Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10® to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies
Abstract
:1. Background
2. Results
2.1. Sample and Assay Characteristics
2.2. Imprecision
2.3. Comparison of IFX and ADAL trough Levels
2.3.1. Comparison between i-TRACK10®and Lisa Tracker® for IFX and ADAL Quantification
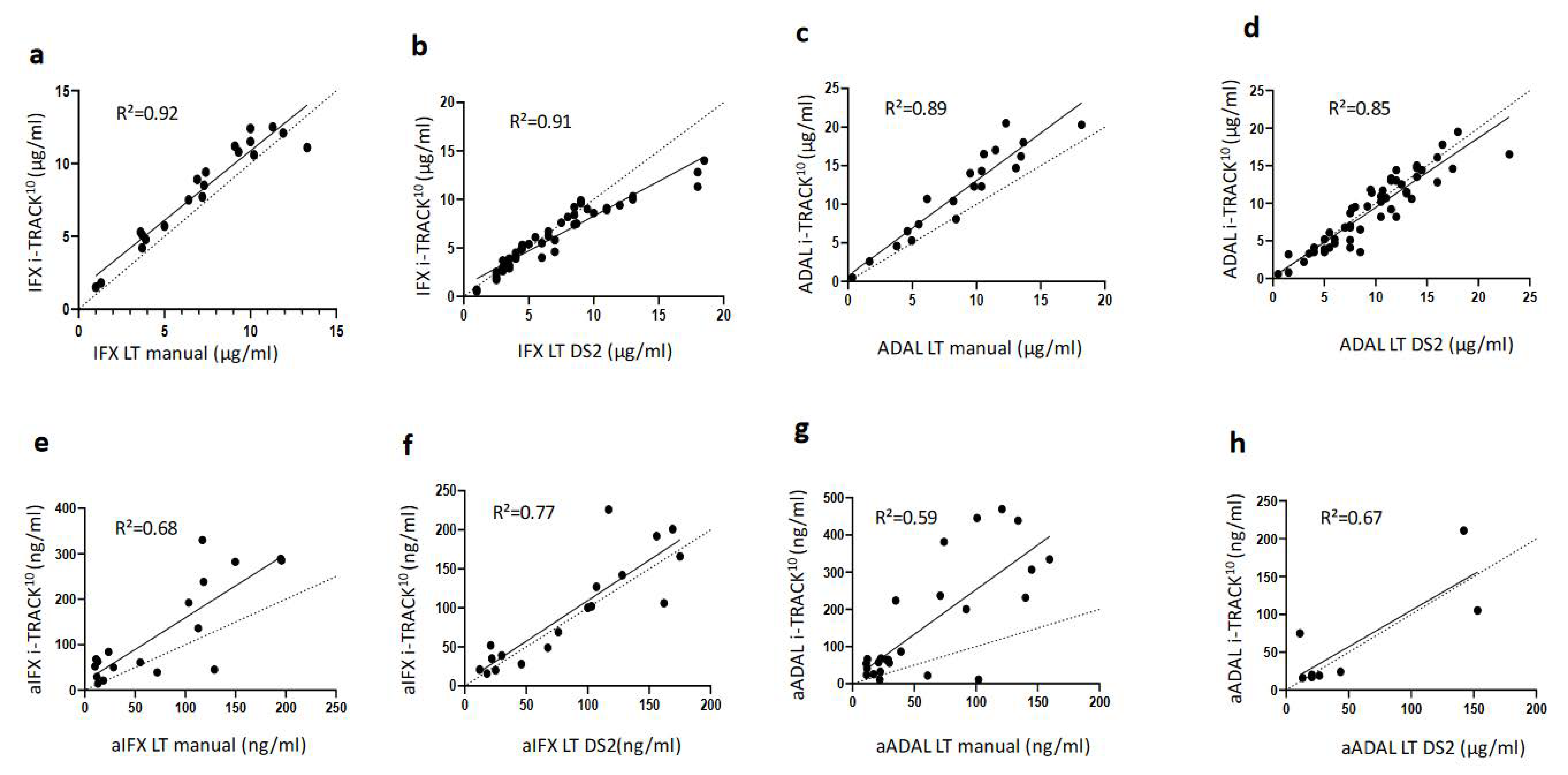
2.3.2. Correlation between i-TRACK10®and Lisa-Tracker® Assay Results
2.4. Comparison of Anti-IFX and Anti-ADAL ADA Testing
2.4.1. Good Agreement between i-TRACK10®and Lisa-Tracker® Measurements of aIFX and aADAL
2.4.2. Correlations between i-TRACK10®and Lisa-Tracker® Assays for ADA Detection and Measurements
2.5. Sample to Sample Carryover
3. Discussion
4. Methods
4.1. Patients and Samples
4.2. Methods
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. AMS 2014, 10, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Udalova, I.; Monaco, C.; Nanchahal, J.; Feldmann, M. Anti-TNF Therapy. Microbiol. Spectr. 2016, 4, 4.4.46. [Google Scholar] [CrossRef]
- Atiqi, S.; Hooijberg, F.; Loeff, F.C.; Rispens, T.; Wolbink, G.J. Immunogenicity of TNF-Inhibitors. Front. Immunol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Brynskov, J.; Thomsen, O.Ø.; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Pedersen, G.; Kjeldsen, J.; Jacobsen, B.A.; Oxholm, A.S.; et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut 2014, 63, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Martelli, L.; Olivera, P.; Roblin, X.; Attar, A.; Peyrin-Biroulet, L. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: A systematic review. J. Gastroenterol. 2017, 52, 19–25. [Google Scholar] [CrossRef]
- Gorovits, B.; Baltrukonis, D.J.; Bhattacharya, I.; Birchler, M.A.; Finco, D.; Sikkema, D.; Vincent, M.S.; Lula, S.; Marshall, L.; Hickling, T.P. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin. Exp. Immunol. 2018, 192, 348–365. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Kadian, N.; Raju KS, R.; Rashid, M.; Malik, M.Y.; Taneja, I.; Wahajuddin, M. Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry. J. Pharm. Biomed. Anal. 2016, 126, 83–97. [Google Scholar] [CrossRef]
- Noguier, G.; Montaillier, C.; Daviere, S.; Colombeau, L.; Parussini, E. Development and evaluation of i-Tracker Infliximab and i-Tracker Anti-Infliximab kits: Fast and innovative chemiluminescent assays for the monitoring of patients treated with Infliximab. J. Crohns & Colitis 2020, 14, S563. [Google Scholar]
- Ruiz-Argüello, B.; del Agua, A.R.; Torres, N.; Monasterio, A.; Martínez, A.; Nagore, D. Comparison study of two commercially available methods for the determination of infliximab, adalimumab, etanercept and anti-drug antibody levels. Clin. Chem. Lab. Med. CCLM 2013, 51, e287–e289. [Google Scholar] [CrossRef] [PubMed]
