Peptidylarginine Deiminase 2 Gene Polymorphisms in Subjects with Periodontitis Predispose to Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Associations between PADI SNPs and Periodontitis
2.3. Associations between PADI SNPs and RA in Subjects with Self-Reported Periodontitis
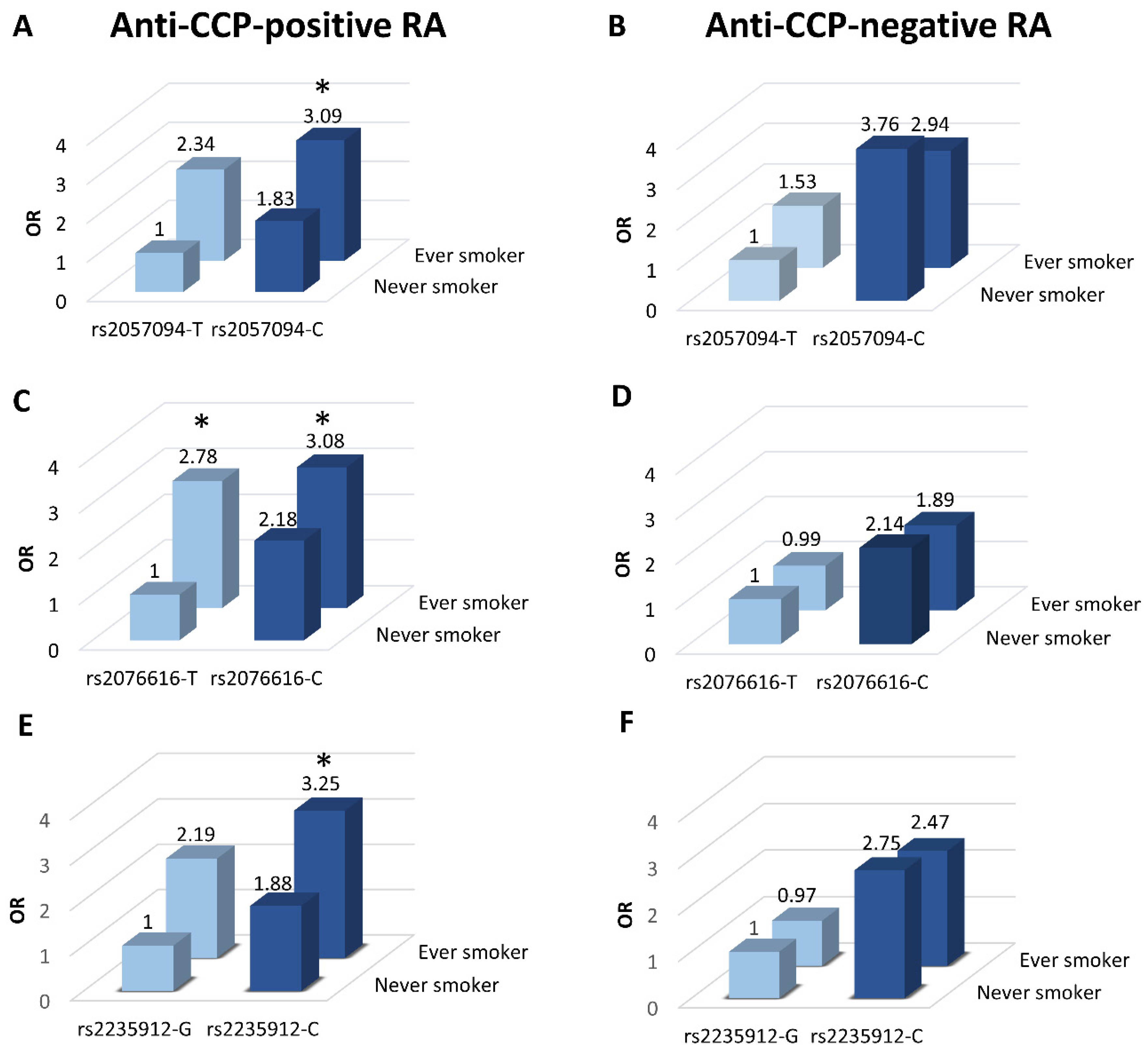
2.4. Associations between PADI SNPs and RA by Smoking Status
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. SNP Selection
4.3. Genotyping
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.A.; Kim, J.H.; Kim, Y.M.; Lee, J.Y.; Kim, K.H.; Lee, E.Y.; Lee, E.B.; Lee, Y.M.; Song, Y.W. Periodontitis is associated with rheumatoid arthritis: A study with longstanding rheumatoid arthritis patients in Korea. Korean J. Intern. Med. 2016, 31, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Äyräväinen, L.; Leirisalo-Repo, M.; Kuuliala, A.; Ahola, K.; Koivuniemi, R.; Meurman, J.H.; Heikkinen, A.M. Periodontitis in early and chronic rheumatoid arthritis: A prospective follow-up study in Finnish population. BMJ Open 2017, 7, e011916. [Google Scholar] [CrossRef] [Green Version]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to mouth: A systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front. Immunol. 2016, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Holmstrup, P.; Damgaard, C.; Olsen, I.; Klinge, B.; Flyvbjerg, A.; Nielsen, C.H.; Hansen, P.R. Comorbidity of periodontal disease: Two sides of the same coin? An introduction for the clinician. J. Oral. Microbiol. 2017, 9, 1332710. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019, 8, 1309. [Google Scholar] [CrossRef] [Green Version]
- Marotte, H.; Farge, P.; Gaudin, P.; Alexandre, C.; Mougin, B.; Miossec, P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann. Rheum. Dis. 2006, 65, 905–909. [Google Scholar] [CrossRef] [Green Version]
- Gehlot, P.; Volk, S.L.; Rios, H.F.; Jepsen, K.J.; Holoshitz, J. Spontaneous destructive periodontitis and skeletal bone damage in transgenic mice carrying a human shared epitopecoding HLA-DRB1 allele. RMD Open 2016, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef]
- Gravallese, E.M. Bone destruction in arthritis. Ann. Rheum. Dis. 2002, 61, 84–86. [Google Scholar] [CrossRef]
- Hill, J.; Southwood, S.; Sette, A.; Jevnikar, A.M.; Bell, D.; Cairns, E. Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 2003, 171, 538–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Padyukov, L.; Alfredsson, L. Smoking as a trigger for inflammatory rheumatic diseases. Curr. Opin. Rheumatol. 2007, 19, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Asma, S. Smoking-attributable periodontitis in the United States: Findings from NHANES III. J. Periodontol. 2005, 71, 743–751. [Google Scholar] [CrossRef]
