Sequestration of Inflammation in Parkinson’s Disease via Stem Cell Therapy
Abstract
:1. Introduction
2. Pathophysiology and Therapeutics for Parkinson’s Disease
3. Rewiring the Inflammatory Responses
3.1. Anti-Inflammatory Based Therapies
3.2. Cell-Based Therapies
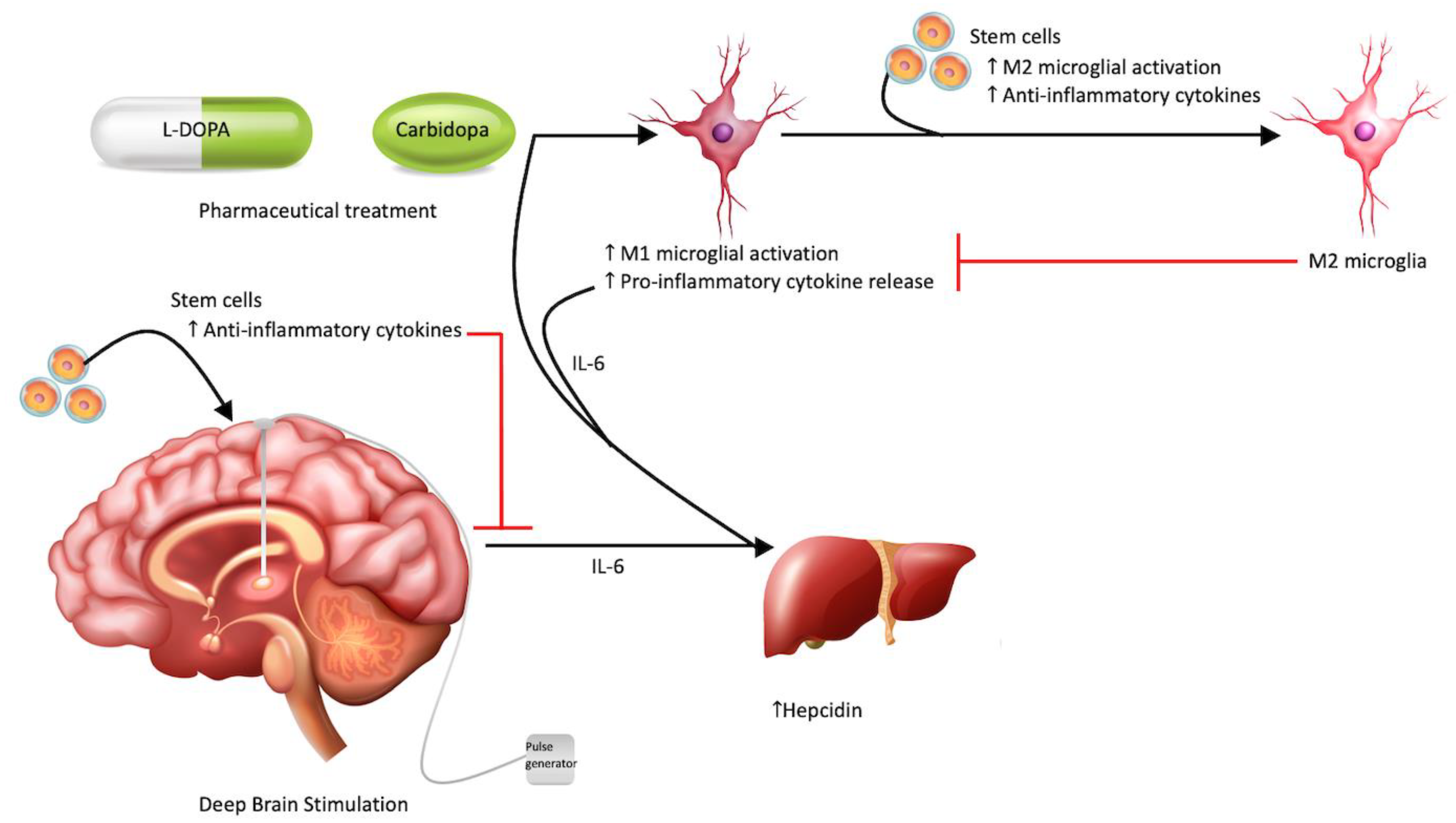
3.3. A Potential Synergistic Niche
3.4. Neurotrophic Factors and Stem Cell Therapy
4. Lifestyle Factors Facilitating the Stem Cell Response
4.1. Exercise and Physical Therapy
4.2. Diet and the Gastrointestinal Microbiome
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Tanner, C.M.; Goldman, S.M.; Ross, G.W.; Grate, S.J. The disease intersection of susceptibility and exposure: Chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014, 10, S213–S225. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Lue, L.; Sue, L.I.; Bachalakuri, J.; Henry-Watson, J.; Sasse, J.; Boyer, S.; Shirohi, S.; Brooks, R.; et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009, 117, 613–634. [Google Scholar] [CrossRef]
- Lionnet, A.; Leclair-Visonneau, L.; Neunlist, M.; Murayama, S.; Takao, M.; Adler, C.H.; Derkinderen, P.; Beach, T.G. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018, 135, 1–12. [Google Scholar] [CrossRef]
- Brundin, P.; Melki, R. Prying into the Prion Hypothesis for Parkinson’s Disease. J. Neurosci. 2017, 37, 9808–9818. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; Pelech, S.; Giasson, B.I.; Maguire, J.; Zhang, H.; McGeer, E.G.; McGeer, P.L. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging 2008, 29, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, S.H.; Kim, K.Y.; Shin, K.Y.; Kim, H.S.; Park, C.H.; Chang, K.A.; Lee, S.H.; Cho, D.; Suh, Y.H. Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J. Neurochem. 2009, 109, 1483–1496. [Google Scholar] [CrossRef]
- Sugama, S.; Yang, L.; Cho, B.P.; DeGiorgio, L.A.; Lorenzl, S.; Albers, D.S.; Beal, M.F.; Volpe, B.T.; Joh, T.H. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003, 964, 288–294. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Broom, L.; Marinova-Mutafchieva, L.; Sadeghian, M.; Davis, J.B.; Medhurst, A.D.; Dexter, D.T. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic. Biol. Med. 2011, 50, 633–640. [Google Scholar] [CrossRef]
- Wu, D.C.; Teismann, P.; Tieu, K.; Vila, M.; Jackson-Lewis, V.; Ischiropoulos, H.; Przedborski, S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 6145–6150. [Google Scholar] [CrossRef]
- Miklossy, J.; Doudet, D.D.; Schwab, C.; Yu, S.; McGeer, E.G.; McGeer, P.L. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006, 197, 275–283. [Google Scholar] [CrossRef]
- Benner, E.J.; Banerjee, R.; Reynolds, A.D.; Sherman, S.; Pisarev, V.M.; Tsiperson, V.; Nemachek, C.; Ciborowski, P.; Przedborski, S.; Mosley, R.L.; et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE 2008, 3, e1376. [Google Scholar] [CrossRef] [Green Version]
