Abstract
The application of immobilized sulfuric acid on silica gel (H2SO4-SiO2) as an efficient and easily reusable solid catalyst was explored in the synthesis of novel α-acyloxycarboxamide derivatives via a Passerini reaction of benzoic acid, aldehyde/ketone, and isocyanides. The Passerini adducts were obtained in high to excellent yields within 10 min in aqueous media under catalytic conditions. The key advantages of the process include a short reaction time, high yields, the catalyst’s low cost, and the catalyst’s reusability.
1. Introduction
Multicomponent reactions (MCRs) involve more than two starting components to produce a new product that incorporates structural features of each reagent [1]. MCRs have the advantage of simplicity and synthetic efficiency over conventional chemical reactions, in addition to developing structural complexity in a single step. Selectivity, synthetic convergency, and atom economy are all advantages of the most useful MCRs [2,3]. MCRs are the foundations of both combinatorial chemistry and diversity-oriented synthesis and have thus played a key role in the development of current synthetic methodologies for pharmaceutical and drug discovery research [4]. Combinatorial chemistry approaches can be utilized to introduce or broaden structural variations in a target lead compound [5]. Therefore, diversity-oriented synthesis is valuable for probing large areas of chemical structure space in the search for new bioactive small molecules that may be undetected by traditional natural product screening tests. The two approaches are complementary, and they both contribute to the ability of MCRs to generate structural complexity [6].
Isocyanide-based MCRs have become a common organic chemical process in the pharmaceutical sector for the production of compound libraries of low-molecular-weight drug-like molecules. Isocyanide-based MCRs have been frequently used in the Ugi and Passerini reactions [7]. The range of bond-forming mechanisms, their functional group tolerance, and the high levels of chemo-, regio-, and stereoselectivity typically observed in isocyanides all contribute to their considerable potential for multicomponent reaction development [8]. The Passerini three-component reaction (P-3CR), first reported in 1921, is a multicomponent reaction involving an isocyanide, an aldehyde, and a carboxylic acid to produce, in one step, an esterified hydroxycarboxamide, also known as a depsipeptide, an essential intermediate in organic synthesis [9]. Medicinal chemists, particularly those working in the discipline of combinatorial chemistry, frequently use multicomponent reactions such as the Passerini because huge libraries of compounds may be rapidly synthesized from a commercial pool of starting materials [10]. This class of molecules is also present in many natural products and pharmacologically active depsipeptides, including enterochelin and pumilacidins [7].
The Passerini reaction has also been utilized to synthesize a variety of valuable structural motifs, including cyclic lipopeptides, which are antibacterial natural products with a broad spectrum of biological activities [11]. In just two steps, the Passerini reaction also yields the fungicidal compound mandipropamid, which is formed by the reaction of an in situ-synthesized isocyanide, an aldehyde, and a carboxylic acid (Figure 1). Micora (mandipropamid) is obtained by alkylation with propargyl bromide in the second step [11,12].
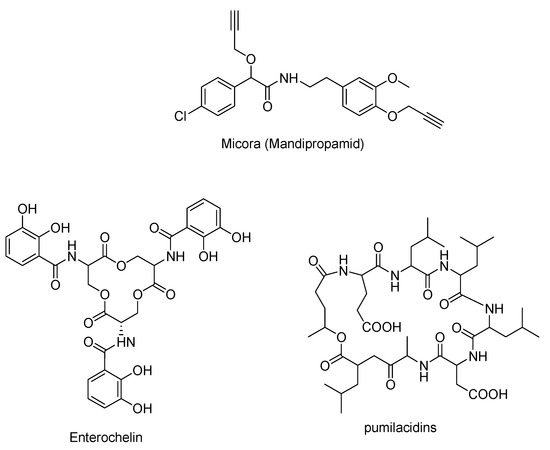
Figure 1.
A few examples of pharmacologically active Passerini adducts.
Water, on the other hand, is a safe, nontoxic, low-cost, and environmentally friendly solvent for organic transformations. Since Breslow’s seminal work in 1980, a wide range of organic reactions have been demonstrated to take place in aqueous media, sometimes with notable improvements, such as faster reaction rates and higher selectivities, when compared to results obtained using traditional organic-solvent-based systems [13,14]. Water is the medium through which all life operates and has distinct physical and chemical properties. It is surprising that this medium, which has been mostly disregarded throughout the last 150–200 years of modern organic chemistry, is yielding novel and unexpected experimental results [1]. Using water as a solvent for organic transformations has several environmental advantages. As a result, water as a reaction medium has received a lot of attention in synthetic organic chemistry because it is a cheap, nontoxic, and nonflammable solvent. The use of water as a medium for organic reactions has properties that may be beneficial in a variety of reactions [15].
Furthermore, as an example of a one-pot multicomponent reaction, the formation of α-acyloxycarboxamides from a carboxylic acid, an aldehyde, and substituted isocyanide was investigated in aqueous media. Usually, Passerini reactions are performed in an organic solvent such as CH2Cl2 or MeOH, and they can take 24 h or more for completion [16]. In addition to the negative environmental impact of using organic solvents and the inconvenience of the longer reaction time, this also makes them impractical for use in a typical three-hour undergraduate laboratory session. In an attempt to find suitable conditions we could use to introduce MCR methodologies to our undergraduate classes, we substituted the organic solvent with water. Surprisingly, the Passerini reaction was completed in 15 min, and excellent yields were observed. This method is significantly greener than the classical Passerini reaction because water is an ideal nontoxic, nonflammable, environmentally friendly, cheap, and readily available solvent. In aqueous media, several organic processes have shown significant increases in the rates of reaction, even though their origins are still a mystery [17,18]. Water might not be the best solvent for many of these reactions because they are biphasic. However, heterogeneous aqueous reaction rates are frequently related to mixing speed and method, and these rates are frequently inversely proportional to reaction temperature [16]. The effects have been interpreted as a result of the aqueous media’s cohesive energy density, the hydrophobicity of the reagents, increased hydrogen bonding in the transition state, or a decrease in the transition state’s volume [19].
Over the past few decades, simple and practical examples of Passerini reactions have been used in a variety of transformations to uncover possible drug-like compounds. These reactions have occasionally been carried out in noncatalytic conditions using excessive amounts of reactants with low efficiency over lengthy periods of time, sometimes up to several days. In addition to diverse methods, the Passerini reaction has been broadened using a variety of catalysts, including Cu(II) [20], Al(III) [21], aluminum-organophosphate [22], SiCl4 [23], In(OTf)3 [24], Ti(i-PrO) [25], and tetramethylguanidine-functionalized silica nanoparticles (TMG-SiO2 NPs) [25]. These catalysts have a high degree of efficiency, and some of them are simple to separate, but they also have some drawbacks, including a high cost, toxicity, non-reusability, lengthy reaction times, environmental pollution, and difficult reaction conditions. As a result, it is crucial to develop new catalytic methodologies with minimal fouling effects, catalyst thermal stability, easy operation, and catalyst recovery processes for the Passerini reaction.
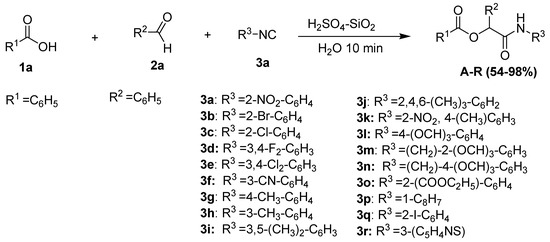
As part of our ongoing research on the synthesis of novel organic compounds utilizing immobilized sulfuric acid on silica gel (H2SO4-SiO2) [26], we report the preparation of a new class of α-aryloxy amide derivatives by a novel three-component Passerini reaction of benzoic acid (1a), aldehydes (2a), and isocyanides (3a) in the presence of immobilized sulfuric acid on silica gel in aqueous media (Scheme 1). A clean, effective, and environmentally safe chemical reaction to generate novel α-acyloxycarboxamide derivatives was carried out in the presence of immobilized sulfuric acid on silica gel. This heterogeneous catalyst was used in a Passerini reaction for the first time in aqueous media under mild conditions.
