Differential Performance and Lung Deposition of Levofloxacin with Different Nebulisers Used in Cystic Fibrosis
Abstract
1. Introduction
2. Results
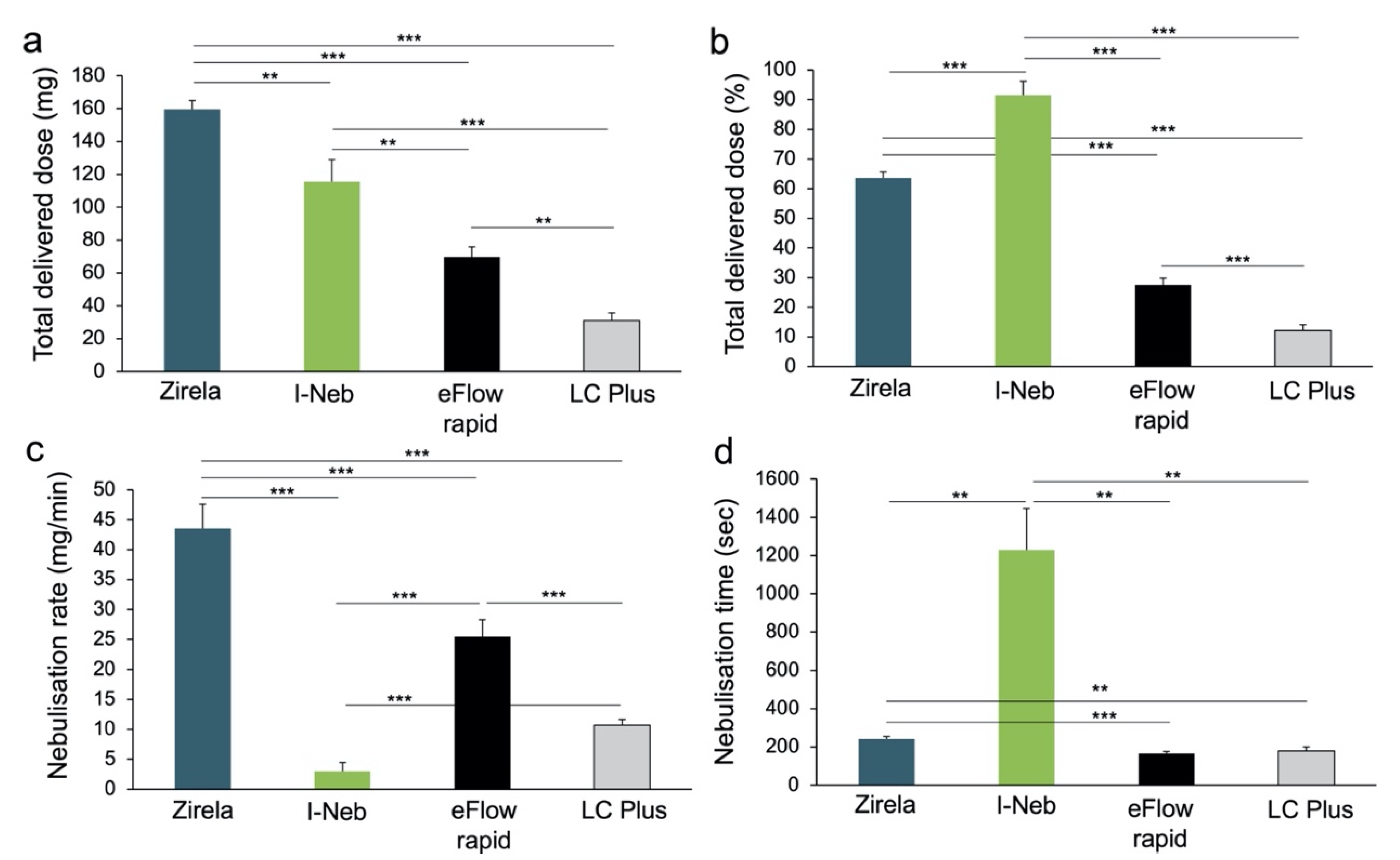
2.1. Delivered Dose and Delivery Rate of Nebulised Levofloxacin
2.2. Aerodynamic Particle Size Characterisation of Levofloxacin Aerosols
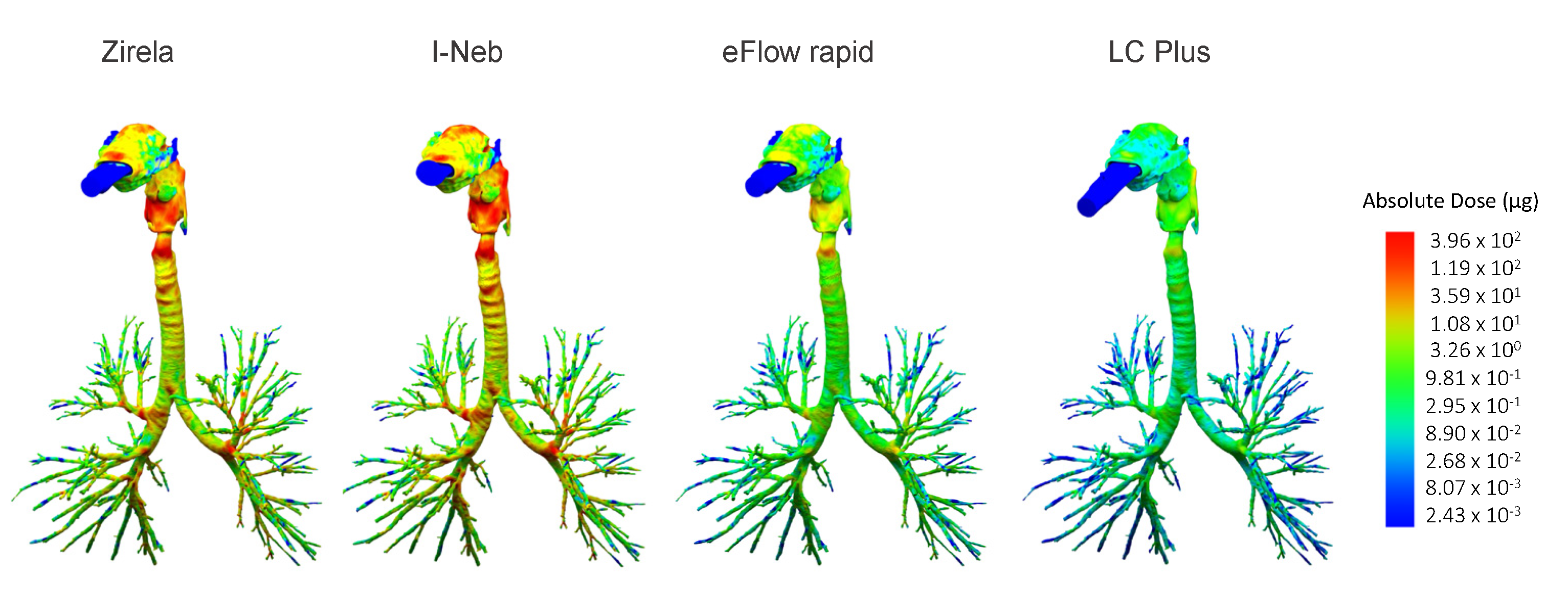
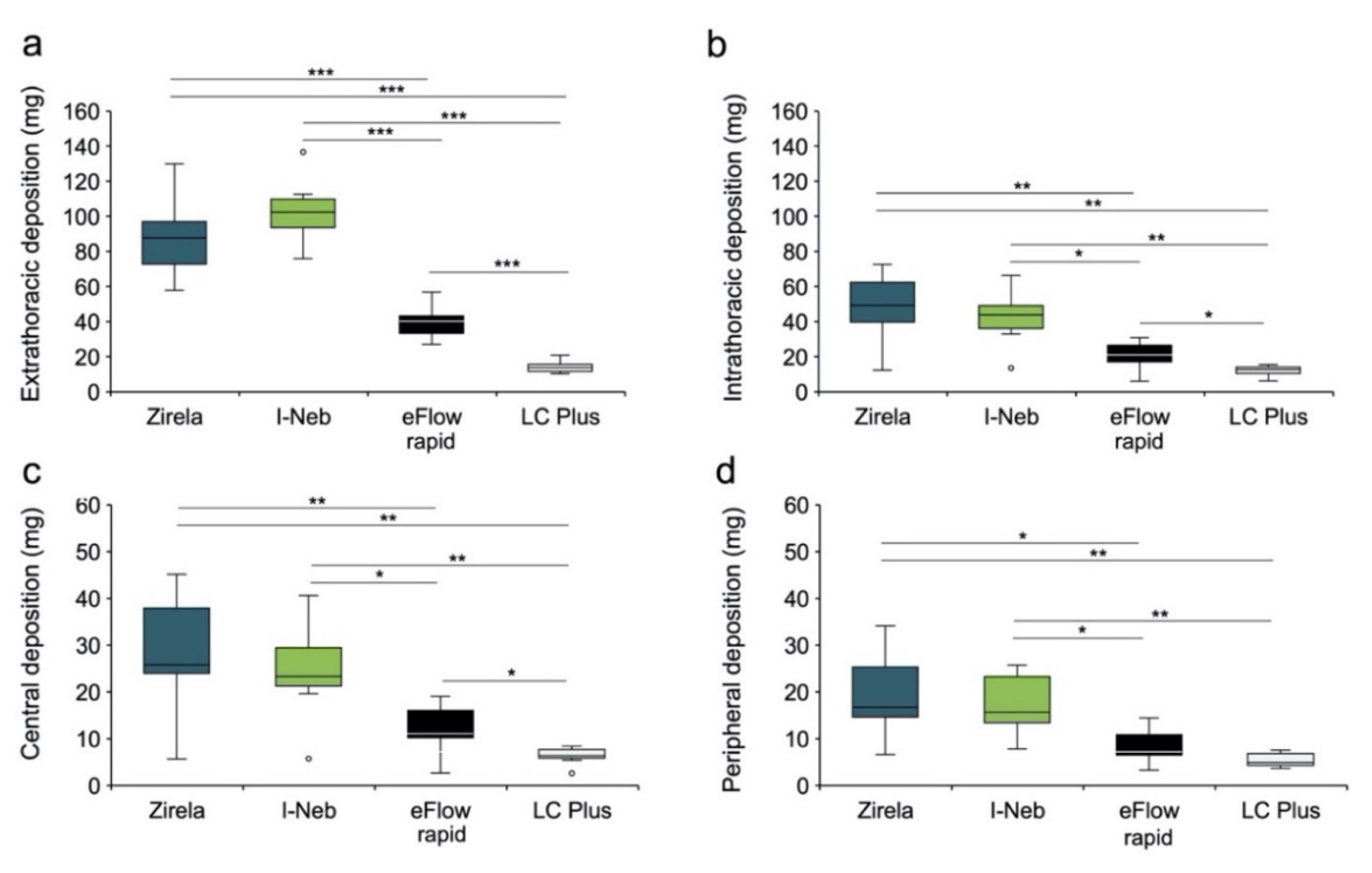
2.3. Functional Respiratory Imaging Assessment of Levofloxacin Lung Deposition
3. Discussion
4. Materials and Methods
4.1. Nebulisers
4.2. Delivered Dose and Delivery Rate of Nebulised Levofloxacin
4.3. Delivered Dose and Delivery Rate of Nebulised Levofloxacin
4.4. Functional Respiratory Imaging Assessment of Levofloxacin Lung Deposition
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elborn, J.S. Cystic fibrosis. Lancet 2017, 388, 2519–2531. [Google Scholar] [CrossRef]
- Haq, I.J.; Gray, M.A.; Garnett, J.P.; Ward, C.; Brodlie, M. Airway surface liquid homeostasis in cystic fibrosis: Pathophysiology and therapeutic targets. Thorax 2016, 71, 284–287. [Google Scholar] [CrossRef]
- Tramper-Stranders, G.A.; van der Ent, C.K.; Molin, S.; Yang, L.; Hansen, S.K.; Rau, M.H.; Ciofu, O.; Johansen, H.K.; Wolfs, T.F.W. Initial Pseudomonas aeruginosa infection in patients with cystic fibrosis: Characteristics of eradicated and persistent isolates. Clin. Microbiol. Infect. 2012, 18, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Mitri, C.; Xu, Z.; Bardin, P.; Corvol, H.; Touqui, L.; Tabary, O. Novel Anti-Inflammatory Approaches for Cystic Fibrosis Lung Disease: Identification of Molecular Targets and Design of Innovative Therapies. Front. Pharmacol. 