- Bertin, D.; Serrero, M.; Grimaud, J.C.; Desjeux, A.; Desplat-Jégo, S. Monitoring of infliximab trough levels and anti-infliximab antibodies in inflammatory bowel diseases: A comparison of three commercially available ELISA kits. Cytokine 2020, 126, 154859. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Salvador-Martín, S.; Marín-Jiménez, I.; Menchén, L.A.; López-Cauce, B.; López-Fernández, L.A.; Lucendo, A.J. Comparison of a new rapid method for determination of serum anti-adalimumab and anti-infliximab antibodies with two established ELISA kits. J. Pharm. Biomed. Anal. 2021, 198, 114003. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Labetoulle, R.; Harzallah, I.; Berger, A.E.; Roblin, X.; Paul, S. Comparison of Point-of-Care and Classical Immunoassays for the Monitoring Infliximab and Antibodies Against Infliximab in IBD. Dig. Dis. Sci. 2018, 63, 2714–2721. [Google Scholar] [CrossRef]
- Steenholdt, C.; Ainsworth, M.A.; Tovey, M.; Klausen, T.W.; Thomsen, O.Ø.; Brynskov, J.; Bendtzen, K. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn’s disease. Ther. Drug Monit. 2013, 35, 530–538. [Google Scholar] [CrossRef]
- Nakase, H.; Motoya, S.; Matsumoto, T.; Watanabe, K.; Hisamatsu, T.; Yoshimura, N.; Ishida, T.; Kato, S.; Nakagawa, T.; Esaki, M.; et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: A subanalysis of the DIAMOND trial. Aliment. Pharmacol. Ther. 2017, 46, 873–882. [Google Scholar] [CrossRef]
- Roblin, X.; Marotte, H.; Rinaudo, M.; Del Tedesco, E.; Moreau, A.; Phelip, J.M.; Genin, C.; Peyrin–Biroulet, L.; Paul, S. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, 80–84.e2. [Google Scholar] [CrossRef]
- Pouw, M.F.; Krieckaert, C.L.; Nurmohamed, M.T.; van der Kleij, D.; Aarden, L.; Rispens, T.; Wolbink, G. Key findings towards optimising adalimumab treatment: The concentration-effect curve. Ann. Rheum. Dis. 2015, 74, 513–518. [Google Scholar] [CrossRef]
- Bortlik, M.; Duricova, D.; Malickova, K.; Machkova, N.; Bouzkova, E.; Hrdlicka, L.; Komarek, A.; Lukas, M. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J. Crohns Colitis 2013, 7, 736–743. [Google Scholar] [CrossRef]
- Moore, C.; Corbett, G.; Moss, A.C. Systematic Review and Meta-Analysis: Serum Infliximab Levels During Maintenance Therapy and Outcomes in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 619–625. [Google Scholar] [CrossRef]
- Paul, S.; Del Tedesco, E.; Marotte, H.; Rinaudo-Gaujous, M.; Moreau, A.; Phelip, J.M.; Genin, C.; Peyrin-Biroulet, L.; Roblin, X. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2013, 19, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Pérez, I.; Fernández, L.; Sánchez-Ramón, S.; Alba, C.; Zatarain, A.; Cañas, M.; López, O.N.; Olivares, D.; Rey, E.; Taxonera, C. Reliability evaluation of four different assays for therapeutic drug monitoring of infliximab levels. Ther. Adv. Gastroenterol. 2018, 11, 1756284818783613. [Google Scholar] [CrossRef] [PubMed]