- Schellekens, G.A.; De Jong, B.A.W.; Van Den Hoogen, F.H.J.; Van De Putte, L.B.A.; Van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Nesse, W.; Westra, J.; Van Der Wal, J.E.; Abbas, F.; Nicholas, A.P.; Vissink, A.; Brouwer, E. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J. Clin. Periodontol. 2012, 39, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Harvey, G.P.; Fitzsimmons, T.R.; Dhamarpatni, A.A.S.S.K.; Marchant, C.; Haynes, D.R.; Bartold, P.M. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J. Periodontal. Res. 2013, 48, 252–261. [Google Scholar] [CrossRef]
- Engström, M.; Eriksson, K.; Lee, L.; Hermansson, M.; Johansson, A.; Nicholas, A.P.; Gerasimcik, N.; Lundberg, K.; Klareskog, L.; Catrina, A.I.; et al. Increased citrullination and expression of peptidylarginine deiminases independently of P. gingivalis and A. actinomycetemcomitans in gingival tissue of patients with periodontitis. J. Transl. Med. 2018, 16, 214. [Google Scholar] [CrossRef]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Méchin, M.C.; Vincent, C.; Nachat, R.; Yamada, M.; Takahara, H.; et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef]
- De Rycke, L.; Nicholas, A.P.; Cantaert, T.; Kruithof, E.; Echols, J.D.; Vandekerckhove, B.; Veys, E.M.; De Keyser, F.; Baeten, D. Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis Rheum. 2005, 52, 2323–2330. [Google Scholar] [CrossRef]
- Chang, X.; Yamada, R.; Suzuki, A.; Sawada, T.; Yoshino, S.; Tokuhiro, S.; Yamamoto, K. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology 2005, 44, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 62, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Ricardo, P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Jon, T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2017, 8, 369ra176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryzek, D.; Ciaston, I.; Dobosz, E.; Gasiorek, A.; Makarska, A.; Sarna, M.; Eickid, S.; Puklo, M.; Lech, M.; Potempa, B.; et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. 2019, 15, e1007773. [Google Scholar] [CrossRef]
- Spengler, J.; Lugonja, B.; Jimmy Ytterberg, A.; Zubarev, R.A.; Creese, A.J.; Pearson, M.J.; Grant, M.M.; Milward, M.; Lundberg, K.; Buckley, C.D.; et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015, 67, 3135–3145. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamada, R.; Chang, X.; Tokuhiro, S.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Nakayama-Hamada, M.; Kawaida, R.; Ono, M.; et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 2003, 34, 395–402. [Google Scholar] [CrossRef]