- Depboylu, C.; Stricker, S.; Ghobril, J.P.; Oertel, W.H.; Priller, J.; Höglinger, G.U. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 2012, 238, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yang, M.S.; Choi, D.; Kim, J.H.; Kim, H.S.; Seol, W.; Choi, S.; Jou, I.; Kim, E.Y.; Joe, E.H. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS ONE 2012, 7, e34693. [Google Scholar] [CrossRef] [PubMed]
- Waak, J.; Weber, S.S.; Waldenmaier, A.; Görner, K.; Alunni-Fabbroni, M.; Schell, H.; Vogt-Weisenhorn, D.; Pham, T.T.; Reumers, V.; Baekelandt, V.; et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. Faseb. J. 2009, 23, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Lücking, C.B.; Dürr, A.; Bonifati, V.; Vaughan, J.; De Michele, G.; Gasser, T.; Harhangi, B.S.; Meco, G.; Denèfle, P.; Wood, N.W.; et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N. Engl. J. Med. 2000, 342, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Byun, J.W.; Choi, I.; Kim, B.; Jeong, H.K.; Jou, I.; Joe, E. PINK1 Deficiency Enhances Inflammatory Cytokine Release from Acutely Prepared Brain Slices. Exp. Neurobiol. 2013, 22, 38–44. [Google Scholar] [CrossRef]
- Frank-Cannon, T.C.; Tran, T.; Ruhn, K.A.; Martinez, T.N.; Hong, J.; Marvin, M.; Hartley, M.; Treviño, I.; O’Brien, D.E.; Casey, B.; et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 2008, 28, 10825–10834. [Google Scholar] [CrossRef]
- Albe-Fessard, D. Electrophysiological methods for the identification of thalamic nuclei. Z. Neurol. 1973, 205, 15–28. [Google Scholar] [CrossRef]
- Groiss, S.J.; Wojtecki, L.; Südmeyer, M.; Schnitzler, A. Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2009, 2, 20–28. [Google Scholar] [CrossRef]
- Gerhard, A. TSPO imaging in parkinsonian disorders. Clin. Transl. Imaging 2016, 4, 183–190. [Google Scholar] [CrossRef]
- Koshimori, Y.; Ko, J.H.; Mizrahi, R.; Rusjan, P.; Mabrouk, R.; Jacobs, M.F.; Christopher, L.; Hamani, C.; Lang, A.E.; Wilson, A.A.; et al. Imaging Striatal Microglial Activation in Patients with Parkinson’s Disease. PLoS ONE 2015, 10, e0138721. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Glial reactions in Parkinson’s disease. Mov. Disord. 2008, 23, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Yagi, S.; Yokokura, M.; Sakamoto, M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15 (Suppl. S3), S200–S204. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 373–381. [Google Scholar] [CrossRef]
- Barcia, C.; Hunot, S.; Guillemin, G.J.; Pitossi, F. Inflammation and Parkinson’s disease. Parkinsons Dis. 2011, 2011, 729054. [Google Scholar] [CrossRef]
- Joers, V.; Tansey, M.G.; Mulas, G.; Carta, A.R. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 2017, 155, 57–75. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Green, H.F.; Khosousi, S.; Svenningsson, P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 705–709. [Google Scholar] [CrossRef]
- Pereira, J.R.; Santos, L.V.D.; Santos, R.M.S.; Campos, A.L.F.; Pimenta, A.L.; de Oliveira, M.S.; Bacheti, G.G.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; et al. IL-6 serum levels are elevated in Parkinson’s disease patients with fatigue compared to patients without fatigue. J. Neurol. Sci. 2016, 370, 153–156. [Google Scholar] [CrossRef]