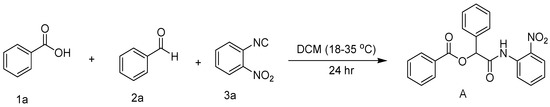
Scheme 1.
Passerini reaction involving benzoic acid (1a), benzaldehyde (2a), and 2-nitrophenylisocyanide (3a) in DCM.
2. Results and Discussion
Multicomponent reactions have been characterized as efficient methods for synthesizing polyfunctional compounds with high diversity and complexity in a few steps. The reaction of benzoic acid (1a), benzaldehyde (2a), and 2-nitrophenylisocyanide (3a) was chosen as the model reaction, and they were subjected to a typical Passerini reaction in DCM at room temperature (18–35 °C) for 24 h (Table 1, entry 5). The desired product (A) was obtained in 85% yield.

Table 1.
Effect of different solvents on the scope of the Passerini reaction.
To improve the efficiency of reaction, various reaction solvents were screened, such as diethyl ether, toluene, acetonitrile, ethyl acetate, DCM, ethanol, and methanol. Although the reaction occurs with all the solvents used, dichloromethane and methanol were the best among the tested solvents, producing 85 and 78% yields, respectively (Table 1).
When the reaction was carried out in diethyl ether, toluene, acetonitrile, ethyl acetate, and ethanol, low to moderate amounts of the product were observed, producing yields of 43, 65, 58, 32, and 74%, respectively which may have been due to solubility issues. (Table 1, entries 1–7). Therefore, DCM was found to be the best organic solvent for the current reaction, as it provided the desired product in 85% yield within 24 h (Table 1, entry 5). At this point, we thought of carrying out these reactions under green conditions. To our delight, when the solvent was replaced with water at room temperature, the Passerini reaction was completed in 15 min (Scheme 2), and the product was obtained in 90% yield (Table 1, entry 8). It was also discovered that extending the reaction time had little or no influence on the yield, as 15 min was found to be sufficient for completing the reaction with the highest possible yield.
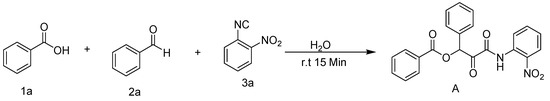
Scheme 2.
Passerini reaction in aqueous media.
Isocyanide has been found to undergo slow spontaneous hydrolysis in the presence of water [27]. Following that observation, we then focused on the optimization of the water content. The reaction was explored by increasing the volume of water from 1 to 5 mL to acquire a better understanding of the effect of water on the model Passerini reaction. The best result, of 90% yield, was observed when 2 mL of water by volume was introduced to the reaction (Table 2, entry 2). The effectiveness of the reaction declined dramatically when the amount of water was reduced (Table 2, entry 1), while increasing the amount of water also resulted in a lower product yield (Table 2, entries 3–5).

Table 2.
Investigating the effect of water on the optimized Passerini reaction.
To investigate the effect of a catalyst on the model Passerini reaction and to establish the best catalytic conditions, we started the condensation of benzoic acid (1 mmol), benzaldehyde (1 mmol), and 2-nitrophenylisocyanide (1 mmol) in the presence of H2SO4 immobilized on SiO2 (0.01 g) as a catalyst at room temperature (18–35 °C) in aqueous media. The reaction furnished the corresponding Passerini adducts in 10 min, and a good yield (93%) of the product was obtained.
To enhance the reaction yields of the products, catalytic studies were carried out by increasing the catalyst amount from 0.01 g to 0.05 g at room temperature (18–35 °C) in aqueous media while keeping the other experimental parameters constant. The conversion rate gradually increased from 93% to 98% within 10 min when the catalyst amount was increased to 0.02 g (Scheme 3). This is because more acidic sites became available as the catalyst concentration increased. The yield was unaffected by further increasing the catalyst loading (0.03–0.04 g). Further loading with 0.05 g of catalyst had a detrimental effect on the yield. As the catalyst amount was increased, the percentage conversion increased to a high point before gradually declining. An excessive amount of catalyst may obstruct conversion due to catalyst poisoning caused by an excessive amount of reactants adhering to the catalyst surface. The amount of catalyst determines the availability of acidic sites, which when present in excess, may produce undesirable byproducts or promote the occurrence of the reversible reaction [28]. When the reaction was carried out in the presence of silica gel (without catalyst) the Passerini adducts were obtained in 64% yield within 15 min, which implies that the reaction rate depends greatly on the catalyst used. The optimal quantity of catalyst for the successful Passerini reaction in aqueous media was determined to be 0.02 g.
Scheme 3.
Passerini reaction under the optimized catalytic conditions.
To demonstrate the catalytic significance of H2SO4-SiO2 for this reaction, we applied this catalyst to synthesize α-acyloxycarboxamide derivatives under aqueous conditions utilizing a variety of aromatic isocyanides with a wide range of ortho-, meta-, and para-substitutions. Generally, the synthetic procedure involved stirring the mixture of benzoic acid (1a, 1 mmol), benzaldehyde (2a, 1 mmol), isocyanide (3a, 1 mmol), and H2SO4 immobilized on SiO2 (0.02 g) in water (2 mL) for 10 min at room temperature (18–35 °C). The corresponding results are given in Figure 2. We discovered that the reaction worked exceptionally well with either electron-releasing or electron-withdrawing substituents on the aryl ring of the isocyanide. Various functional groups, such as fluoro, chloro, methyl, and methoxy groups, were found to be well-tolerated.
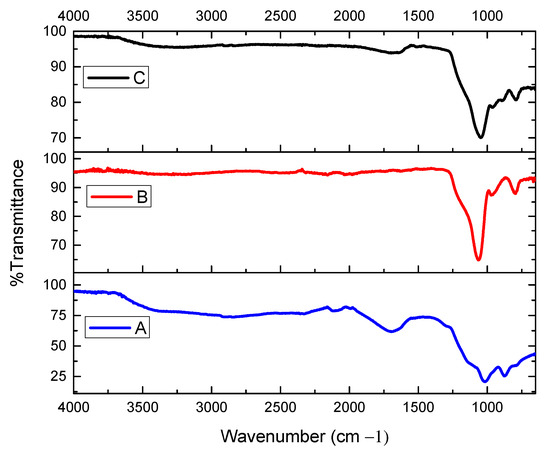
Figure 2.
The FT-IR spectra of (A) the immobilized sulfuric acid on silica gel, (B) silica gel without catalyst, and (C) recovered catalyst after five cycles.
The obtained α-acyloxycarboxamide derivatives (A-R) (Figure 2) were confirmed based on their NMR spectral data (Figures S1–S24). The 1H NMR spectra of this series showed NH signals ranging from δH 8.00 to 11.00 ppm. The aromatic protons of carboxylic acid and aldehyde moieties also appeared in the expected range (δH 7.80–6.40 ppm). 13C NMR spectra exhibited signals ranging from δC 161.0 to 173.0 ppm, corresponding to the two amido-ester C=O groups.
The Passerini reaction under the above optimized condition did not afford the expected bis α-acyloxycarboxamide derivatives in the case of phthalic acid (1b) with 2a and 3a. This may be attributed to the planarity of the phenyl ring preventing the second Passerini reaction due to steric hindrance. According to a literature review, succinic acid could be used to synthesize bis α-acyloxycarboxamides [28]. The free rotation of the C-C bond between the two methylene groups was suggested as a strategy to increase the flexibility and avoid steric restriction around the -COOH. However, the synthesis of bis α-acyloxycarboxamides utilizing succinic acid (1c) under the above experimental condition was unsuccessful; all trials led to the hydrolysis of the corresponding isocyanide (Scheme 4).
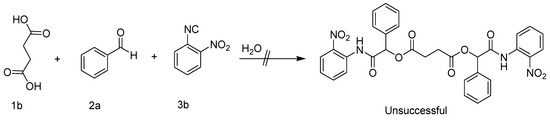
Scheme 4.
Unsuccessful attempts to synthesize bis α-acyloxycarboxamides.