| Antigen | Method | Measurement Range | Interference | |||||
|---|---|---|---|---|---|---|---|---|
| IFX/ADAL | aIFX/aADAL ADAs | IFX | ADAL | aIFX | aADAL | |||
| Lisa Tracker® assays | Human recombinant TNF | Infliximab Adalimumab | Manual or automated (DS2) ELISA | 0.3–20 µg/mL | 10–200 ng/mL | 10–160 ng/mL | No influence onhaemolysis, bilirubin, triglyceride, RF. No cross-reaction with other anti-TNF molecules nor with rituximab. | |
| i-TRACK10® assay | Human recombinant TNF | Infliximab Adalimumab | CLIA | 0.3–24 µg/mL | 0.5–24 µg/mL | 10–2000 ng/mL | No influence of haemolysis (2 mg/mL), bilirubin (0.2 mg/mL), triglyceride (10 mg/mL), RF (1000 UI/mL), biotin (2000 ng/mL). No cross-reactivity with other anti-TNF biologics, ustekinumab/vedolizumab, nor with aADAL ADAs (for IFX), nor with aIFX ADAs (for ADAL). | |
| Laboratory A | Laboratory B | |||
|---|---|---|---|---|
| Low or Medium | High | Low or Medium | High | |
| Mean IFX—µg/mL (Intra-run—CV%) | 1.6 (8.2) | 9.1 (11.3) | 2.1 (5.8) | 10.7 (8.3) |
| Mean IFX—µg/mL (Inter-run—CV%) | 2.3 (11.7) | 11.7 (7.3) | 2.3 (16.1) | 10.3 (10) |
| Mean ADAL—µg/mL (Intra-run—CV%) | 1 (8.1) | 13.3 (10.3) | ND | ND |
| Mean ADAL—µg/mL (Inter-run—CV%) | 3.7 (9.3) | 12.5 (12.8) | ||
| Mean aIFX ADAs—ng/mL (Intra-run—CV%) | 30 (3.7) | 102 (3.7) | 56 (1.8) | 586 (2.6) |
| Mean aIFX ADAs—ng/mL (Inter-run—CV%) | 60 (6.3) | 603 (4.8) | 61 (13.5) | 594 (10.2) |
| Mean aADAL ADAs—ng/mL (Intra-run—CV%) | 37 (1.8) | 212 (2.2) | ND | ND |
| Mean aADAL ADAs—ng/mL (Inter-run—CV%) | 53 (4.1) | 505 (8.6) | ||
| IFX | I-TRACK10 | |||
|---|---|---|---|---|
| Manual LT DS2 LT | <3 µg/mL | 3–7 µg/mL | >7 µg/mL | TOTAL |
| <3 µg/mL | 2 7 | 0 0 | 0 0 | 2 7 |
| 3–7 µg/mL | 0 1 | 5 24 | 2 0 | 7 25 |
| >7 µg/mL | 0 0 | 0 0 | 11 18 | 11 18 |
| TOTAL | 2 8 | 5 24 | 13 18 | 20 50 |
| Kappa value | 0.81 0.97 | |||
| ADAL | I-TRACK10 | |||
| Manual LT DS2 LT | <5 µg/mL | 5–8 µg/mL | >8 µg/mL | TOTAL |
| <5 µg/mL | 3 7 | 2 0 | 0 0 | 5 7 |
| 5–8 µg/mL | 0 6 | 1 8 | 1 2 | 2 16 |
| >8 µg/mL | 0 0 | 0 2 | 13 30 | 13 32 |
| TOTAL | 3 13 | 3 10 | 14 32 | 20 55 |
| Kappa value | 0.7 0.66 | |||
| aIFX | I-TRACK10 | |||
| Manual LT DS2 LT | <100 ng/mL | ≥100 ng/mL | TOTAL | |
| <100 ng/mL | 10 9 | 0 0 | 10 9 | |
| ≥100 ng/mL | 1 0 | 7 9 | 8 9 | |
| TOTAL | 11 9 | 7 9 | 18 18 | |
| Kappa value | 0.89 1 | |||
| aADAL | I-TRACK10 | |||
| Manual LT DS2 LT | <100 ng/mL | ≥100 ng/mL | TOTAL | |
| <100 ng/mL | 35 4 | 4 0 | 39 4 | |
| ≥100 ng/mL | 2 0 | 9 2 | 11 2 | |
| TOTAL | 37 4 | 13 2 | 50 6 | |