2020, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Flume, P.A.; Van Devanter, D.R.; Procaccianti, C. Management of chronic Pseudomonas aeruginosa infection with inhaled levofloxacin in people with cystic fibrosis. Future Microbiol. 2021, 16, 1087–1104. [Google Scholar] [CrossRef]
- Ho, D.-K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- Janssens, H.; Tiddens, H.A.W.M. Aerosol therapy for CF lung disease: The basics. In Optimizing Pharmaceutical Care in Cystic Fibrosis; Bentley, S., Castellani, C., Peckham, D., Shaw, N., Eds.; European Cystic Fibrosis Society: Karup, India, 2020; pp. 119–137. [Google Scholar]
- Bianco, F.; Salomone, F.; Milesi, I.; Murgia, X.; Bonelli, S.; Pasini, E.; Dellacà, R.; Ventura, M.L.; Pillow, J. Aerosol drug delivery to spontaneously-breathing preterm neonates: Lessons learned. Respir. Res. 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.N.; Hatley, R.H.M.; Denyer, J.; Von Hollen, D. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther. Deliv. 2018, 9, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Boe, J.; Dennis, J.H.; O’Driscoll, B.R. European respiratory society guidelines on the use of nebulizers. Eur. Respir. J. 2001, 18, 228–242. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Sellers, D.E.; Robinson, W.M. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J. Cyst. Fibros. 2009, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, K.; Salvas, B.; Stevens, V.; Brown, J. Aztreonam lysine for inhalation: New formulation of an old antibiotic. Am. J. Health-Syst. Pharm. 