The versatility of the standardized Passerini reaction conditions for the synthesis of novel α-acyloxycarboxamides was also examined for the carbonyl component of the Passerini reaction while employing 5-bromo isatin (2a) (5-bromo Indole 2,3-dione) as the carbonyl component and 4-bromo benzoic acid (1c) (Scheme 5). It was observed that the developed reaction conditions were robust for accepting indole 2,3-dione. Thus, the reaction of isatin (2b), benzoic acid (1a), and 3-methoxyphenylisocyanide (3b) under optimized reaction conditions led to the formation of (3-methoxyphenylcarbamoyl)(2-oxoindolin-3-yl)methyl benzoate in 90% yield (Scheme 6).
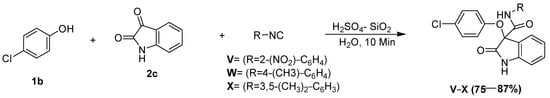
Scheme 5.
Passerini reaction in aqueous media, employing 4-chlorophenol as the acid surrogate under catalytic conditions.
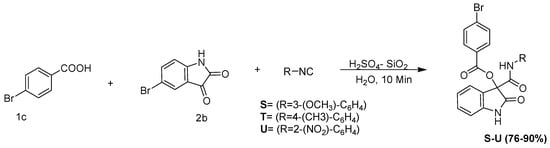
Scheme 6.
Passerini reaction in aqueous media, employing 5-bromo isatin (5-bromo indole 2,3-dione) as the carbonyl surrogate.
Utility of 4-chlorophenol as an acid surrogate
The synthetic potential of this protocol was examined by utilizing electron-deficient phenols as the acid component in the reaction. Thus, the Passerini reaction involving isatins (2c) with 4-chlorophenol (1b) and 2-nitrophenylisocyanide (3a) under optimized reaction conditions resulted in the synthesis of an O-arylated oxindole derivative in good to excellent yields (Scheme 5). In this reaction, the irreversible Smiles rearrangement of the intermediate phenoxyimidate adducts resulted in the formation of the O-arylated product, and this is an example of the application of the Smiles rearrangement in a Passerini reaction.
We also studied the recyclability and reusability effect of a H2SO4·SiO2 catalyst in these reactions. After each reaction, the catalyst was filtered, cleaned with ethyl acetate, dried at 120 °C for three hours, and then utilized in another cycle of reactions. The catalyst reusability demonstrated that it was extremely effective in the synthesis of Passerini products. After five cycles, the percentage conversion efficiency under the optimum conditions dropped from 98% to 87%. The higher catalytic efficiency was due to the higher acidity produced by the H-bond and hydrophobic core of the mesoporous catalyst (Table 3).

Table 3.
Efficiency of the recycled catalyst in the model Passerini reaction.
Scheme 7 illustrates the proposed mechanism for the formation of α-acyloxycarboxamide derivatives in the presence of SiO2-H2SO4. The catalyst first activates the carbonyl group of the aldehydes (4). Then, the isocyanide attacks the activated carbonyl (5) group nucleophilically, forming an intermediate known as nitrilium (6). The intermediate (6) is then attacked by the carboxylate of (7), which is followed by an acyl transfer by a Mumm rearrangement to generate derivatives of oxindole-based acyloxycarboxamides (9).
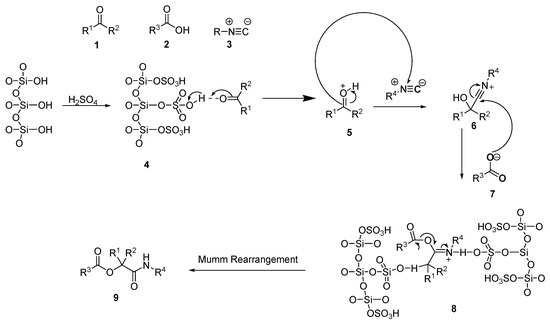
Scheme 7.
Proposed mechanism for the formation of α-acyloxycarboxamides in the presence of the immobilized sulfuric acid on silica gel (SiO2-H2SO4).
Characterization of the catalyst
FT-IR spectral analysis
The FT-IR spectrum of the catalyst is shown in Figure 2. The catalyst was solid, and the solid-state IR spectrum was obtained by the attenuated total reflection (ATR) method. For each experiment, 16 scans were performed in the frequency range from 650 to 4000 cm−1. The wide bands detected in the spectra (A) at 3511 and (C) 3486 cm−1 were attributed to superimposed stretching modes of Si–OH groups and hydroxyl groups (O–H). The peaks from Si–O–Si groups, which occur in the range of 1250–1000 cm−1 for antisymmetric stretching, are a characteristic indicator of the silica structure. For silica (B, SiO2) without a catalyst, the major peaks were broad antisymmetric Si–O–Si stretching from 1200 to 1000 cm−1 and symmetric Si–O–Si stretching near 800 cm−1.
Powder X-ray diffraction (XRD)
As shown in Figure 3, the XRD pattern of (A) the immobilized sulfuric acid on silica gel displayed a noticeable signal at a 2θ angle of 22.91° (a broad diffraction band), which is reminiscent of a typical amorphous silica diffraction pattern. The same broad diffraction band was noticed for the recovered catalyst (C) after five cycles (Figure 3C). A certain conclusion can be made about the crystalline structure of the support, i.e., silica was not distorted during the preparation of the catalysts.
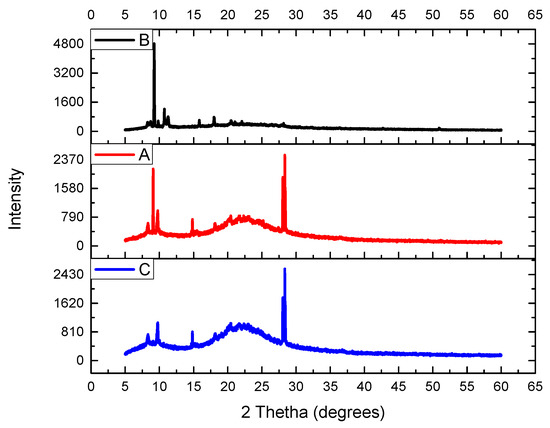
Figure 3.
The PXRD of (A) the immobilized sulfuric acid on silica gel, (B) silica gel without catalyst, and (C) recovered catalyst after five cycles.
3. Experimental Section
3.1. Materials and Methods
A PerkinElmer Spectrum 100 FT-IR Spectrometer was used for the FT-IR analysis. The IR spectra were obtained by the attenuated total reflection (ATR) method. For each experiment, 16 scans were performed in the frequency range from 650 to 4000 cm−1. The melting points of all the compounds were determined using a Koffler hot-stage apparatus and are uncorrected. The NMR spectra were recorded on a Bruker Advance III 400 spectrometer using CDCl3 or DMSO-d6 as a solvent with tetramethyl silane used as an internal standard. LC-MS/MS data were recorded on a Bruker Compact quadrupole time of flight (QToF) mass spectrometer. Raw mass spectrometry data were processed using MZmine free online software (version 2.38). Powder X-ray diffraction measurements were performed using a D8 Advance diffractometer made by a Bruker AXS company in Germany. The solvents and chemicals used were of analytical grade, purchased from Sigma Aldrich, and used without further purification. The purity determination of the starting materials and reaction monitoring were performed by thin-layer chromatography (TLC) on Merck silica gel G F254 plates.
3.2. Preparation of Sulfuric Acid Adsorbed on Silica Gel (SiO2-H2SO4)
This was previously reported [29]. To a suspension of silica gel (29.5 g, 230–400 mesh size) in EtOAc (60 mL), H2SO4 (1.5 g, 15.5 mmol, 0.8 mL of a 98% aq. solution of H2SO4) was added, and the mixture was stirred magnetically for 30 min at room temperature. EtOAc was removed under reduced pressure (rotary evaporator), and the residue was heated at 100 °C for 72 h under vacuum to afford SiO2-H2SO4 as a free-flowing powder.
General Experimental Procedures
A mixture of benzoic acid (0.122 g, 1 mmol), benzaldehyde (0.10 mL, 1 mmol), and isocyanide (0.15 g 1 mmol) was vigorously stirred in 2 mL of water at room temperature for 10 min in the presence of 0.02 g of immobilized sulfuric acid on silica gel (SiO2-H2SO4). Upon completion, the organic layer was separated and collected by a separating funnel. The combined organic phases were concentrated under reduced pressure, and the residue was purified by column chromatography using DCM/hexane (3:1) as an eluent to afford the desired products. Typical yields ranged from 60 to 98%. All other products (A-X) were obtained by a similar approach.
