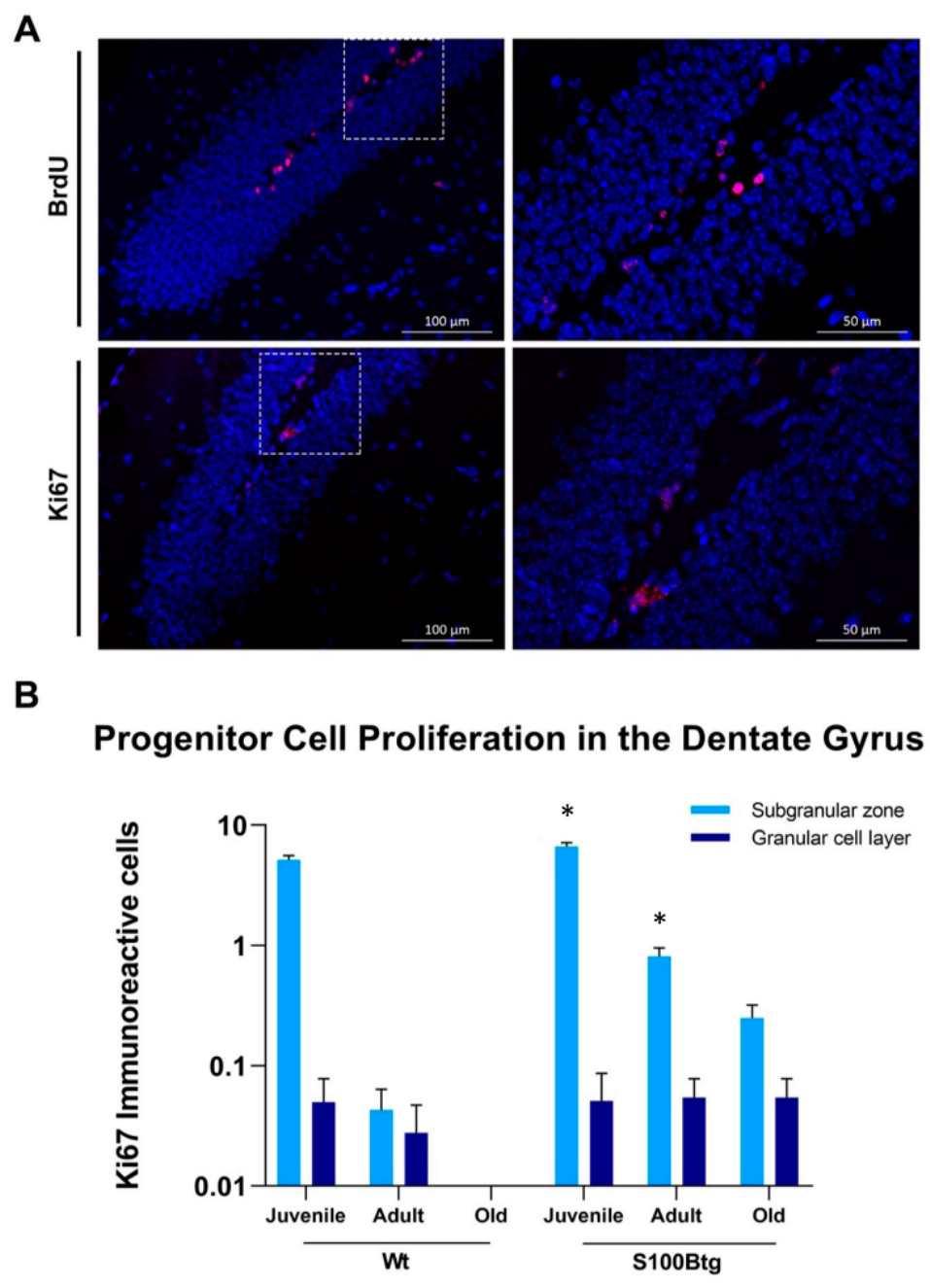
2.1. Effect of Long-Term Increased S100B Levels on Hippocampal Progenitor Cell Proliferation and Migration
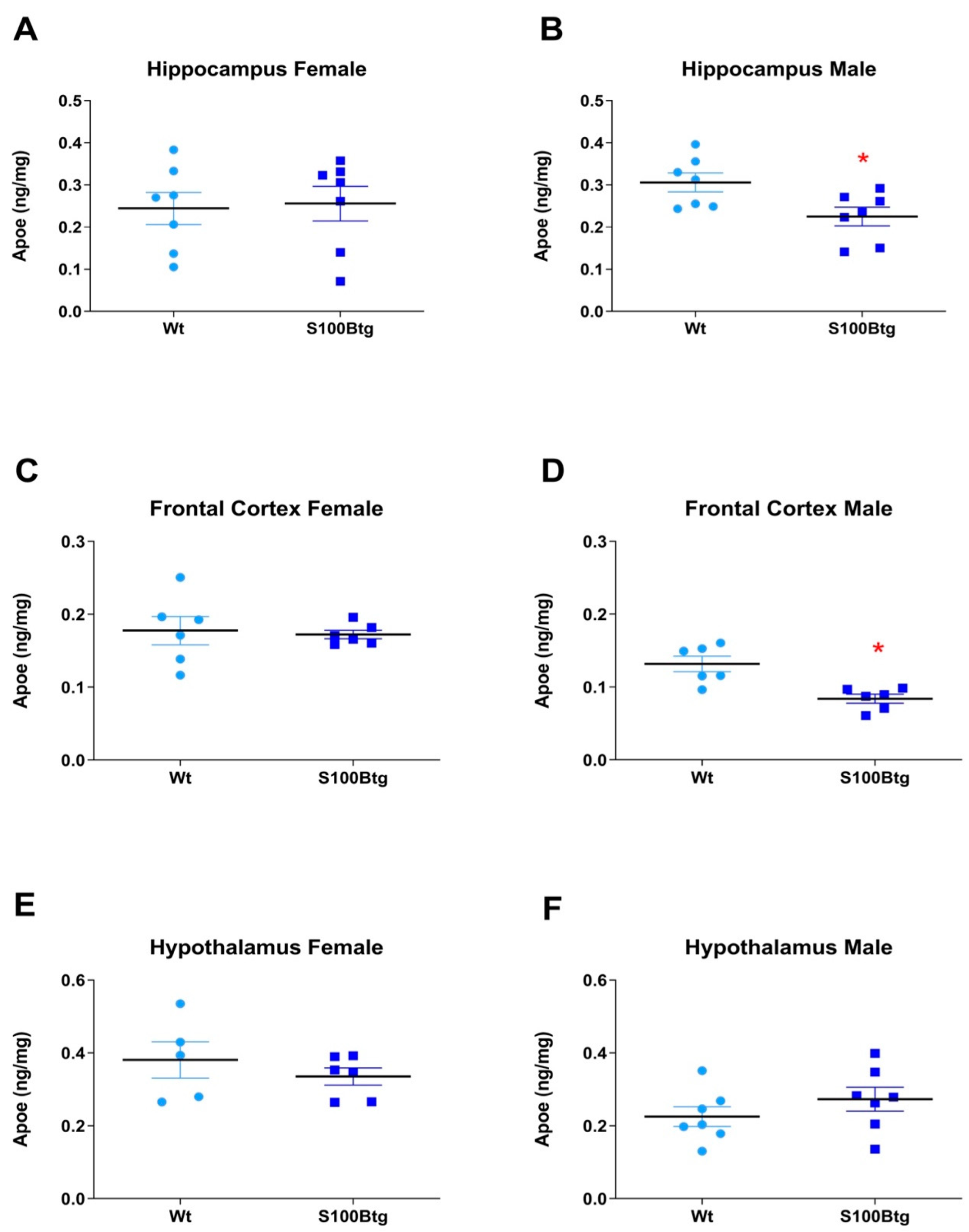
2.2. Effect of Long-Term Increased S100B Levels on Central and Peripheral ApoE Content
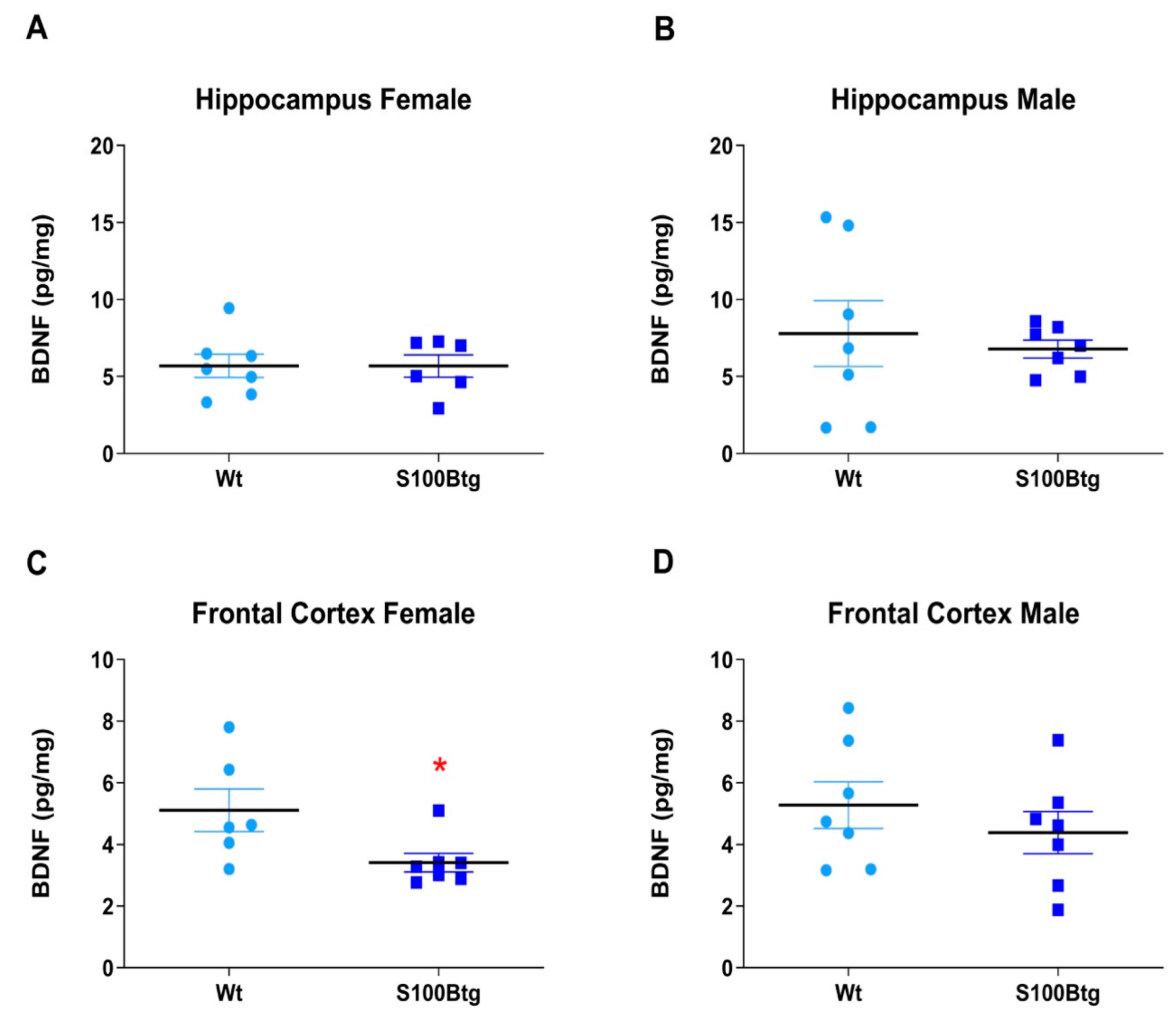
2.3. Effect of Long-Term Increased S100B Levels on Central BDNF Content
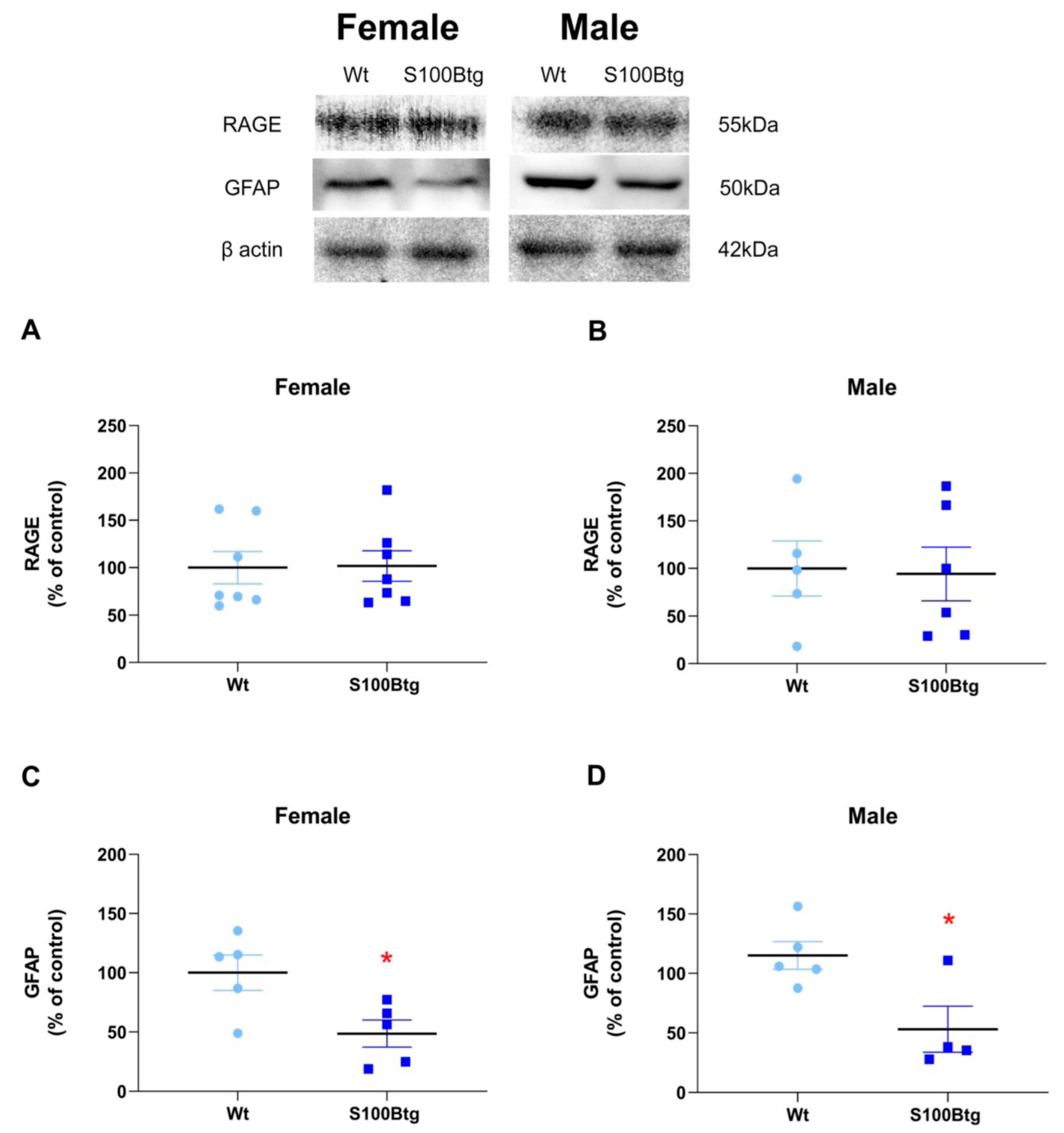
2.4. Effect of Long-Term Increased S100B Levels on Hippocampal RAGE and GFAP Content
3. Discussion
3.1. Long-Term Increased S100B Levels Promote Hippocampal Neurogenesis
3.2. Long-Term Increased S100B Levels Attenuate Hippocampal and Frontal ApoE Content in Male S100Btg Mice
3.3. Long-Term Increased S100B Levels Are Not Associated with Increased Hippocampal BDNF Content
3.4. Long-Term Increased S100B Levels Are Not Accompanied by Enhanced Hippocampal RAGE
3.5. Long-Term Increased S100B Levels Are Associated with a Reduced Hippocampal GFAP Content
3.6. Limitations
4. Materials and Methods
4.1. Effect of Long-Term Increased S100B Levels on Hippocampal Progenitor Cell Proliferation and Migration
4.2. Quantification of Hippocampal Progenitor Cell Proliferation
4.3. Enzyme-Linked Immunosorbent Assay for ApoE Content
4.4. Enzyme-Linked Immunosorbent Assay for BDNF Content
4.5. Western Blot Analysis for RAGE and GFAP
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Heizmann, C.W.; Fritz, G.; Schafer, B.W. S100 proteins: Structure, functions and pathology. Front. Biosci. 2002, 7, 1356–1368. [Google Scholar] [PubMed]
- Netto, C.B.; Siqueira, I.R.; Fochesatto, C.; Portela, L.V.; da Purificacao Tavares, M.; Souza, D.O.; Giugliani, R.; Gonçalves, C.-A. S100B content and SOD activity in amniotic fluid of pregnancies with Down syndrome. Clin. Biochem. 2004, 37, 134–137. [Google Scholar] [CrossRef]