- Veselý, B.; Dufek, M.; Thon, V.; Brozman, M.; Királová, S.; Halászová, T.; Koriťáková, E.; Rektor, I. Interleukin 6 and complement serum level study in Parkinson’s disease. J. Neural. Transm. (Vienna) 2018, 125, 875–881. [Google Scholar] [CrossRef]
- Hofmann, K.W.; Schuh, A.F.; Saute, J.; Townsend, R.; Fricke, D.; Leke, R.; Souza, D.O.; Portela, L.V.; Chaves, M.L.; Rieder, C.R. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 2009, 34, 1401–1404. [Google Scholar] [CrossRef] [Green Version]
- Pisanu, A.; Lecca, D.; Mulas, G.; Wardas, J.; Simbula, G.; Spiga, S.; Carta, A.R. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol. Dis. 2014, 71, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Boi, L.; Mulas, G.; Spiga, S.; Fenu, S.; Carta, A.R. Neuroinflammation in L-DOPA-induced dyskinesia: Beyond the immune function. J. Neural. Transm. (Vienna) 2018, 125, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Zecca, L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox Res. 2000, 1, 181–195. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Feyder, M.; Bonito-Oliva, A.; Fisone, G. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front. Behav. Neurosci. 2011, 5, 71. [Google Scholar] [CrossRef]
- Barnum, C.J.; Eskow, K.L.; Dupre, K.; Blandino, P., Jr.; Deak, T.; Bishop, C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: Role for interleukin-1beta. Neuroscience 2008, 156, 30–41. [Google Scholar] [CrossRef]
- Kwiatek-Majkusiak, J.; Geremek, M.; Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Higher serum levels of pro-hepcidin in patients with Parkinson’s disease treated with deep brain stimulation. Neurosci. Lett. 2018, 684, 205–209. [Google Scholar] [CrossRef]
- Kwiatek-Majkusiak, J.; Geremek, M.; Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Serum levels of hepcidin and interleukin 6 in Parkinson’s disease. Acta Neurobiol. Exp. (Wars) 2020, 80, 297–304. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Zella, M.A.S.; Metzdorf, J.; Ostendorf, F.; Maass, F.; Muhlack, S.; Gold, R.; Haghikia, A.; Tonges, L. Novel Immunotherapeutic Approaches to Target Alpha-Synuclein and Related Neuroinflammation in Parkinson’s Disease. Cells 2019, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Lindestam Arlehamn, C.S.; Dhanwani, R.; Pham, J.; Kuan, R.; Frazier, A.; Rezende Dutra, J.; Phillips, E.; Mallal, S.; Roederer, M.; Marder, K.S.; et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 2020, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Sabahi, M.; Joshaghanian, A.; Dolatshahi, M.; Jabbari, P.; Rahmani, F.; Rezaei, N. Modification of Glial Cell Activation through Dendritic Cell Vaccination: Promises for Treatment of Neurodegenerative Diseases. J. Mol. Neurosci. 2021, 71, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Englund, E.; Holton, J.L.; Soulet, D.; Hagell, P.; Lees, A.J.; Lashley, T.; Quinn, N.P.; Rehncrona, S.; Bjorklund, A.; et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008, 14, 501–503. [Google Scholar] [CrossRef]
