Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection
Abstract
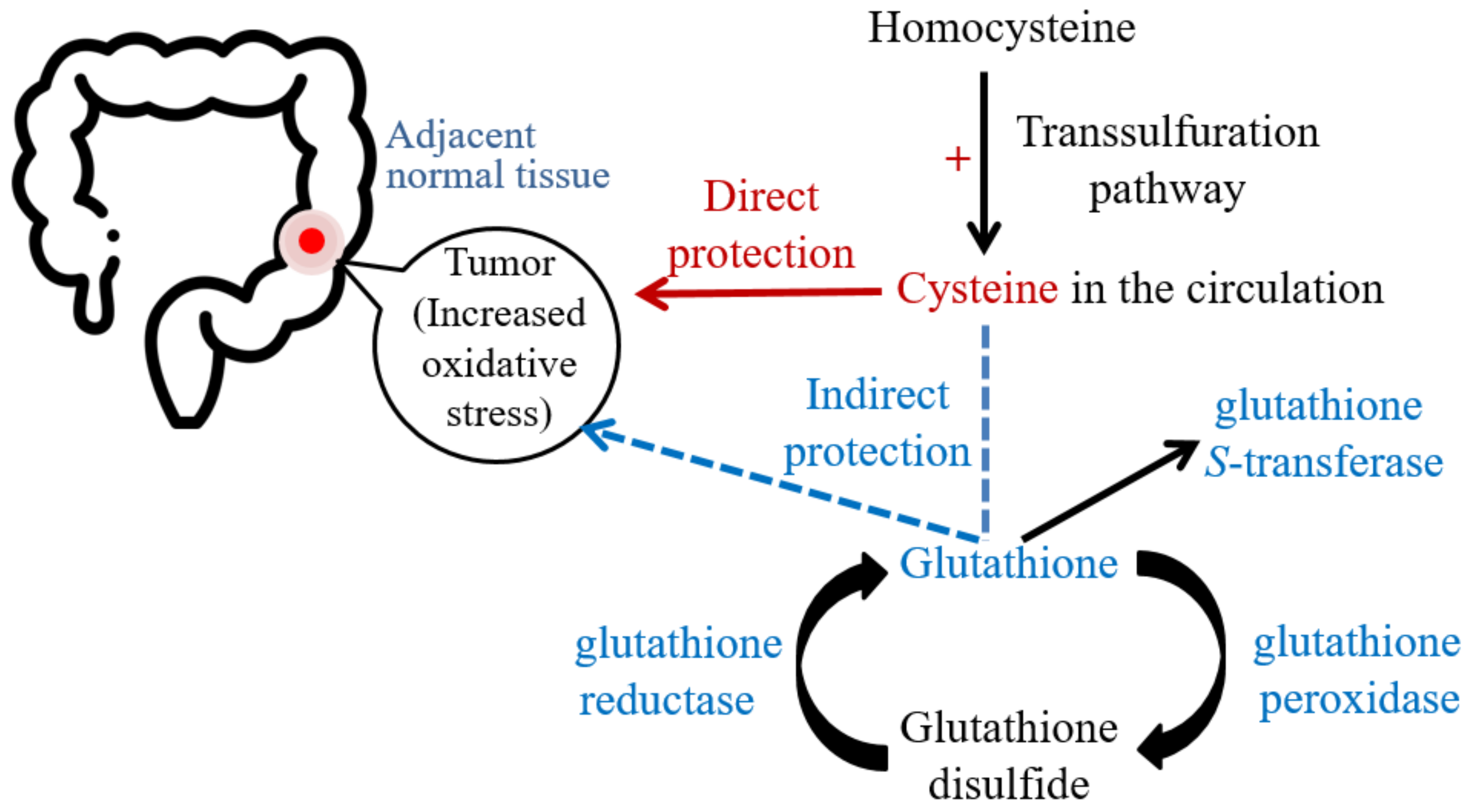
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Size Calculation
4.2. Subjects
4.3. Data Collection and Biochemical Measurements
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gandomani, H.S.; Yousefi, S.M.; Aghajani, M.; Mohammadian-Hafshejani, A.; Tarazoj, A.A.; Pouyesh, V.; Salehiniya, H. Colorectal cancer in the world: Incidence, mortality and risk factors. Biomed. Res. Ther. 2017, 4, 1656–1675. [Google Scholar] [CrossRef] [Green Version]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Wang, F.; Zhao, Y.S.; Pan, H.Z. Evaluation of oxidative stress in colorectal cancer patients. Biomed. Environ. Sci. 2008, 21, 286–289. [Google Scholar] [CrossRef]
- Leufkens, A.M.; van Duijnhoven, F.J.; Woudt, S.H.; Siersema, P.D.; Jenab, M.; Jansen, E.H.J.M.; Pischon, T.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: A cohort-nested case-control study in the European Prospective Investigation into Cancer and Nutrition. Am. J. Epidemiol. 2012, 175, 653–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelczyk, J.K.; Wielkoszyński, T.; Krakowczyk, Ł.; Adamek, B.; Zalewska-Ziob, M.; Gawron, K.; Kasperczyk, J.; Wiczkowski, A. The activity of antioxidant enzymes in colorectal adenocarcinoma and corresponding normal mucosa. Acta Biochim. Pol. 2012, 59, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopčević, K.R.; Rovčanin, B.R.; Tatić, S.B.; Krivokapić, Z.V.; Gajić, M.M.; Dragutinović, V.V. Activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in different stages of colorectal carcinoma. Dig. Dis. Sci. 2013, 58, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Perše, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? Biomed. Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [Green Version]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińnska-Ustymowicz, K. Antioxidant barrier, redox status, and oxidative damage to biomolecules in patients with colorectal cancer. Can malondialdehyde and catalase be markers of colorectal cancer advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Kundaktepe, B.P.; Sozer, V.; Durmus, S.; Kocael, P.C.; Kundaktepe, F.O.; Papila, C.; Gelisgen, R.; Uzun, H. The evaluation of oxidative stress parameters in breast and colon cancer. Medicine 2021, 100, e25104. [Google Scholar] [CrossRef]
- Salehi, S.S.; Mirmiranpour, H.; Rabizadeh, S.; Esteghamati, A.; Tomasello, G.; Alibakhshi, A.; Najafi, N.; Rajab, A.; Nakhjavani, M. Improvement in redox homeostasis after cytoreductive surgery in colorectal adenocarcinoma. Oxid. Med. Cell Longev. 2021, 2021, 8864905. [Google Scholar] [CrossRef]