- Rothermundt, M.; Peters, M.; Prehn, J.H.; Arolt, V. S100B in brain damage and neurodegeneration. Microsc. Res. Tech. 2003, 60, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Wartchow, K.M.; Rodrigues, L.; Swierzy, I.; Buchfelder, M.; de Souza, D.O.; Gonçalves, C.-A.; Kleindienst, A. Amyloid-β Processing in Aged S100B Transgenic Mice Is Sex Dependent. Int. J. Mol. Sci. 2021, 22, 10823. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Kuja-Panula, J.; Sorci, G.; Agneletti, A.L.; Donato, R.; Rauvala, H. Coregulation of Neurite Outgrowth and Cell Survival by Amphoterin and S100 Proteins through Receptor for Advanced Glycation End Products (RAGE) Activation. J. Biol. Chem. 2000, 275, 40096–40105. [Google Scholar] [CrossRef]
- Reeves, R.H.; Yao, J.; Crowley, M.R.; Buck, S.; Zhang, X.; Yarowsky, P.; Gearhart, J.D.; Hilt, D.C. Astrocytosis and axonal proliferation in the hippocampus of S100b transgenic mice. Proc. Natl. Acad. Sci. USA 1994, 91, 5359–5363. [Google Scholar] [CrossRef]
- Mrak, R.E.; Sheng, J.G.; Griffin, W.S. Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 273–279. [Google Scholar] [CrossRef]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S.T. S100β protein expression in Alzheimer disease: Potential role in the pathogenesis of neuritic plaques. J. Neurosci. Res. 1994, 39, 398–404. [Google Scholar] [CrossRef]
- Kleindienst, A.; McGinn, M.J.; Harvey, H.B.; Colello, R.J.; Hamm, R.J.; Bullock, M.R. Enhanced Hippocampal Neurogenesis by Intraventricular S100B Infusion Is Associated with Improved Cognitive Recovery after Traumatic Brain Injury. J. Neurotrauma 2005, 22, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Baecker, J.; Wartchow, K.; Sehm, T.; Ghoochani, A.; Buchfelder, M.; Kleindienst, A. Treatment with the Neurotrophic Protein S100B Increases Synaptogenesis after Traumatic Brain Injury. J. Neurotrauma 2020, 37, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, A.; Grünbeck, F.; Buslei, R.; Emtmann, I.; Buchfelder, M. Intraperitoneal treatment with S100B enhances hippocampal neurogenesis in juvenile mice and after experimental brain injury. Acta Neurochir. 2013, 155, 1351–1360. [Google Scholar] [CrossRef]
- Kleindienst, A.; Harvey, H.B.; Rice, A.C.; Müller, C.; Hamm, R.J.; Gaab, M.R.; Bullock, M.R. Intraventricular Infusion of the Neurotrophic Protein S100B Improves Cognitive Recovery after Fluid Percussion Injury in the Rat. J. Neurotrauma 2004, 21, 541–547. [Google Scholar] [CrossRef]
- Hicks, R.R.; Smith, D.H.; Lowenstein, D.H.; Saint Marie, R.; McIntosh, T.K. Mild Experimental Brain Injury in the Rat Induces Cognitive Deficits Associated with Regional Neuronal Loss in the Hippocampus. J. Neurotrauma 1993, 10, 405–414. [Google Scholar] [CrossRef]
- McIntosh, T.; Vink, R.; Noble, L.; Yamakami, I.; Fernyak, S.; Soares, H.; Faden, A. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience 1989, 28, 233–244. [Google Scholar] [CrossRef]
- Povlishock, J.T. Pathobiology of traumatically induced axonal injury in animals and man. Ann. Emerg. Med. 1993, 22, 980–986. [Google Scholar] [CrossRef]
- Kuhn, H.-G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef] [Green Version]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001, 63, 313–319. [Google Scholar] [CrossRef]