- Ugen, K.E.; Lin, X.; Bai, G.; Liang, Z.; Cai, J.; Li, K.; Song, S.; Cao, C.; Sanchez-Ramos, J. Evaluation of an alpha synuclein sensitized dendritic cell based vaccine in a transgenic mouse model of Parkinson disease. Hum. Vaccin. Immunother. 2015, 11, 922–930. [Google Scholar] [CrossRef]
- Ciaramella, A.; Salani, F.; Bizzoni, F.; Pontieri, F.E.; Stefani, A.; Pierantozzi, M.; Assogna, F.; Caltagirone, C.; Spalletta, G.; Bossu, P. Blood dendritic cell frequency declines in idiopathic Parkinson’s disease and is associated with motor symptom severity. PLoS ONE 2013, 8, e65352. [Google Scholar] [CrossRef]
- Tran, H.T.; Chung, C.H.; Iba, M.; Zhang, B.; Trojanowski, J.Q.; Luk, K.C.; Lee, V.M. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell Rep. 2014, 7, 2054–2065. [Google Scholar] [CrossRef]
- Shahaduzzaman, M.; Nash, K.; Hudson, C.; Sharif, M.; Grimmig, B.; Lin, X.; Bai, G.; Liu, H.; Ugen, K.E.; Cao, C.; et al. Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson’s disease. PLoS ONE 2015, 10, e0116841. [Google Scholar] [CrossRef]
- Games, D.; Valera, E.; Spencer, B.; Rockenstein, E.; Mante, M.; Adame, A.; Patrick, C.; Ubhi, K.; Nuber, S.; Sacayon, P.; et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci. 2014, 34, 9441–9454. [Google Scholar] [CrossRef]
- Bae, E.J.; Lee, H.J.; Rockenstein, E.; Ho, D.H.; Park, E.B.; Yang, N.Y.; Desplats, P.; Masliah, E.; Lee, S.J. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012, 32, 13454–13469. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.B.; Koller, M.; Ness, D.K.; Griffith, S.G.; Grundman, M.; Zago, W.; Soto, J.; Atiee, G.; Ostrowitzki, S.; Kinney, G.G. First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Mov. Disord. 2017, 32, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Brys, M.; Fanning, L.; Hung, S.; Ellenbogen, A.; Penner, N.; Yang, M.; Welch, M.; Koenig, E.; David, E.; Fox, T.; et al. Randomized phase I clinical trial of anti-alpha-synuclein antibody BIIB054. Mov. Disord. 2019, 34, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Goodman, I.; Safirstein, B.; Marmon, T.K.; Schenk, D.B.; Koller, M.; Zago, W.; Ness, D.K.; Griffith, S.G.; Grundman, M.; et al. Safety and Tolerability of Multiple Ascending Doses of PRX002/RG7935, an Anti-alpha-Synuclein Monoclonal Antibody, in Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1206–1214. [Google Scholar] [CrossRef]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Tanna, T.; Sachan, V. Mesenchymal stem cells: Potential in treatment of neurodegenerative diseases. Curr. Stem Cell Res. Ther. 2014, 9, 513–521. [Google Scholar] [CrossRef]
- Yen, B.L.; Chang, C.J.; Liu, K.J.; Chen, Y.C.; Hu, H.I.; Bai, C.H.; Yen, M.L. Brief report--human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 2009, 27, 451–456. [Google Scholar] [CrossRef]
- Trivedi, P.; Hematti, P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 2008, 36, 350–359. [Google Scholar] [CrossRef]