2012, 69, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Perkins, W.; Cipolla, D. Robustness of aerosol delivery of amikacin liposome inhalation suspension using the eFlow® Technology. Eur. J. Pharm. Biopharm. 2021, 166, 10–18. [Google Scholar] [CrossRef]
- Schwarz, C.; Procaccianti, C.; Mignot, B.; Sadafi, H.; Schwenck, N.; Murgia, X.; Bianco, F. Deposition of Inhaled Levofloxacin in Cystic Fibrosis Lungs Assessed by Functional Respiratory Imaging. Pharmaceutics 2021, 13, 2051. [Google Scholar] [CrossRef]
- Flume, P.A.; VanDevanter, D.R.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S.; Bell, S.C.; Kerem, E.; Fischer, R.; Smyth, A.R.; Aaron, S.D.; et al. A phase 3, multi-center, multinational, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT-1026) in stable cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 495–502. [Google Scholar] [CrossRef]
- Elborn, J.S.; Flume, P.A.; Cohen, F.; Loutit, J.; VanDevanter, D.R. Safety and efficacy of prolonged levofloxacin inhalation solution (APT-1026) treatment for cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. J. Cyst. Fibros. 2016, 15, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Stuart Elborn, J.; Geller, D.E.; Conrad, D.; Aaron, S.D.; Smyth, A.R.; Fischer, R.; Kerem, E.; Bell, S.C.; Loutit, J.S.; Dudley, M.N.; et al. A phase 3, open-label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT-1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 507–514. [Google Scholar] [CrossRef]