- A.
- (2-nitrophenylcarbamoyl)(phenyl)methyl benzoate
Prepared from 1-isocyano-2-nitrobenzene (0.15 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol,) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (98% yield); mp 112–114 °C; 1H NMR (400 MHz, CDCl3) δ 10.26 (s, 1H, NH), 8.71 (d, J = 8.4 Hz, 1H, Ar-H), 8.51 (s, 1H, Ar-H), 8.15 (d, J = 9.8 Hz, 1H, Ar-H), 8.04 (d, J = 8.4 Hz, 3H, Ar-H), 7.64–7.48 (m, 2H, Ar-H), 7.39 (t, J = 7.7 Hz, 3H, Ar-H), 7.26 (t, J = 7.7 Hz, 1H, Ar-H), 7.14 (t, J = 7.8 Hz, 1H, Ar-H), 6.72 (d, J = 8.4 Hz, 1H, Ar-H), 6.60 (t, J = 7.2 Hz, 1H, (O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 172.40, (NH-CO), 159.83, (O-C=O), 144.69, 136.24, 135.71, 133.88, 133.63, 130.23, 129.33, 128.54, 126.21, 125.91, 124.07, 122.81, 118.77, 116.86. HR-MS (ESI): [M+H+] calculated for C21H17N2O5: 377.1211, found: 377.2603.
- B.
- (2-bromophenylcarbamoyl)phenyl)methyl benzoate
Prepared from 1-bromo-2-isocyanobenzene (0.18 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol,) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (76% yield); mp 100–102 °C; IR (NaCl) v(cm−1) 3253 (NH), 1712 (CO) ester, and 1628 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 11.18 (s, 1H), 8.31 (d, J = 8.2 Hz, 1H, NH), 8.13 (d, J = 7.3 Hz, 1H, Ar-H), 8.03 (d, J = 7.2 Hz, 2H, Ar-H), 7.52 (dd, J = 15.8, 8.3 Hz, 1H, Ar-H), 7.44–7.29 (m, 3H, Ar-H), 7.20 (t, J = 7.7 Hz, 1H, Ar-H), 6.89 (t, J = 8.3 Hz, 1H, Ar-H), 6.45 (s, 1H, O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 166.75, (NH-CO), 164.62, (O-C=O), 135.18, 134.84, 134.00, 133.90, 132.28, 130.47, 130.27, 130.04, 129.39, 129.35, 129.07, 128.86, 128.77, 128.62, 128.55, 128.19, 128.16, 127.44, 125.79, 121.67, 113.53, 76.18 (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C21H16BrNO3: 410,0325, found: 410.0331.
- C.
- (2-chlorophenylcarbamoyl)(phenyl)methyl benzoate
Prepared from 1-chloro-2-isocyanobenzene (0.14 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (96% yield); mp 143–145 °C; IR (NaCl) v(cm−1) 3300 (NH), 1715 (CO) ester, and 1675 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H, NH), 8.35 (d, J = 8.2 Hz, 1H, Ar-H), 8.12 (d, J = 7.4 Hz, 2H, Ar-H), 7.56 (t, J = 8.0 Hz, 3H, Ar-H), 7.43 (t, J = 7.7 Hz, 2H, Ar-H), 7.37 (s, 1H, Ar-H), 7.33 (dd, J = 9.8, 7.3 Hz, 3H, Ar-H), 7.28 (d, J = 8.0 Hz, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.19–7.15 (m, 1H, Ar-H), 6.97 (dd, J = 11.2, 4.3 Hz, 1H, Ar-H), 6.44 (s, 1H, (O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 166.45, (NH-CO), 164.35, (O-C=O), 135.27, 133.92, 133.84, 129.92, 129.32, 129.01, 128.93, 128.75, 127.94, 127.37, 125.16, (Ar-Cl), 122.88, 121.33, 76.18 (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C21H16ClNO3: 366.0831, found: 366.1302.
- D.
- (3,4-difluorophenylcarbamoyl)(phenyl)methyl benzoate
Prepared from 1,2-difluoro-4-isocyanobenzene (0.14 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (66% yield); mp 150–151 °C; IR (NaCl) v(cm−1) 3273 (NH), 1732 (CO) ester, and 1671 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.49 (s, 1H, (NH), 8.17 (d, J = 7.7 Hz, 1H, Ar-H), 8.11 (d, J = 8.3 Hz, 1H, Ar-H), 7.72–7.57 (m, 2H, Ar-H), 7.52 (dd, J = 12.6, 4.8 Hz, 1H, Ar-H), 7.46 (dd, J = 10.9, 5.5 Hz, 2H, Ar-H), 7.15–6.98 (m, 1H, Ar-H), 6.45 (s, 1H, (O-(CH)CO).). 13C NMR (101 MHz, CDCl3) δ 166.46, (NH-CO), 165.40, (O-C=O), 151.01 (NH-R-C-F), 148.91, 134.67, 134.00, 133.71, 130.19, 129.94, 129.47, 129.06, 128.89, 128.78, 128.50, 127.47, 117.31, 117.13, 110.07, 109.85, 76.13, (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C21H15F2NO3: 368,1015, found: 368.1022.
- E.
- (3,4-dichlorophenylcarbamoyl)phenyl)methyl benzoate
Prepared from 1,2-dichloro-4-isocyanobenzene (0.17 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow solid (73% yield); mp 152–153 °C; IR (NaCl) v(cm−1) 3273 (NH), 1722 (CO) ester, and 1671 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H, NH), 8.05 (t, J = 8.2 Hz, 3H, Ar-H), 7.65 (s, 1H, Ar-H), 7.54 (t, J = 7.1 Hz, 3H, Ar-H), 7.41 (dd, J = 15.7, 7.9 Hz, 4H, Ar-H), 7.34 (d, J = 6.4 Hz, 2H, Ar-H), 7.27–7.14 (m, 2H, Ar-H), 6.34 (s, 1H, O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 166.75, (NH-CO), 165.68, (O-C=O), 136.54, 134.51, 134.04, 133.75, 132.86, 130.46, 130.22, 129.98, 129.53, 129.33, 129.09, 128.84, 128.79, 128.51, 128.07, 127.50, 121.72, 119.24, 76.25, (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C21H15Cl2NO3: 427.0346, found: 427.1105.
- F.
- (3-cyanophenylcarbamoyl)phenyl)methyl benzoate
Prepared from 3-isocyanobenzonitrile (0.13 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (89% yield); mp 136–137 °C; IR (NaCl) v(cm−1) 3350 (NH), 1730 (CO) ester, and 1687 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 11.30 (s, 1H, NH), 8.14–7.82 (m, 2H, Ar-H), 7.66–7.57 (m, 1H, Ar-H), 7.57–7.51 (m, 2H, Ar-H), 7.48 (t, J = 7.9 Hz, 1H, Ar-H), 7.40 (t, J = 7.7 Hz, 2H, Ar-H), 6.36 (s, 1H, O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 172.55, (NH-CO), 167.48, (O-C=O), 133.84, 132.96, 130.77, 130.24, 129.80, 129.40, 128.55, 127.62, 116.65, (C-CN), 113.96, 76.31, (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C22H16N2O3: 357.1172, found: 356.9086.
- G.
- (p-tolycarbamoyl)phenyl)methyl benzoate
Prepared from 1-isocyano-4-methylbenzene (0.12 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (94% yield); mp 148–149 °C; IR (NaCl) v(cm−1) 3285 (NH), 1730 (CO) ester, and 1642 (CO) amide. 1H NMR (400 MHz, DMSO) δ 10.44 (s, 1H, NH), 8.07 (s, 2H, Ar-H), 7.96 (s, 1H, Ar-H), 7.71 (s, 3H, Ar-H), 7.57 (s, 3H Ar-H), 7.48 (s, 6H, Ar-H), 7.10 (s, 2H, Ar-H), 6.26 (s, 1H, O-(CH)CO), 2.23 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 167.82 (NH-C=O), 166.82 (O-C=O), 136.42, 135.78, 134.23, 133.31, 133.22, 131.26, 129.93, 129.74, 129.66, 129.56, 129.37, 129.33, 129.17, 129.02, 127.86, 119.81, 76.30, (O-(CH)CO), 20.88 (CH3). HR-MS (ESI): [M+H+] calculated for C22H19NO3: 346.1318, found: 346.1315.