- Tan, Z.; Su, Z.Y.; Wu, R.R.; Gu, B.; Liu, Y.K.; Zhao, X.L.; Zhang, M. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J. Zhejiang Univ. Sci. B 2011, 12, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, M.; Cimini, A.; Castelli, V. Insights into the Effects of Mesenchymal Stem Cell-Derived Secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 5241. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Paterno, G.; Vivarelli, S.; L’Episcopo, F.; Tirolo, C.; Raciti, G.; Pappalardo, F.; Giachino, C.; Caniglia, S.; Serapide, M.F.; et al. Extracellular Vesicles as Nanotherapeutics for Parkinson’s Disease. Biomolecules 2020, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulou, V.; Ivanyuk, D.; De Cicco, S.; Haq, W.; Arsic, A.; Yu, C.; Messelodi, D.; Oldrati, M.; Schondorf, D.C.; Perez, M.J.; et al. Interferon-gamma signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 5163. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Kordower, J.H.; Goetz, C.G.; Chu, Y.; Halliday, G.M.; Nicholson, D.A.; Musial, T.F.; Marmion, D.J.; Stoessl, A.J.; Sossi, V.; Freeman, T.B.; et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann. Neurol. 2017, 81, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Goetz, C.G.; Kordower, J.H.; Stoessl, A.J.; Sossi, V.; Brin, M.F.; Shannon, K.M.; Nauert, G.M.; Perl, D.P.; Godbold, J.; et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003, 54, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheung, H.H. Stem Cell-Based Therapies for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 8060. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Dezawa, M. Parkinson’s disease and mesenchymal stem cells: Potential for cell-based therapy. Parkinsons Dis. 2012, 2012, 873706. [Google Scholar] [CrossRef]
- Lindvall, O.; Sawle, G.; Widner, H.; Rothwell, J.C.; Bjorklund, A.; Brooks, D.; Brundin, P.; Frackowiak, R.; Marsden, C.D.; Odin, P.; et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann. Neurol. 1994, 35, 172–180. [Google Scholar] [CrossRef]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Freed, C.R.; Breeze, R.E.; Rosenberg, N.L.; Schneck, S.A.; Kriek, E.; Qi, J.X.; Lone, T.; Zhang, Y.B.; Snyder, J.A.; Wells, T.H.; et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N. Engl. J. Med. 1992, 327, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Vinuela, A.; Astradsson, A.; Mukhida, K.; Hallett, P.; Robertson, H.; Tierney, T.; Holness, R.; Dagher, A.; Trojanowski, J.Q.; et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 2008, 14, 507–509. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, M.; Dong, Y.; Haberman, A.; Cao, J.; Liu, H.; Zhou, W.; Zhang, S.C. Chemical Control of Grafted Human PSC-Derived Neurons in a Mouse Model of Parkinson’s Disease. Cell Stem Cell 2016, 18, 817–826. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cerdeno, V.; Noctor, S.C.; Espinosa, A.; Ariza, J.; Parker, P.; Orasji, S.; Daadi, M.M.; Bankiewicz, K.; Alvarez-Buylla, A.; Kriegstein, A.R. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 2010, 6, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Bjorklund, A.; et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014, 15, 653–665. [Google Scholar] [CrossRef]