- Stockmann, C.; Sherwin, C.M.T.; Ampofo, K.; Spigarelli, M.G. Development of levofloxacin inhalation solution to treat Pseudomonas aeruginosa in patients with cystic fibrosis. Ther. Adv. Respir. Dis. 2014, 8, 13–21. [Google Scholar] [CrossRef]
- Schwarz, C.; Grehn, C.; Temming, S.; Holz, F.; Eschenhagen, P.N. Clinical impact of levofloxacin inhalation solution in cystic fibrosis patients in a real-world setting. J. Cyst. Fibros. 2021, 20, 1035–1039. [Google Scholar] [CrossRef]
- Geller, D.E.; Kesser, K.C. The I-neb Adaptive Aerosol Delivery System Enhances Delivery of α1-Antitrypsin with Controlled Inhalation. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S-55–S-59. [Google Scholar] [CrossRef] [PubMed]
- Naehrig, S.; Lang, S.; Schiffl, H.; Huber, R.M.; Fischer, R. Lung function in adult patients with cystic fibrosis after using the eFlow® rapid for one year. Eur. J. Med. Res. 2011, 16, 63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hogan, A.; Bonney, M.-A.; Brien, J.; Karamy, R.; Aslani, P. Factors affecting nebulised medicine adherence in adult patients with cystic fibrosis: A qualitative study. Int. J. Clin. Pharm. 2015, 37, 86–93. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.W.; Vos, W.G.; Vinchurkar, S.C.; Claes, R.; Drollmann, A.; Wulfrank, D.; Parizel, P.M.; Germonpré, P.; De Backer, W. Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology 2010, 257, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Kozuka, T.; Nakanishi, J.; Yamada, K.; Nishiyama, O.; Sano, H.; Murakami, T.; Tohda, Y. Aerosol Deposition of Inhaled Corticosteroids/Long-Acting β2-Agonists in the Peripheral Airways of Patients with Asthma Using Functional Respiratory Imaging, a Novel Imaging Technology. Pulm. Ther. 2017, 3, 219–231. [Google Scholar] [CrossRef]