- H.
- (m-tolylcarbamoyl)(phenyl)methyl benzoate
Prepared from 1-isocyano-3-methylbenzene (0.12 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (75% yield); IR (NaCl) v(cm−1) 3273 (NH), 1718 (CO) ester, and 1675 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 7.4 Hz, 1H, (NH), 7.80 (s, 1H, Ar-H), 7.55 (dd, J = 14.0, 6.9 Hz, 2H, Ar-H), 7.43 (t, J = 7.7 Hz, 1H, Ar-H), 7.40–7.26 (m, 2H, Ar-H), 7.21 (d, J = 8.1 Hz, 1H, Ar-H), 7.11 (t, J = 7.8 Hz, 1H, Ar-H), 6.86 (d, J = 7.5 Hz, 1H), 6.37 (s, 1H, (O-(CH)CO), 2.23 (s, 2H, CH3), 2.09 (s, 1H, CH3). 13C NMR (101 MHz, CDCl3) δ 166.32, (NH-CO), 165.02, (O-C=O), 139.07, 136.84 (NH-R-CH3), 135.20, 133.83, 129.91, 129.25, 129.13, 128.96, 128.86, 128.75, 127.48, 125.71, 120.71, 117.13, 76.14, (O-(CH)CO), 21.43, (CH3). HR-MS (ESI): [M+H+] calculated for C22H19NO3: 346.1340, found: 346.1836.
- I.
- (3,5-dimethylphenylcarbamoyl)(phenyl)methyl benzoate
Prepared from 3,5-dimethyl phenyl isocyanide (0.13 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (94% yield); mp 148–149 °C; 1H NMR (400 MHz, CDCl3) δ 10.45 (s, 1H, NH), 8.03 (dd, J = 14.1, 7.5 Hz, 3H, Ar-H), 7.54 (t, J = 7.4 Hz, 2H, Ar-H), 7.40 (t, J = 7.7 Hz, 3H, Ar-H), 7.28 (d, J = 7.1 Hz, 1H, Ar-H), 6.95–6.84 (m, 1H, Ar-H), 6.82 (t, J = 7.4 Hz, 1H, Ar-H), 6.75 (d, J = 8.1 Hz, 1H, Ar-H), 6.32 (s, 1H, (O-(CH)CO), 4.44 (d, J = 5.9 Hz, 1H, CH3), 3.61 (s, 1H, CH3). 13C NMR (101 MHz, CDCl3) δ 168.04, NH(CO), 164.79, (O-C=O), 135.58, 133.77, 130.24, 129.83, 129.37, 129.10, 128.96, 128.75, 128.60, 128.51, 127.52, 125.52, 120.84, 110.27, 75.90, (O-(CH)CO), 55.11, 40.00. HR-MS (ESI): [M+H+] calculated for C23H21NO3: 361.1522, found: 361.1936.
- J.
- (mesitylcarbamoyl)(phenyl)methyl benzoate
Prepared from 2-isocyano-1,3,5-trimethylbenzene (0.15 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a colorless liquid (60% yield); IR (NaCl) v(cm−1) 3257 (NH), 1730 (CO) ester, and 1604 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 8.05 (d, J = 7.5 Hz, 1H), 7.56 (t, J = 7.4 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H), 6.77 (s, 3H), 2.25 (s, 7H), 2.17 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 166.96, 162.35, 138.81, 134.55, 131.32, 130.56, 129.61, 128.86, 128.46, 126.94, 21.17, 18.77. HR-MS (ESI): [M+H+] calculated for C24H23NO3: 374.1655, found: 374.2118.
- K.
- (4-methyl-2-nitrophenylcarbamoyl)phenyl)methylbenzoate
Prepared from 4-methyl-2-nitro phenyl isocyanide (0.16 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow solid (92% yield); mp 126–129 °C; IR (NaCl) v(cm−1) 3343 (NH), 1694 (CO) ester, and 1641 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 11.19 (s, 1H, NH), 8.60 (d, J = 8.6 Hz, 1H, Ar-H), 8.19 (d, J = 7.3 Hz, 2H, Ar-H), 7.95 (s, 1H, Ar-H), 7.56 (d, J = 7.7 Hz, 3H Ar-H), 7.35 (d, J = 7.6 Hz, 4H, Ar-H), 7.11 (d, J = 10.2 Hz, 2H, Ar-H), 6.66 (d, J = 8.5 Hz, 2H, Ar-H), 6.43 (s, 1H, O-(CH)CO), 2.42 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 172.76, (NH-CO), 167.52, (O-C=O), 143.91, 142.77, 137.23, 136.37, 135.07, 134.39, 133.87, 131.89, 130.09, 129.56, 129.01, 128.77, 127.15, 126.62, 125.85, 125.71, 125.27, 122.02, 118.80, 76.36, (O-(CH)CO), 21.32. HR-MS (ESI): [M+H+] calculated for C22H18N2O5: 391.1383, found: 391.3062.
- L.
- (4-methoxyphenylcarbamoyl)phenyl)methyl benzoate
Prepared from 1-isocyano-4-methoxy benzene (0.13 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (94% yield); IR (NaCl) v(cm−1) 3293 (NH), 1734 (CO) ester, and 1671 (CO) amide. 1H NMR (400 MHz, DMSO) δ 10.42 (s, 1H, NH), 8.08 (d, J = 7.2 Hz, 2H, Ar-NH), 7.70 (d, J = 7.3 Hz, 3H), 7.58 (t, J = 7.7 Hz, 2H), 7.52–7.46 (m, 4H), 7.43 (dd, J = 14.5, 7.4 Hz, 2H), 6.88 (d, J = 9.1 Hz, 2H), 6.22 (s, 1H, O-(CH)CO), 3.70 (s, 3H, OCH3). 13C NMR (101 MHz, DMSO) δ 166.57 (NHC=O), 165.69 (O-C=O), 155.95 (R-C-O-CH3), 135.81, 134.28, 132.01, 129.94, 129.74, 129.50, 129.37, 129.19, 129.03, 127.84, 121.25, 114.39, 76.27 (O-(CH)CO), 55.60 (OCH3). HR-MS (ESI): [M+H+] calculated for C22H19NO4: 362.1346, found: 362.1319.
- M.
- (2-methoxybenzylcarbamoyl)phenyl)methyl benzoate
Prepared from 1-(isocyanomethyl)-2-methoxybenzene (0.15 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a colorless liquid (94% yield); IR (NaCl) v(cm−1) 3273 (NH), 1722 (CO) ester, and 1663 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.10–8.04 (m, 1H, NH), 8.02 (dd, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.60–7.49 (m, 1H, Ar-H), 7.48–7.35 (m, 2H, Ar-H), 7.29 (d, J = 7.0 Hz, 1H, Ar-H), 7.23–7.12 (m, 1H, Ar-H), 6.83 (td, J = 7.4, 0.7 Hz, 1H, Ar-H), 6.76 (d, J = 8.2 Hz, 1H, Ar-H), 6.30 (s, 1H, O-(CH)CO)), 4.44 (d, J = 5.9 Hz, 1H, (NH-CH-R), 3.67 (d, J = 6.2 Hz, 1H, (NH-CH-R), 3.63 (s, 1H, O-CH3). 13C NMR (101 MHz, CDCl3) δ 171.76, (NH-CO), 167.97, (O-C=O), 157.61, (C-O-CH3), 135.74, 133.79, 133.59, 130.22, 129.86, 129.81, 129.37, 129.31, 129.07, 128.93, 128.73, 128.60, 128.51, 127.48, 125.57, 120.80, 110.26, 75.90, (O-(CH)CO), 55.10, (O-CH3) 39.95, (NH-(CH2)R. HR-MS (ESI): [M+H+] calculated for C23H21NO4: 376.1435, found: 376.1560.
- N.