- Özdemirler, G.; Pabuççuoğlu, H.; Bulut, T.; Buğra, D.; Uysal, M.; Toker, G. Increased lipoperoxide levels and antioxidant system in colorectal cancer. J. Cancer Res. Clin. Oncol. 1998, 124, 555–559. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef]
- Rainis, T.; Maor, I.; Lanir, A.; Shnizer, S.; Lavy, A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Dig. Dis. Sci. 2007, 52, 526–530. [Google Scholar] [CrossRef]
- Kekec, Y.; Paydas, S.; Tuli, A.; Zorludemir, S.; Sakman, G.; Seydaoglu, G. Antioxidant enzyme levels in cases with gastrointestinal cancer. Eur. J. Intern. Med. 2009, 20, 403–406. [Google Scholar] [CrossRef]
- Ighodar, O.M.; Akinoloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX); The fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Fra, A.; Yoboue, E.D.; Sitia, R. Cysteine as redox molecular switches and targets of disease. Front. Mol. Neurosci. 2017, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, X.; Wei, C.; Zheng, D.; Lu, X.; Yang, Y.; Luo, A.; Zhang, K.; Duan, X.; Wang, Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur. J. Pharm. Sci. 2020, 152, 105450. [Google Scholar] [CrossRef]
- Wu, J.; Yeung, S.C.J.; Liu, S.; Qdaisat, A.; Jiang, D.; Liu, W.; Cheng, Z.; Liu, W.; Wang, H.; Li, L.; et al. Cyst(e)ine in nutrition formulation promotes colon cancer growth and chemoresistance by activating mTORC1 and scavenging ROS. Sig. Transduct. Target Ther. 2021, 6, 188. [Google Scholar] [CrossRef]
- Zhang, H.F.; Klein Geltink, R.I.; Parker, S.J.; Sorensen, P.H. Transsulfuration, minor player or crucial for cysteine homeostasis in cancer. Trends Cell Biol. 2022, 32, 800–814. [Google Scholar] [CrossRef]
- Le Marchand, L.; White, K.K.; Nomura, A.M.; Wilkens, L.R.; Selhub, J.S.; Tiirikainen, M.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N. Plasma levels of B vitamins and colorectal cancer risk: The multiethnic cohort study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2195–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, F.F.; Wang, H.M.; Lan, Y.C.; Yang, M.H.; Huang, S.C.; Huang, Y.C. High homocysteine is associated with an increased risk of colorectal cancer independently of oxidative stress and antioxidant capacities. Clin. Nutr. 2014, 33, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Myte, R.; Gylling, B.; Haggstrom, J.; Schneede, J.; Ueland, P.M.; Hallmans, G.; Johansson, I.; Palmqvist, R.; Van Guelpen, B. Untangling the role of one-carbon metabolism in colorectal cancer risk: A comprehensive Bayesian network analysis. Sci. Rep. 2017, 7, 43434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gylling, B.; Myte, R.; Ulvik, A.; Ueland, P.M.; Middttun, Ø.; Schnede, J.; Hallmans, G.; Häggstrőm, J.; Hohansson, I.; Van Guelpen, B.; et al. One-carbon metabolite ratios as functional vitamin markers and in