- Too, C.L.; Murad, S.; Dhaliwal, J.S.; Larsson, P.; Jiang, X.; Ding, B.; Alfredsson, L.; Klareskog, L.; Padyukov, L. Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: Evidence from MyEIRA study and meta-analysis. Arthritis Res. Ther. 2012, 14, R250. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Xia, Y.; Pan, J.; Meng, Q.; Zhao, Y.; Yan, X. PADI2 is significantly associated with rheumatoid arthritis. PLoS ONE 2013, 8, e81259. [Google Scholar] [CrossRef]
- Naranbhai, V.; Fairfax, B.P.; Makino, S.; Humburg, P.; Wong, D.; Ng, E.; Hill, A.V.S.; Knight, J.C. Genomic modulators of gene expression in human neutrophils. Nat. Commun. 2015, 6, 7545. [Google Scholar] [CrossRef] [Green Version]
- Massarenti, L.; Enevold, C.; Damgaard, D.; Ødum, N.; Garred, P.; Frisch, M.; Shelef, M.A.; Jacobsen, S.; Nielsen, C.H. PADI4 Polymorphisms Confer Risk of Anti-CCP-Positive Rheumatoid Arthritis in Synergy With HLA-DRB1*04 and Smoking. Front. Immunol. 2021, 12, 707690. [Google Scholar] [CrossRef]
- Pedersen, M.; Jacobsen, S.; Klarlund, M.; Pedersen, B.V.; Wiik, A.; Wohlfahrt, J.; Frisch, M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res. Ther. 2006, 8, R133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochi, Y.; Thabet, M.M.; Suzuki, A.; Okada, Y.; Daha, N.A.; Toes, R.E.M.; Huizinga, T.W.J.; Myouzen, K.; Kubo, M.; Yamada, R.; et al. PADI4 polymorphism predisposes male smokers to rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Nise, L.; Kats, A.; Luttropp, E.; Catrina, A.I.; Askling, J.; Jansson, L.; Alfredsson, L.; Klareskog, L.; Lundberg, K.; et al. Prevalence of periodontitis in patients with established rheumatoid arthritis: A swedish population based case-control study. PLoS ONE 2016, 11, e0155956. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, K.; Fei, G.; Lundmark, A.; Benchimol, D.; Lee, L.; Hu, Y.O.O.; Kats, A.; Saevarsdottir, S.; Catrina, A.I.; Klinge, B.; et al. Periodontal Health and Oral Microbiota in Patients with Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolt, P.; Bengtsson, C.; Nordmark, B.; Lindblad, S.; Lundberg, I.; Klareskog, L.; Alfredsson, L. Quantification of the influence of cigarette smoking on rheumatoid arthritis: Results from a population based case-control study, using incident cases. Ann. Rheum. Dis. 2003, 62, 835–841. [Google Scholar] [CrossRef]
- Damgaard, C.; Danielsen, A.K.; Enevold, C.; Reinholdt, J.; Holmstrup, P.; Nielsen, C.H.; Massarenti, L. Circulating antibodies against leukotoxin A as marker of periodontitis Grades B and C and oral infection with Aggregatibacter actinomycetemcomitans. J. Periodontol. 2021, 92, 1795–1804. [Google Scholar] [CrossRef]