- (4-methoxybenzylcarbamoyl)phenyl)methyl benzoate
Prepared from 1-(isocyanomethyl)-4-methoxybenzene (0.15 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (98% yield); mp 112–114 °C; 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H, NH), 8.09–7.93 (m, 2H, Ar-H), 7.47 (dt, J = 15.6, 9.4 Hz, 1H, Ar-H), 7.43–7.31 (m, 2H, Ar-H), 7.26–7.16 (m, 1H, Ar-H), 7.08 (dd, J = 14.6, 9.1 Hz, 1H, Ar-H), 6.80–6.55 (m, 1H, Ar-H), 6.25 (d, J = 30.0 Hz, 1H, (O-(CH)CO), 4.69 (s, 1H, OCH3), 4.62 (s, 1H), 4.36–4.28 (m, 1H, (NH-CH-R), 3.68–3.62 (m, 1H, (NH-CH-R). 13C NMR (101 MHz, CDCl3) δ 164.35, (NH-CO), 161.83, (O-C=O), 159.17, 143.09, 133.71, 130.46, 130.19, 129.53, 129.26, 129.02, 128.88, 128.49, 127.81, 127.48, 123.36, 114.46, 114.33, 114.18, 114.13, 113.97, 76.08, (O-(CH)CO), 55.26, (OCH3), 41.88, (NH-CH2). HR-MS (ESI): [M+H+] calculated for C23H21NO4: 376.1402, found: 376.2408.
- O.
- ethyl 2-[2-(benzoyloxy)-2-phenylacetamido]benzoate
Prepared from ethyl-2 isocyanobenzoate (0.18 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (88% yield); mp 130–132 °C; IR (NaCl) v(cm−1) 3242 (NH), 1730 (CO) ester, and 1683 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 12.02 (s, 1H, NH), 8.65 (d, J = 8.6 Hz, 1H, Ar-H), 8.27 (d, J = 7.5 Hz, 2H, Ar-H), 7.93 (dd, J = 14.6, 8.6 Hz, 1H, Ar-H), 7.59 (d, J = 7.2 Hz, 2H Ar-H), 7.53 (t, J = 7.4 Hz, 1H, Ar-H), 7.48–7.36 (m, 4H, Ar-H), 7.37–7.24 (m, 3H, Ar-H), 7.01 (t, J = 7.6 Hz, 1H, Ar-H), 6.38 (s, 1H, O-(CH)CO), 4.37 (q, J = 7.1 Hz, 1H, CH), 4.31–4.19 (m, 2H, CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 168.12 O-C=O benzoate), 167.48 (NH-CO), 165.09 (O-C=O ester), 140.87, 135.72, 134.55, 133.54, 131.30, 130.75, 129.24, 128.85, 128.37, 127.24, 123.05, 120.54, 115.87, 62.02, 61.44, 14.18. HR-MS (ESI): [M+H+] calculated for C25H21NO6: 431.1317, found: 431.1020.
- P.
- (naphthalen-3-ylcarbamoyl)(phenyl)methyl benzoate
Prepared from 1-isocyanonaphthalene (0.15 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white powder (80% yield); mp 171–172 °C; IR (NaCl) v(cm−1) 3422 (NH), 1713 (CO) ester, and 1623 (CO) amide. 1H NMR (400 MHz, DMSO) δ 8.15 (d, J = 7.4 Hz, 1H, (NH), 7.99–7.90 (m, 1H, Ar-H), 7.85 (d, J = 7.3 Hz, 1H, Ar-H), 7.80 (d, J = 8.0 Hz, 1H, Ar-H), 7.77–7.67 (m, 1H, Ar-H), 7.59 (dd, J = 9.7, 5.6 Hz, 2H, Ar-H), 7.57–7.51 (m, 1H, Ar-H), 7.52–7.43 (m, 1H, Ar-H), 6.53 (s, 1H, O-(CH)CO). 13C NMR (101 MHz, DMSO) δ 168.14, (NH-CO), 165.81, (O-C=O), 136.03, 134.25, 134.19, 133.11, 130.03, 129.62, 129.44, 129.34, 129.25, 128.66, 128.63, 127.93, 126.61, 126.49, 126.00, 122.98, 122.83, 76.44, (O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C25H20NO3: 382.1339, found: 382.1316.
- Q.
- (2-iodophenylcarbamoyl)phenyl)methyl benzoate
Prepared from 1-iodo-2-isocyanobenzene (0.23 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a green solid (85% yield); mp 104–106 °C; IR (NaCl) v(cm−1) 3249 (NH), 1730 (CO) ester, and 1679 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 8.45 (s, 1H, NH), 8.24 (d, J = 8.3 Hz, 1H, Ar-H), 8.18 (d, J = 7.2 Hz, 2H, Ar-H), 7.82 (d, J = 8.0 Hz, 1H, Ar-H), 7.69 (d, J = 6.7 Hz, 2H, Ar-H), 7.57 (t, J = 7.6 Hz, 4H, Ar-H), 7.43 (t, J = 7.7 Hz, 3H, Ar-H), 7.27 (dd, J = 12.1, 4.8 Hz, 2H, Ar-H), 7.08–7.01 (m, 1H, Ar-H), 6.79 (td, J = 7.9, 1.5 Hz, 2H, Ar-H), 6.46 (s, 1H, O-(CH)CO). 13C NMR (101 MHz, CDCl3) δ 166.92, (NH-CO), 164.85, (O-C=O), 139.58, 138.85, 137.32, 135.20, 133.97, 130.48, 130.20, 129.45, 129.35, 129.14, 128.88, 128.69, 127.67, 127.47, 126.43, 121.66, 76.13, O-(CH)CO). HR-MS (ESI): [M+H+] calculated for C21H16INO3: 458.0158, found: 458.0715.
- R.
- (benzo[d]thiazol-2-ylcarbamoyl)(phenyl)methyl benzoate
Prepared from 2-isocyanobenzo[d]thiazole (0.16 g, 1 mMol), benzaldehyde (0.11 mL, 1 mMol), and benzoic acid (0.122 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a white solid (63% yield); mp 122–124 °C; IR (NaCl) v(cm−1) 3286 (NH), 1710 (CO) ester, and 1679 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 9.01 (s, 1H, NH), 8.09 (d, J = 7.4 Hz, 1H, Ar-H), 7.96 (d, J = 7.4 Hz, 1H, Ar-H), 7.79 (d, J = 7.6 Hz, 1H, Ar-H), 7.61 (t, J = 7.5 Hz, 1H, Ar-H), 7.57–7.50 (m, 1H, Ar-H), 7.45 (dd, J = 16.4, 8.4 Hz, 2H, Ar-H), 7.33 (t, J = 7.6 Hz, 1H, Ar-H), 7.25 (t, J = 9.0 Hz, 1H, Ar-H). 13C NMR (101 MHz, CDCl3) δ 165.18, (NH-CO), 164.31, (O-C=O), 161.31, (Ar-C=S), 133.52, 132.17, 130.96, 130.76, 129.56, 129.23, 128.95, 128.80, 128.06, 127.93, 126.72, 125.26, 123.07, 122.07, 120.68, 120.50, 119.74. HR-MS (ESI): [M+H+] calculated for C22H17N2O3S: 389.0861, found: 389.2442.
- S.
- 3-(3-methoxyphenylcarbamoyl)-5-bromo-2-oxoindolin-3-yl 4-bromobenzoate
Prepared from 1-isocyano-3-methoxybenzene (1.33 mL, 1 mMol), 5-bromo isatin (0.23 g, 1 mMol), and 4-bromobenzoic acid (0.21 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (90% yield); IR (NaCl) v(cm−1) 3286 (NH), 1710 (CO) ester, and 1679 (CO) amide. 1H NMR (400 MHz, CDCl3) δ 9.09 (s, 1H, (NH-amide)), 8.75 (s, 1H, (NH-indole)), 7.98 (dd, J = 14.9, 7.7 Hz, 3H, (Ar-H), 7.49 (dd, J = 12.7, 7.2 Hz, 2H, (Ar-H)), 7.35 (dd, J = 16.1, 8.2 Hz, 6H, (Ar-H)), 7.16 (dt, J = 23.6, 8.0 Hz, 3H, (Ar-H)), 6.97 (dd, J = 17.5, 8.8 Hz, 2H, (Ar-H)), 6.85 (d, J = 7.8 Hz, 1H, (Ar-H)), 6.62 (dd, J = 8.2, 1.7 Hz, 1H, (Ar-H)), 5.18 (s, NH-indole), 3.68 (s, 3H, (O-CH3)), 2.08 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 173.55, (-CO-indole), 171.03, (-CO-ester), 163.84, (CO-amide), 161.87, (C-OCH3), 160.17, 142.31, 138.36, 134.36, 133.41, 131.12, 130.14, 129.77, 129.61, 128.75, 128.46, 128.08, 124.91, 124.85, 123.44, 112.37, 111.47, 111.22, 105.66, 55.39, 31.00. HR-MS (ESI): [M+H+] calculated for C23H18Br2N2O6: 575.9202, found: 575.6714.