relation to colorectal cancer risk. Int. J. Cancer 2019, 144, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.W.; Beresford, S.A.A.; Neuhouser, M.L.; Cheng, T.Y.D.; Song, X.; Brown, E.C.; Zheng, Y.; Rodriguez, B.; Green, R.; Ulrich, C.M. Homocysteine, cysteine, and risk of incident colorectal cancer in the women’s health initiative observational cohort. Am. J. Clin. Nutr. 2013, 97, 827–834. [Google Scholar] [CrossRef]
- Rio, D.D.; Stwart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. Dis. 2005, 15, 316–328. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeldt, F.; Wilson, M.; Lee, G.; Kure, C.; Ou, R.; Braun, L.; de Haan, J. Oxidative stress in surgery in an ageing population: Pathophysiology and therapy. Exp. Gerontol. 2013, 48, 45–54. [Google Scholar] [CrossRef]
- Stipancic, I.; Zarkovic, N.; Servis, D.; Sabolović, S.; Tatzber, F.; Busic, Z. Oxidative stress markers after laparoscopic and open cholecystectomy. J. Laparoendosc. Adv. Surg. Tech. 2005, 15, 347–352. [Google Scholar] [CrossRef]
- Veljković, A.; Stanojević, G.; Branković, B.; Pavlović, D.; Stojanović, I.; Cvetković, T. Parameters of oxidative stress in colon cancer tissue. Acta Med. Median. 2016, 55, 32–37. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Brit. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Synder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Brit. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef]
- Chombs, J.A.; DeNicola, G.M. The non-essential amino acid cysteine becomes essential for tumor proliferation and survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Chay, K.O.; Kwon, J.; Kwon, S.O.; Park, Y.K.; Lee, T.H. Comparative proteomic analysis of cysteine oxidation in colorectal cancer patients. Mol. Cells 2013, 35, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. List. 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Araki, A.; Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 1987, 422, 43–52. [Google Scholar] [CrossRef]
- Radha Rama Devi, A.; Naushad, S.M.; Prasad, K.C. Evaluation of total plasma homocysteine in Indian newborns using heel-prick samples. Indian J. Pediatr. 2006, 73, 503–508. [Google Scholar] [CrossRef]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic. Biol. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernandez-Ruiz, J.; Garcia-Canovas, F.; Acosta, M. Inhibition by L-ascorbic acid and other antioxidants of the 2.2′ -azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] oxidation catalyzed by peroxidase: A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
| Parameters | Pre-Resection | Post-Resection |
|---|---|---|
| Age (y) | 61.8 ± 11.1 (64.5) | |
| Male/Female | 34/32 | |
| BMI (kg/m2) | 24.6 ± 4.6 (24) | 23.3 ± 4.5 † (23.2) |