- Sur Chowdhury, C.; Giaglis, S.; Walker, U.A.; Buser, A.; Hahn, S.; Hasler, P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: Analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 2014, 16, R122. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Qu, S.; Alam, H.B.; Williams, A.M.; Wu, Z.; Deng, Q.; Pan, B.; Zhou, J.; Liu, B.; Duan, X.; et al. Peptidylarginine deiminase 2 has potential as both a biomarker and therapeutic target of sepsis. JCI Insight 2020, 5, e138873. [Google Scholar] [CrossRef]
- Abbood, H.M.; Hinz, J.; Cherukara, G.; Macfarlane, T.V. Validity of self-reported periodontal disease: A systematic review and meta-analysis. J. Periodontol. 2016, 87, 1474–1483. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Massarenti, L.; Enevold, C.; Damgaard, D.; Ødum, N.; Nielsen, C.H.; Jacobsen, S. Peptidylarginine deiminase-4 gene polymorphisms are associated with systemic lupus erythematosus and lupus nephritis. Scand. J. Rheumatol. 2019, 48, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Frisch, M.; Madsen, H.O.; Garred, P.; Jacobsen, S. Smoking and polymorphisms of genes encoding mannose-binding lectin and surfactant protein-D in patients with rheumatoid arthritis. Rheumatol. Int. 2014, 34, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Enevold, C.; Oturai, A.B.; Sørensen, P.S.; Ryder, L.P.; Koch-Henriksen, N.; Bendtzen, K. Multiple sclerosis and polymorphisms of innate pattern recognition receptors TLR1-10, NOD1-2, DDX58, and IFIH1. J. Neuroimmunol. 2009, 212, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.A.; Graves-Lindsay, T.; Howe, K.; Bouk, N.; Chen, H.C.; Kitts, P.A.; Murphy, T.D.; Pruitt, K.D.; Thibaud-Nissen, F.; Albracht, D.; et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome. Res. 2017, 27, 849–864. [Google Scholar] [CrossRef] [Green Version]

| Anti-CCP-Positive RA (n = 300) | Anti-CCP-Negative RA (n = 134) | Controls (n = 524) | ||
|---|---|---|---|---|
| Age at study inclusion, median (range), years | 52 (19–68) | 53 (21–69) | 53 (19–69) | |
| Women, n (%) | 202 (67%) | 103 (77%) | 321 (61%) | |
| Ever smoker, n (%) | 222 (74%) | 83 (62%) | 319 (61%) | |
| European ancestry (%) | 99% | 99% | 99% | |
| Self-reported periodontitis, n (%) | 93 (31%) | 44 (33%) | 161 (31%) | |
| Age at periodontitis onset, median (range), years | 38 (7–61) * | 35 (10–65) ** | 35 (8–66) *** | |
| Age at RA diagnosis, median (range), years | 51 (18–65) | 50 (22–66) | NA | |
| HLA-DRB1*04 | Self-reported periodontitis yes, n (%) | 68 (73.1%) | 12 (27.3%) | 52 (32.3%) |
| Self-reported periodontitis no, n (%) | 153 (73.9%) | 36 (40%) | 136 (37.5%) | |