- Yoshimura, S.; Teramoto, T.; Whalen, M.J.; Irizarry, M.C.; Takagi, Y.; Qiu, J.; Harada, J.; Waeber, C.; Breakefield, X.O.; Moskowitz, M.A. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J. Clin. Investig. 2003, 112, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Mello e Souza, T.; Rohden, A.; Meinhardt, M.; Goncalves, C.A.; Quillfeldt, J.A. S100B infusion into the rat hippocampus facilitates memory for the inhibitory avoidance task but not for the open-field habituation. Physiol. Behav. 2000, 71, 29–33. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Ma, Y. Molecular mechanisms of altered adult hippocampal neurogenesis in Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111452. [Google Scholar] [CrossRef]
- Nathan, B.P.; Bellosta, S.; Sanan, D.A.; Weisgraber, K.H.; Mahley, R.W.; Pitas, R.E. Differential Effects of Apolipoproteins E3 and E4 on Neuronal Growth in Vitro. Science 1994, 264, 850–852. [Google Scholar] [CrossRef]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol. Brain 2017, 10, 19. [Google Scholar] [CrossRef]
- Morgan, T.E.; Xie, Z.; Goldsmith, S.; Yoshida, T.; Lanzrein, A.-S.; Stone, D.; Rozovsky, I.; Perry, G.; Smith, M.; Finch, C. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 1999, 89, 687–699. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A.; Olsen, M.L. AP-1 and the injury response of the GFAP gene. J. Neurosci. Res. 2019, 97, 149–161. [Google Scholar] [CrossRef]
- Garcia, A.D.R.; Doan, N.B.; Imura, T.; Bush, T.G.; Sofroniew, M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004, 7, 1233–1241. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011, 345, 1–19. [Google Scholar] [CrossRef]
- Zhao, X.; van Praag, H. Steps towards standardized quantification of adult neurogenesis. Nat. Commun. 2020, 11, 4275. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J.M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef]