| Blood pressure (mmHg) | ||
| systolic | 133.8 ± 14.3 (135) | 135.2 ± 17.8 (135) |
| diastolic | 77.1 ± 10.9 (75) | 79.4 ± 11.4 (79.5) |
| White blood cell (103/μL) | 7.6 ± 3.7 (6.8) | 7.1 ± 2.4 (6.5) |
| Neutrophil-to-lymphocyte ratio | 4.5 ± 4.4 (3.3) | 3.5 ± 3.4 (2.5) |
| Serum C-reactive protein (mg/dL) | 1.0 ± 1.8 (0.3) | 1.2 ± 2.7 (0.3) |
| Serum albumin (g/dL) | 4.1 ± 0.5 (4.1) | 4.2 ± 0.4 ** (4.3) |
| Serum ALT (U/L) | 21.8 ± 22.0 (16) | 30.9 ± 36.8 * (20) |
| Serum creatinine (mg/dL) | 0.9 ± 0.3 (0.8) | 0.9 ± 0.3 (0.9) |
| Serum carcinoembryonic antigen (ng/mL) | 56.8 ± 264.1 (4.7) | 52.0 ± 353.3 † (2.3) |
| Serum carbohydrate antigen 19-9 (U/mL) | 66.7 ± 294.5 (12.4) | 36.3 ± 110.9 ** (10.3) |
| Family history of colorectal cancer (n, %) | 11, 16.7% | |
| Stage at diagnosis (n, %) | ||
| Stage 0 | 2, 3% | |
| Stage I | 8, 12.1% | |
| Stage II | 15, 22.7% | |
| Stage III | 27, 40.9% | |
| Stage IV | 14, 21.2% | |
| Cancer location | ||
| Colon | 32 (48.5%) | |
| Rectum | 34 (51.5%) | |
| Smoking habit (n, %) | ||
| Current | 6, 9.1% | |
| Past | 8, 12.1% | |
| No | 52, 78.8% | |
| Drinking habit (n, %) | ||
| Current | 5, 7.6% | |
| Past | 5, 7.6% | |
| No | 56, 84.8% | |
| Pre-Resection | Post-Resection | p Value | |
|---|---|---|---|
| Cysteine (μmol/L) | 167.42 ± 35.07 | 186.09 ± 40.50 | <0.001 |
| Homocysteine (μmol/L) | 9.94 ± 3.66 | 11.48 ± 3.88 | <0.001 |
| Oxidative stress indicators | |||
| MDA (μmol/L) | 0.97 ± 0.27 | 1.03 ± 0.30 | 0.044 |
| AOPP (μmol/L) | 385.06 ± 157.71 | 479.40 ± 244.00 | 0.012 |
| Antioxidant capacities | |||
| TEAC (μmol/L) | 3909.91 ± 326.45 | 4486.69 ± 395.27 | <0.001 |
| GSH (μmol/L) | 79.28 ± 45.11 | 114.69 ± 47.99 | <0.001 |
| GSSG (μmol/L) | 482.33 ± 64.16 | 578.331 ± 76.43 | <0.001 |
| GSH/GSSG ratio | 0.16 ± 0.07 | 0.20 ± 0.07 | <0.001 |
| GPx (nmol/mL/min) | 166.74 ± 55.28 | 232.44 ± 66.93 | <0.001 |
| GR (nmol/mL/min) | 55.96 ± 18.43 | 67.34 ± 29.07 | <0.001 |
| GST (nmol/mL/min) | 26.01 ± 19.65 | 37.79 ± 21.62 | <0.001 |
| SOD (U/mL/min) | 3.47 ± 1.76 | 3.61 ± 1.41 | 0.220 |
| Tumor Tissue | Adjacent Normal Tissue | p Value | |
|---|---|---|---|
| Homocysteine (μmol/g protein) | 1.00 ± 0.44 | 0.65 ± 0.28 | <0.001 |
| Cysteine (μmol/g protein) | 35.03 ± 20.26 | 18.95 ± 10.01 | <0.001 |
| Oxidative stress indicators | |||
| MDA (μmol/g protein) | 0.20 ± 0.20 | 0.28 ± 0.51 | 0.017 |
| AOPP (μmol/protein) | 130.03 ± 42.34 | 86.42 ± 29.57 | <0.001 |
| Antioxidant capacities | |||
| TEAC (μmol/g protein) | 445.00 ± 177.28 | 394.59 ± 156.99 | 0.020 |
| GSH (μmol/g protein) | 10.90 ± 12.50 | 7.55 ± 4.09 | 0.001 |
| GSSG (μmol/g protein) | 187.96 ± 83.87 | 141.00 ± 42.97 | <0.001 |
| GSH/GSSG ratio | 5.72 ± 4.08 | 5.33 ± 2.20 | 0.448 |