- T.
- 3-(p-tolylcarbamoyl)-5-bromo-2-oxoindolin-3-yl 4-bromobenzoate
Prepared from 1-isocyano-4-methylbenzene (0.13 mL, 1 mMol) 5-bromo isatin (0.23 g, 1 mMol), and 4-bromobenzoic acid (0.21 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as yellow liquid (76% yield); IR (NaCl) v(cm−1) 3244 (NH), 1718 (CO) ester, and 1682 (CO) amide. 1H NMR (400 MHz, DMSO) δ 11.12 (d, J = 40.1 Hz, 1H, NH-amide), 10.09 (d, J = 23.3 Hz, 1H, NH-indole), 8.71 (d, J = 11.1 Hz, 1H, (Ar-H), 8.23 (s, 1H, Ar-H), 7.87 (d, J = 8.4 Hz, 1H, Ar-H), 7.77–7.61 (m, 1H, Ar-H), 7.47 (d, J = 8.3 Hz, 2H, Ar-H), 7.09 (dd, J = 17.5, 8.0 Hz, 3H, Ar-H), 6.87 (d, J = 8.3 Hz, 1H, Ar-H), 2.25 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 167.13 (CO-indole), 162.97 (CO-amide), 159.82 (CO-ester), 159.49, 140.46, 136.22, 133.16, 133.00, 132.17, 131.77, 130.25, 129.70, 127.37, 119.52, 118.10, 114.71, 20.92. HR-MS (ESI): [M+H+] calculated for C23H16Br2N2O4: 541.9416, found: 541.4483.
- U.
- 3-(2-nitrophenylcarbamoyl)-5-bromo-2-oxoindolin-3-yl 4-bromobenzoate
Prepared from 1-isocyano-2-nitrobenzene (0.15 g, 1 mMol), 5-bromo isatin (0.23 g, 1 mMol), and 4-bromobenzoic acid (0.21 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (85% yield); IR (NaCl) v(cm−1) 3280 (NH), 1712 (CO) ester, and 1676 (CO) amide. 1H NMR (400 MHz, DMSO) δ 11.07 (s, 1H, NH-amide), 8.20 (d, J = 8.2 Hz, 1H NH-indole), 7.93 (d, J = 8.6 Hz, 3H, Ar-H), 7.88–7.80 (m, 3H, Ar-H), 7.73 (t, J = 7.6 Hz, 1H, Ar-H), 7.66 (d, J = 8.4 Hz, 1H, Ar-H), 7.55 (t, J = 7.7 Hz, 1H, Ar-H), 7.49–7.41 (m, 6H, Ar-H), 7.36 (t, J = 7.7 Hz, 4H, Ar-H), 7.01 (t, J = 6.8 Hz, 4H, Ar-H), 6.87 (t, J = 11.5 Hz, 1H, Ar-H), 6.58 (t, J = 7.7 Hz, 3H, Ar-H). 13C NMR (101 MHz, DMSO) δ 184.86 (-CO-indole), 172.49 (-CO-ester), 167.11 (-CO-amide), 159.84 (-C-NO2), 151.16, 146.68, 144.17, 138.74, 136.10, 135.54, 132.08, 131.71, 131.44, 130.69, 130.43, 130.40, 127.35, 126.12, 125.80, 125.09, 123.15, 119.62, 118.23, 115.85, 112.63. HR-MS (ESI): [M+H+] calculated for C22H13Br2N3O6: 575.9320, found: 575.6714.
- V.
- 3-(4-chlorophenoxy)-N-(naphthalen-1-yl)-2-oxoindoline-3-carboxamide
Prepared from 1-isocyano-naphthalein (0.15 mL, 1 mMol), isatin (0.147 g, 1 mMol), and 4-chlorophenol (0.13 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (80% yield); IR (NaCl) v(cm−1) 3295 (NH), 1053 (CO), and 1679 (CO) amide. 1H NMR (400 MHz, DMSO) δ 11.06 (s, 2H, NH-amide), 10.34 (s, 1H, NH-indole), 9.71 (s, 1H, Ar-H), 8.59 (d, J = 10.5 Hz, 1H, Ar-H), 8.49 (s, 1H, Ar-H), 8.22–8.05 (m, 1H, Ar-H), 7.98 (dd, J = 25.7, 7.5 Hz, 1H, Ar-H), 7.83 (dd, J = 26.9, 7.8 Hz, 1H, Ar-H), 7.72 (dd, J = 16.1, 7.8 Hz, 1H, Ar-H), 7.58 (t, J = 7.6 Hz, 3H, Ar-H), 7.50 (d, J = 7.3 Hz, 2H, Ar-H), 7.19 (d, J = 8.8 Hz, 2H, Ar-H), 7.06 (t, J = 7.5 Hz, 2H, Ar-H), 6.91 (d, J = 7.9 Hz, 2H, Ar-H), 6.77 (d, J = 8.8 Hz, 2H, Ar-H). 13C NMR (101 MHz, DMSO) δ 184.87 (CO-indole), 160.79 (CO-amide), 159.85, 156.76, 151.15, 138.83, 134.11, 133.03, 129.60, 128.80, 126.59, 126.53, 126.13, 125.25, 125.16, 123.22, 122.76, 122.23, 119.78, 118.29, 117.37, 112.65. HR-MS (ESI): [M+H+] calculated for C22H13Br2N3O6: 573.93, found: 573.80.
- W.
- 3-(4-chlorophenoxy)-2-oxo-N-p-tolylindoline-3-carboxamide
Prepared from 1-isocyano-4-methylbenzene (0.13 mL, 1 mMol), isatin (0.147 g, 1 mMol), and 4-chlorophenol (0.13 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (87% yield); IR (NaCl) v (cm−1) 3259 (NH), 1048 (CO), and 1680 (CO) amide. 1H NMR (400 MHz, DMSO) δ 11.07 (s, 1H, NH-amide), 10.11 (s, 1H, NH-indole), 9.72 (s, 2H, Ar-H), 8.71 (d, J = 11.1 Hz, 1H, Ar-H), 8.24 (s, 1H, Ar-H), 7.58 (t, J = 7.7 Hz, 1H, Ar-H), 7.55–7.41 (m, 2H, Ar-H), 7.19 (d, J = 8.7 Hz, 4H, Ar-H), 7.14–6.99 (m, 3H, Ar-H), 6.91 (d, J = 7.9 Hz, 1H, Ar-H), 6.77 (d, J = 8.7 Hz, 4H, Ar-H), 2.25 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 184.87 (CO-indole), 162.96 (CO-amide), 159.86, 159.81, 156.76, 151.16, 138.82, 136.22, 133.17, 133.00, 130.25, 129.69, 129.60, 125.16, 123.22, 122.77, 119.54, 118.29, 118.12, 117.37, 112.65, 20.92. HR-MS (ESI): [M+H+] calculated for C22H17ClN2O3: 573.0911, found: 393.2902.
- X.