- McAdory, B.S.; Van Eldik, L.J.; Norden, J.J. S100B, a neurotropic protein that modulates neuronal protein phosphorylation, is upregulated during lesion-induced collateral sprouting and reactive synaptogenesis. Brain Res. 1998, 813, 211–217. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Wainwright, M.S. The Janus face of glial-derived S100B: Beneficial and detrimental functions in the brain. Restor. Neurol. Neurosci. 2003, 21, 97–108. [Google Scholar]
- Rice, A.C.; Khaldi, A.; Harvey, H.B.; Salman, N.J.; White, F.; Fillmore, H.; Bullock, M.R. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp. Neurol. 2003, 183, 406–417. [Google Scholar] [CrossRef]
- Mirescu, C.; Gould, E. Stress and adult neurogenesis. Hippocampus 2006, 16, 233–238. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar]
- Klempin, F.; Kempermann, G. Adult hippocampal neurogenesis and aging. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 271–280. [Google Scholar] [CrossRef]
- Rickmann, M.; Wolff, J.R.; Meyer, D.L. Expression of S100 protein in the vestibular nuclei during compensation of unilateral labyrinthectomy symptoms. Brain Res. 1995, 688, 8–14. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Sheng, J.G.; Liu, L.; Barger, S.W.; Jones, R.A.; Van Eldik, L.J.; Mrak, R.E.; Griffin, W.S. S100 beta increases levels of beta-amyloid precursor protein and its encoding mRNA in rat neuronal cultures. J. Neurochem. 1998, 71, 1421–1428. [Google Scholar] [CrossRef]
- Yu, T.S.; Tensaouti, Y.; Stephanz, E.P.; Chintamen, S.; Rafikian, E.E.; Yang, M.; Kernie, S.G. Astrocytic ApoE underlies maturation of hippocampal neurons and cognitive recovery after traumatic brain injury in mice. Commun. Biol. 2021, 4, 1303. [Google Scholar] [CrossRef] [PubMed]