| GPx (nmol/min/g protein) | 136.01 ± 53.24 | 88.21 ± 35.71 | <0.001 |
| GR (nmol/min/g protein) | 157.71 ± 76.98 | 134.20 ± 59.25 | 0.012 |
| GST (nmol/min/g protein) | 32.75 ± 11.44 | 28.49 ± 10.03 | 0.019 |
| SOD (U/min/g protein) | 17.10 ± 6.46 | 14.62 ± 4.15 | 0.003 |
| Plasma Cysteine at Pre-Resection | Plasma Cysteine at Post-Resection | Cysteine in Tumor Tissue | Cysteine in Adjacent Normal Tissue | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma Level at Pre-Resection | Plasma Level at Post-Resection | Tumor Tissue | Adjacent Normal Tissue | Plasma Level at Post-Resection | Tumor Tissue | Adjacent Normal Tissue | Tumor Tissue | Adjacent Normal Tissue | |
| Homocysteine | 0.61 † | 0.36 ** | 0.18 | 0.07 | 0.39 ** | 0.74 † | 0.35 ** | 0.34 ** | 0.63 † |
| Cysteine | − | 0.45 † | 0.21 | 0.19 | − | − | 0.59 † | 0.59 † | − |
| MDA | −0.23 | −0.15 | 0.36 ** | 0.37 ** | 0.17 | 0.20 | 0.32 * | 0.34 ** | 0.14 |
| AOPP | 0.24 | −0.09 | 0.15 | −0.01 | 0.03 | 0.05 | 0.32 * | −0.18 | 0.44 † |
| TEAC | 0.35 ** | 0.08 | 0.42 ** | 0.21 | 0.03 | 0.62 † | 0.42 ** | 0.48 † | 0.57 † |
| GSH | 0.00 | 0.00 | 0.29 * | 0.09 | 0.31 * | −0.05 | 0.11 | 0.05 | 0.37 ** |
| GSSG | 0.04 | −0.29 * | 0.50 † | 0.23 | 0.34 ** | 0.56 † | 0.31 * | 0.42 ** | 0.71† |
| GSH/GSSG ratio | −0.01 | −0.06 | −0.02 | −0.14 | 0.25 | −0.40 † | −0.23 | −0.19 | −0.20 |
| GPx | −0.07 | 0.09 | 0.24 | 0.15 | 0.25 | 0.48 † | 0.36 ** | 0.41 ** | 0.49 † |
| GR | 0.11 | −0.08 | −0.00 | 0.01 | 0.06 | 0.63 † | 0.51 † | 0.46 † | 0.80 † |
| GST | 0.22 | −0.02 | 0.14 | 0.01 | 0.02 | 0.46 † | 0.33 * | 0.23 | 0.52 † |
| SOD | 0.16 | −0.03 | 0.26 | 0.07 | −0.11 | 0.27 * | 0.33 * | 0.19 | 0.56 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, F.-F.; Chao, T.-H.; Huang, S.-C.; Cheng, C.-H.; Tseng, Y.-Y.; Huang, Y.-C. Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection. Int. J. Mol. Sci. 2022, 23, 9581. https://doi.org/10.3390/ijms23179581
Chiang F-F, Chao T-H, Huang S-C, Cheng C-H, Tseng Y-Y, Huang Y-C. Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection. International Journal of Molecular Sciences. 2022; 23(17):9581. https://doi.org/10.3390/ijms23179581
Chicago/Turabian StyleChiang, Feng-Fan, Te-Hsin Chao, Shih-Chien Huang, Chien-Hsiang Cheng, Yu-Yao Tseng, and Yi-Chia Huang. 2022. "Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection" International Journal of Molecular Sciences 23, no. 17: 9581. https://doi.org/10.3390/ijms23179581
APA StyleChiang, F.-F., Chao, T.-H., Huang, S.-C., Cheng, C.-H., Tseng, Y.-Y., & Huang, Y.-C. (2022). Cysteine Regulates Oxidative Stress and Glutathione-Related Antioxidative Capacity before and after Colorectal Tumor Resection. International Journal of Molecular Sciences, 23(17), 9581. https://doi.org/10.3390/ijms23179581