- Kappeler, D.; Sommerer, K.; Kietzig, C.; Huber, B.; Woodward, J.; Lomax, M.; Dalvi, P. Lung deposition of fluticasone propionate/formoterol administered via a breath-triggered inhaler. Eur. Respir. J. 2017, 50, PA522. [Google Scholar] [CrossRef]
- Hirst, P.H.; Bacon, R.E.; Pitcairn, G.R.; Silvasti, M.; Newman, S.P. A comparison of the lung deposition of budesonide from Easyhlaler®, Turbuhaler® and pMDI plus spacer in asthmatic patients. Respir. Med. 2001, 95, 720–727. [Google Scholar] [CrossRef]
- Usmani, O.S.; Mignot, B.; Kendall, I.; Maria, R.D.; Cocconi, D.; Georges, G.; Scichilone, N. Predicting Lung Deposition of Extrafine Inhaled Corticosteroid-Containing Fixed Combinations in Patients with Chronic Obstructive Pulmonary Disease Using Functional Respiratory Imaging: An In Silico Study. J. Aerosol Med. Pulm. Drug Deliv. 2020, 34, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sanz-García, F.; Hernando-Amado, S.; Martínez, J.L. Evolution under low antibiotic concentrations: A risk for the selection of Pseudomonas aeruginosa multidrug-resistant mutants in nature. Environ. Microbiol. 2021, 24, 1279–1293. [Google Scholar] [CrossRef]
- Products for Nebulization—Characterization Tests. In The Unites States Pharmacopeia; USP-NF: Rockville, MD, USA, 2018.
- Cystic Fibrosis Foundation 2018 Patient Registry Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2018.