- Struble, R.G.; Nathan, B.P.; Cady, C.; Cheng, X.; McAsey, M. Estradiol regulation of astroglia and apolipoprotein E: An important role in neuronal regeneration. Exp. Gerontol. 2007, 42, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gamache, J.; Yun, Y.; Chiba-Falek, O. Sex-dependent effect of APOE on Alzheimer’s disease and other age-related neurodegenerative disorders. Dis. Model Mech. 2020, 13. [Google Scholar] [CrossRef]
- Koutseff, A.; Mittelhaeuser, C.; Essabri, K.; Auwerx, J.; Meziane, H. Impact of the apolipoprotein E polymorphism, age and sex on neurogenesis in mice: Pathophysiological relevance for Alzheimer’s disease? Brain Res. 2014, 1542, 32–40. [Google Scholar] [CrossRef]
- Abotalebi, H.; Ebrahimi, B.; Shahriyari, R.; Shafieian, R. Sex steroids-induced neurogenesis in adult brain: A better look at mechanisms and mediators. Horm. Mol. Biol. Clin. Investig. 2021, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Herbruggen, O.; Hortnagl, H.; Ponath, G.; Rothermundt, M.; Hellweg, R. Distinct regulation of brain-derived neurotrophic factor and noradrenaline in S100B knockout mice. Neurosci. Lett. 2008, 442, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Buschert, J.; Hohoff, C.; Touma, C.; Palme, R.; Rothermundt, M.; Arolt, V.; Zhang, W.; Ambree, O. S100B overexpression increases behavioral and neural plasticity in response to the social environment during adolescence. J. Psychiatr. Res. 2013, 47, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Bianchi, R.; Giambanco, I.; Donato, R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol. Aging 2010, 31, 665–677. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Loane, D.J. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneghini, V.; Francese, M.T.; Carraro, L.; Grilli, M. A novel role for the Receptor for Advanced Glycation End-products in neural progenitor cells derived from adult SubVentricular Zone. Mol. Cell. Neurosci. 2010, 45, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Moysa, A.; Steczkiewicz, K.; Niedzialek, D.; Hammerschmid, D.; Zhukova, L.; Sobott, F.; Dadlez, M. A model of full-length RAGE in complex with S100B. Structure 2021, 29, 989–1002.e6. [Google Scholar] [CrossRef]
- Moreira, A.P.; Vizuete, A.F.K.; Zin, L.E.F.; de Marques, C.O.; Pacheco, R.F.; Leal, M.B.; Goncalves, C.A. The Methylglyoxal/RAGE/NOX-2 Pathway is Persistently Activated in the Hippocampus of Rats with STZ-Induced Sporadic Alzheimer’s Disease. Neurotox. Res. 2022, 40, 395–409. [Google Scholar] [CrossRef]
- Barger, S.W.; Harmon, A.D. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997, 388, 878–881. [Google Scholar] [CrossRef]
- Hill, S.J.; Barbarese, E.; McIntosh, T.K. Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J. Neuropathol. Exp. Neurol. 1996, 55, 1221–1229. [Google Scholar] [CrossRef]
- Hatten, M.E. Neuronal regulation of astroglial morphology and proliferation in vitro. J. Cell Biol. 1985, 100, 384–396. [Google Scholar] [CrossRef]
- Briones, T.L.; Woods, J.; Wadowska, M.; Rogozinska, M.; Nguyen, M. Astrocytic changes in the hippocampus and functional recovery after cerebral ischemia are facilitated by rehabilitation training. Behav. Brain Res. 2006, 171, 17–25. [Google Scholar] [CrossRef]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.A.; Bialowas-McGoey, L.A.; Whitaker-Azmitia, P.M. Effects of S100B on Serotonergic Plasticity and Neuroinflammation in the Hippocampus in Down Syndrome and Alzheimer’s Disease: Studies in an S100B Overexpressing Mouse Model. Cardiovasc. Psychiatry Neurol. 