- 3-(4-chlorophenoxy)-N-(3,5-dimethylphenyl)-2-oxoindoline-3-carboxamide
Prepared from 1-isocyano-3,5-dimethylbenzene (0.131 mL, 1 mMol), isatin (0.147 g, 1 mMol), and 4-chlorophenol (0.13 g, 1 mMol) according to the general procedure. Purification: column chromatography on silica gel (3:1 DCM/hexane). Isolated as a yellow liquid (87% yield); IR (NaCl) v(cm−1) 3283 (NH), 1050 (CO), and 1679 (CO) amide. 1H NMR (400 MHz, DMSO) δ 11.07 (s, 1H, NH-indole), 10.05 (s, 1H, NH-amide), 9.72 (s, 2H, Ar-H), 8.75 (d, J = 11.0 Hz, 1H, Ar-H), 8.23 (s, 1H, Ar-H), 7.59 (t, J = 7.7 Hz, 1H, Ar-H), 7.50 (d, J = 7.4 Hz, 1H, Ar-H), 7.19 (d, J = 8.8 Hz, 6H, Ar-H), 7.07 (t, J = 7.5 Hz, 1H, Ar-H), 6.91 (d, J = 7.8 Hz, 1H, Ar-H), 6.78 (t, J = 8.7 Hz, 5H, Ar-H), 6.71 (s, 1H, Ar-H), 2.23 (s, 5H, CH3). 13C NMR (101 MHz, DMSO) δ 184.87 (CO-indole), 159.94 (CO-amide), 159.86, 156.76, 151.16, 138.83, 138.57, 138.35, 129.60, 125.60, 125.17, 123.23, 122.77, 118.29, 117.38, 117.27, 115.63, 112.65, 21.52. HR-MS (ESI): [M+H+] calculated for C23H19ClN2O3: 406.1136, found: 406.4155.
4. Conclusions
In this work, we have shown the reactivity, efficiency, and reusability of immobilized sulfuric acid on silica gel. This solid catalyst is applicable in MCRs as a heterogenous catalyst. The Passerini reaction was assayed in the presence of SiO2-H2SO4 for the first time. Our findings also demonstrated that water is a good substitute for volatile organic solvents, which is in line with one of the green chemistry principles and important for future applications. The following are a few benefits of this protocol: quick reaction times, high product yields, a simple workup, mild reaction conditions, environmental compatibility, and a lack of side products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23179529/s1.
Author Contributions
Conceptualization, S.A.S. and R.W.M.K.; methodology, S.A.S.; writing—original draft preparation, S.A.S.; writing—review and editing, M.M. and X.S.-N.; supervision, R.W.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of South Africa, grant number 116109.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pirrung, M.C.; Das Sarma, D. Multicomponent Reactions Are Accelerated in Water. J. Am. Chem. Soc. 2004, 126, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Brioche, J.; Masson, G.; Zhu, J. Passerini three-component reaction of alcohols under catalytic aerobic oxidative conditions. Org. Lett. 2010, 12, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Grolla, A.A.; Podestà, V.; Chini, M.G.; Di Micco, S.; Vallario, A.; Genazzani, A.A.; Canonico, P.L.; Bifulco, G.; Tron, G.C.; Sorba, G.; et al. Synthesis, biological evaluation, and molecular docking of Ugi products containing a zinc-chelating moiety as novel inhibitors of histone deacetylases. J. Med. Chem. 2009, 52, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Ganem, B. Strategies for Innovation in Multicomponent Reaction Design. Account. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, I.A.; Kumar, B.; McCullough, J. Strategies for Innovation in the Design. Innov. Civ. Constr. Eng. 2009, 49, 29–32. [Google Scholar] [CrossRef]
- Vessally, E.; Ramazani, A.; Shabrendi, H.; Ghadimi, R.; Rouhani, M. A novel four-component reaction between secondary amines and hydroxybenzaldehydes with isocyanides in water: An efficient one-pot and green synthesis of benzo [b] furan derivatives. J. Chem. 2013, 2013, 761982. [Google Scholar] [CrossRef] [Green Version]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V.A. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef]
- Zhang, X.; Evanno, L.; Poupon, E. Biosynthetic Routes to Natural Isocyanides. European. J. Org. Chem. 2020, 2020, 1919–1929. [Google Scholar] [CrossRef]
- Ugi, B.; Werner, A.; Dömling, A. The chemistry of isocyanides, their multicomponent reactions and their libraries. Molecules 2003, 8, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Chandgude, A. Multicomponent Reactions: Development, Scope, and Applications. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2017. [Google Scholar]
- Hashizume, H.; Nishimura, Y. Cyclic lipopeptide antibiotics. Stud. Nat. Prod. Chem. 2008, 35, 693–751. [Google Scholar] [CrossRef]
- Váradi, A.; Palmer, T.C.; Dardashti, R.N.; Majumdar, S. Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 2016, 21, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Yang, Q.L.; Liu, Y.; Li, D.W.; Huang, N.; Liu, M.G. A novel and green synthesis of indolone-N-amino acid derivatives via the Passerini three-component reactions in water. Chin. Chem. Lett. 2016, 27, 345–348. [Google Scholar] [CrossRef]
- Kaboudin, B.; Khodamorady, M. Organic reactions in water: A practical and convenient method for the N-formylation of amines in water. Synlett 2010, 19, 2905–2907. [Google Scholar] [CrossRef]
- Hooper, M.M.; De Boef, B. A green multicomponent reaction for the organic chemistry laboratory: The aqueous passerini reaction. J. Chem. Educ. 2009, 86, 1077–1079. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Das Sarma, K.; Wang, J. Hydrophobicity and mixing effects on select heterogeneous, water-accelerated synthetic reactions. J. Org. Chem. 2008, 73, 8723–8730. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Das Sarma, K. Aqueous medium effects on multi-component reactions. Tetrahedron 2005, 61, 11456–11472. [Google Scholar] [CrossRef]
- Sela, T.; Vigalok, A. Salt-Controlled selectivity in ‘on water’ and ‘in Water’ passerini-type multicomponent reactions. Adv. Synth. Catal. 2012, 354, 2407–2411. [Google Scholar] [CrossRef]
- Andreana, P.R.; Liu, C.C.; Schreiber, S.L. Stereochemical control of the passerini reaction. Org. Lett. 2004, 6, 4231–4233. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.X.; Wang, M.X.; Wang, D.X.; Zhu, J. Catalytic enantioselective Passerini three-component reaction. Angew. Chem. Int. Ed. 2008, 47, 388–391. [Google Scholar] [CrossRef]
- Yue, T.; Wang, M.X.; Wang, D.X.; Masson, G.; Zhu, J. Catalytic asymmetric Passerini-type reaction: Chiral aluminum-organophosphate-catalyzed enantioselective α-addition of isocyanides to aldehydes. J. Org. Chem. 2009, 74, 8396–8399. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Fan, Y. Catalytic, enantioselective α-additions of isocyanides: Lewis base catalyzed Passerini-type reactions. J. Org. Chem. 2005, 70, 9667–9676. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Oguchi, T.; Taguchi, T. Direct alkylative Passerini reaction of aldehydes, isocyanides, and free aliphatic alcohols catalyzed by indium (III) triflate. J. Org. Chem. 2009, 74, 3927–3929. [Google Scholar] [CrossRef] [PubMed]
- Kusebauch, U.; Beck, B.; Messer, K.; Herdtweck, E.; Dömling, A. Massive Parallel Catalyst Screening: Toward Asymmetric MCRs. Org. Lett. 2003, 5, 4021–4024. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Siwe-Noundou, X.; Krause, R.W. Catalytic Performance of Immobilized Sulfuric Acid on Silica Gel for N-Formylation of Amines with Triethyl Orthoformate. Molecules 2022, 27, 4213. [Google Scholar] [CrossRef]
- Goldeman, W.; Nasulewicz-Goldeman, A. Synthesis and biological evaluation of aminomethylidenebisphosphonic derivatives of β-arylethylamines. Tetrahedron 2015, 71, 3282–3289. [Google Scholar] [CrossRef]
- Salah Ayoup, M.; Wahby, Y.; Abdel-Hamid, H.; Ramadan, E.S.; Teleb, M.; Abu-Serie, M.M.; Noby, A. Design, synthesis and biological evaluation of novel α-acyloxy carboxamides via Passerini reaction as caspase 3/7 activators. Eur. J. Med. Chem. 2019, 168, 340–356. [Google Scholar] [CrossRef]
- Liu, X.M.; Song, Z.X.; Wang, H.J. Density functional theory study on the –SO3H functionalized acidic ionic liquids. Struct. Chem. 2009, 20, 509–515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).