- Van Holsbeke, C.; De Backer, J.; Vos, W.; Marshall, J. Use of functional respiratory imaging to characterize the effect of inhalation profile and particle size on lung deposition of inhaled corticosteroid/long-acting β2-agonists delivered via a pressurized metered-dose inhaler. Ther. Adv. Respir. Dis. 2018, 12, 1753466618760948. [Google Scholar] [CrossRef]
- Bos, A.C.; Van Holsbeke, C.; De Backer, J.W.; Van Westreenen, M.; Janssens, H.M.; Vos, W.G.; Tiddens, H.A.W.M. Patient-specific modeling of regional antibiotic concentration levels in airways of patients with cystic fibrosis: Are we dosing high enough? PLoS ONE 2015, 10, e0118454. [Google Scholar] [CrossRef]


| Nebuliser | Recovered Levofloxacin (mg) | Emitted Dose (mg) | MMAD (μm) | GSD | FPF (%) |
|---|---|---|---|---|---|
| Zirela | 258.1 ± 4.7 | 243.8 ± 5.8 | 4.5 ± 0.1 | 1.6 ± 0.1 | 58.1 ± 1.7 |
| I-Neb Advance * | 173.4 ± 13.9 | 154.3 ± 15.4 | 5.0 ± 0.3 | 1.9 ± 0.0 | 44.8 ± 2.3 |
| eFlow rapid | 262.2 ± 4.0 | 123.7 ± 8.6 | 4.7 ± 0.2 | 1.7 ± 0.1 | 54.9 ± 1.8 |
| LC Plus | 262.8 ± 6.4 | 90.5 ± 3.9 | 3.5 ± 0.2 | 2.1 ± 0.0 | 66.8 ± 2.5 |
| Device | Type of Device | Type of Nebulisation | Power Supply/Compressor | Reported Uses in CF * |
|---|---|---|---|---|
| Zirela | VM | Continuous | eBase controller |
|
| I-Neb Advance | VM | Breath-coordinated | Built-in battery |
|
| eFlow rapid | VM | Continuous | eBase controller |
|
| LC Plus | Jet | Continuous | Pari Turboboy SX |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, C.; Procaccianti, C.; Costa, L.; Brini, R.; Friend, R.; Caivano, G.; Sadafi, H.; Mussche, C.; Schwenck, N.; Hahn, M.; et al. Differential Performance and Lung Deposition of Levofloxacin with Different Nebulisers Used in Cystic Fibrosis. Int. J. Mol. Sci. 2022, 23, 9597. https://doi.org/10.3390/ijms23179597
Schwarz C, Procaccianti C, Costa L, Brini R, Friend R, Caivano G, Sadafi H, Mussche C, Schwenck N, Hahn M, et al. Differential Performance and Lung Deposition of Levofloxacin with Different Nebulisers Used in Cystic Fibrosis. International Journal of Molecular Sciences. 2022; 23(17):9597. https://doi.org/10.3390/ijms23179597
Chicago/Turabian StyleSchwarz, Carsten, Claudio Procaccianti, Laura Costa, Riccardo Brini, Richard Friend, Grazia Caivano, Hosein Sadafi, Charles Mussche, Nicolas Schwenck, Michael Hahn, and et al. 2022. "Differential Performance and Lung Deposition of Levofloxacin with Different Nebulisers Used in Cystic Fibrosis" International Journal of Molecular Sciences 23, no. 17: 9597. https://doi.org/10.3390/ijms23179597
APA StyleSchwarz, C., Procaccianti, C., Costa, L., Brini, R., Friend, R., Caivano, G., Sadafi, H., Mussche, C., Schwenck, N., Hahn, M., Murgia, X., & Bianco, F. (2022). Differential Performance and Lung Deposition of Levofloxacin with Different Nebulisers Used in Cystic Fibrosis. International Journal of Molecular Sciences, 23(17), 9597. https://doi.org/10.3390/ijms23179597