| HLA-DRB1*01 | Self-reported periodontitis yes, n (%) | 15 (16.1%) | 5 (11.4%) | 29 (18%) |
| Self-reported periodontitis no, n (%) | 63 (30.4%) | 22 (24.4%) | 71 (19.6%) | |
| pp | pq | OR (Trend Test) | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|
| rs2057094 | TT | TC | CC | |||
| Population controls | 66 | 69 | 26 | |||
| RA | 41 | 67 | 29 | 1.42 | [1.02; 1.98] | 0.03 |
| Anti-CCP-positive RA | 31 | 44 | 18 | 1.28 | [0.89; 1.84] | 0.18 |
| Anti-CCP-negative RA | 10 | 23 | 11 | 1.75 | [1.08; 2.84] | 0.02 |
| rs2076616 | TT | TC | CC | |||
| Population controls | 84 | 58 | 19 | |||
| RA | 60 | 56 | 21 | 1.35 | [0.96; 1.88] | 0.08 |
| Anti-CCP-positive RA | 44 | 37 | 12 | 1.21 | [0.83; 1.76] | 0.32 |
| Anti-CCP-negative RA | 16 | 19 | 9 | 1.67 | [1.04; 2.68] | 0.04 |
| rs2235912 | GG | GC | CC | |||
| Population controls | 67 | 69 | 25 | |||
| RA | 39 | 70 | 28 | 1.48 | [1.06; 2.07] | 0.02 |
| Anti-CCP-positive RA | 29 | 49 | 15 | 1.28 | [0.88; 1.86] | 0.20 |
| Anti-CCP-negative RA | 10 | 21 | 13 | 2.04 | [1.25; 3.34] | 0.004 |
| rs1005753 | TT | TG | GG | |||
| Population controls | 61 | 80 | 20 | |||
| RA | 43 | 76 | 18 | 1.12 | [0.79; 1.60] | 0.52 |
| Anti-CCP-positive RA | 28 | 54 | 11 | 1.14 | [0.76; 1.70] | 0.52 |
| Anti-CCP-negative RA | 15 | 22 | 7 | 1.06 | [0.64; 1.77] | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massarenti, L.; Enevold, C.; Damgaard, D.; Hansen, P.R.; Frisch, M.; Ødum, N.; Jacobsen, S.; Nielsen, C.H. Peptidylarginine Deiminase 2 Gene Polymorphisms in Subjects with Periodontitis Predispose to Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 9536. https://doi.org/10.3390/ijms23179536
Massarenti L, Enevold C, Damgaard D, Hansen PR, Frisch M, Ødum N, Jacobsen S, Nielsen CH. Peptidylarginine Deiminase 2 Gene Polymorphisms in Subjects with Periodontitis Predispose to Rheumatoid Arthritis. International Journal of Molecular Sciences. 2022; 23(17):9536. https://doi.org/10.3390/ijms23179536
Chicago/Turabian StyleMassarenti, Laura, Christian Enevold, Dres Damgaard, Peter Riis Hansen, Morten Frisch, Niels Ødum, Søren Jacobsen, and Claus Henrik Nielsen. 2022. "Peptidylarginine Deiminase 2 Gene Polymorphisms in Subjects with Periodontitis Predispose to Rheumatoid Arthritis" International Journal of Molecular Sciences 23, no. 17: 9536. https://doi.org/10.3390/ijms23179536
APA StyleMassarenti, L., Enevold, C., Damgaard, D., Hansen, P. R., Frisch, M., Ødum, N., Jacobsen, S., & Nielsen, C. H. (2022). Peptidylarginine Deiminase 2 Gene Polymorphisms in Subjects with Periodontitis Predispose to Rheumatoid Arthritis. International Journal of Molecular Sciences, 23(17), 9536. https://doi.org/10.3390/ijms23179536