2010, 2010, 153657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.S.; Ariah, L.M.; Marks, A.; Azmitia, E.C. Chronic gliosis induced by loss of S-100B: Knockout mice have enhanced GFAP-immunoreactivity but blunted response to a serotonin challenge. Brain Res. 2005, 1031, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guillery, R.W.; Herrup, K. Quantification without pontification: Choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 1997, 386, 2–7. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.; Wartchow, K.M.; Buchfelder, M.; Souza, D.O.; Gonçalves, C.-A.; Kleindienst, A. Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model. Int. J. Mol. Sci. 2022, 23, 9600. https://doi.org/10.3390/ijms23179600
Rodrigues L, Wartchow KM, Buchfelder M, Souza DO, Gonçalves C-A, Kleindienst A. Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model. International Journal of Molecular Sciences. 2022; 23(17):9600. https://doi.org/10.3390/ijms23179600
Chicago/Turabian StyleRodrigues, Leticia, Krista Minéia Wartchow, Michael Buchfelder, Diogo Onofre Souza, Carlos-Alberto Gonçalves, and Andrea Kleindienst. 2022. "Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model" International Journal of Molecular Sciences 23, no. 17: 9600. https://doi.org/10.3390/ijms23179600
APA StyleRodrigues, L., Wartchow, K. M., Buchfelder, M., Souza, D. O., Gonçalves, C.-A., & Kleindienst, A. (2022). Longterm Increased S100B Enhances Hippocampal Progenitor Cell Proliferation in a Transgenic Mouse Model. International Journal of Molecular Sciences, 23(17), 9600. https://doi.org/10.3390/ijms23179600