| Kappa value | 0.67 1 | |||
| Manual Lisa Tracker® | DS2 L Tracker® (Specific to the Automated Process) | I-TRACK10® | |
|---|---|---|---|
| Advantages | Reliable and robust test to quantify drugs and anti-drug antibodies Precision, sensibility and specificity of the dosage Many molecules and their specific antibodies are available | No risk of human error affecting sample dilution and distribution | Random-access instrument: decreased time to access the result for clinicians (about 35 min to obtain the first result) Standardization thanks to the automated process Reducing the chance of data input errors with automated transmission to the informatics lab system Expansion of the measuring range, especially for ADA, useful for therapeutic de-escalation Precision, sensibility and specificity of the dosage Possibility of dosing multiple drugs and anti-drugs antibodies in the same round Cost |
| Disadvantages | Need to work in series Unavoidable risk of technical issues (manual dilution of samples, distribution, results entry) Incompressible technical time linked to manual steps Increased time-to-result (about 120 min to obtain the first result) | Risk of machine failure | Risk of machine failure Delay of the accessibility to the dosage in the instrument (for molecules infrequently prescribed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, A.E.; Gleizes, A.; Waeckel, L.; Roblin, X.; Krzysiek, R.; Hacein-Bey-Abina, S.; Soriano, A.; Paul, S. Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10® to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies. Int. J. Mol. Sci. 2022, 23, 9561. https://doi.org/10.3390/ijms23179561
Berger AE, Gleizes A, Waeckel L, Roblin X, Krzysiek R, Hacein-Bey-Abina S, Soriano A, Paul S. Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10® to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies. International Journal of Molecular Sciences. 2022; 23(17):9561. https://doi.org/10.3390/ijms23179561
Chicago/Turabian StyleBerger, Anne Emmanuelle, Aude Gleizes, Louis Waeckel, Xavier Roblin, Roman Krzysiek, Salima Hacein-Bey-Abina, Alessandra Soriano, and Stephane Paul. 2022. "Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10® to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies" International Journal of Molecular Sciences 23, no. 17: 9561. https://doi.org/10.3390/ijms23179561
APA StyleBerger, A. E., Gleizes, A., Waeckel, L., Roblin, X., Krzysiek, R., Hacein-Bey-Abina, S., Soriano, A., & Paul, S. (2022). Validation Study of a New Random-Access Chemiluminescence Immunoassay Analyzer i-TRACK10® to Monitor Infliximab and Adalimumab Serum trough Levels and Anti-Drug Antibodies. International Journal of Molecular Sciences, 23(17), 9561. https://doi.org/10.3390/ijms23179561