- Wang, Y.K.; Zhu, W.W.; Wu, M.H.; Wu, Y.H.; Liu, Z.X.; Liang, L.M.; Sheng, C.; Hao, J.; Wang, L.; Li, W.; et al. Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson’s Disease. Stem Cell Reports 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]
- Ben-Hur, T.; Idelson, M.; Khaner, H.; Pera, M.; Reinhartz, E.; Itzik, A.; Reubinoff, B.E. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells 2004, 22, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Hargus, G.; Cooper, O.; Deleidi, M.; Levy, A.; Lee, K.; Marlow, E.; Yow, A.; Soldner, F.; Hockemeyer, D.; Hallett, P.J.; et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA 2010, 107, 15921–15926. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, Z.J.; Oldenburg, M.; Ayala, M.; Zhang, S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 2008, 26, 55–63. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Harrison, E.B.; Mahajan, V.; Ahmed, S.; He, Z.; Suresh, P.; Hingtgen, S.D.; Klyachko, N.L.; Mosley, R.L.; et al. Specific transfection of inflamed brain by macrophages: A new therapeutic strategy for neurodegenerative diseases. PLoS ONE 2013, 8, e61852. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, S.C.; Kim, D.Y.; Kim, H.N.; Shin, J.Y.; Ye, B.S.; Lee, P.H. Mesenchymal Stem Cells Stabilize Axonal Transports for Autophagic Clearance of alpha-Synuclein in Parkinsonian Models. Stem Cells 2017, 35, 1934–1947. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Auslander, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Peruzzotti-Jametti, L.; Serapide, M.F.; Testa, N.; Caniglia, S.; Balzarotti, B.; Pluchino, S.; Marchetti, B. Neural Stem Cell Grafts Promote Astroglia-Driven Neurorestoration in the Aged Parkinsonian Brain via Wnt/β-Catenin Signaling. Stem Cells 2018, 36, 1179–1197. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tuazon, J.P.; Ehrhart, J.; Sanberg, P.R.; Borlongan, C.V. Gutting the brain of inflammation: A key role of gut microbiome in human umbilical cord blood plasma therapy in Parkinson’s disease model. J. Cell Mol. Med. 2019, 23, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Tuazon, J.P.; Corey, S.; Bonsack, B.; Acosta, S.; Ehrhart, J.; Sanberg, P.R.; Borlongan, C.V. A Gutsy Move for Cell-Based Regenerative Medicine in Parkinson’s Disease: Targeting the Gut Microbiome to Sequester Inflammation and Neurotoxicity. Stem Cell Rev. Rep. 2019, 15, 690–702. [Google Scholar] [CrossRef]
- Serapide, M.F.; L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Giachino, C.; Marchetti, B. Boosting Antioxidant Self-defenses by Grafting Astrocytes Rejuvenates the Aged Microenvironment and Mitigates Nigrostriatal Toxicity in Parkinsonian Brain via an Nrf2-Driven Wnt/beta-Catenin Prosurvival Axis. Front. Aging Neurosci. 2020, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Serapide, M.F.; Pluchino, S.; Marchetti, B. Wnt/β-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson’s disease. Stem Cells 2014, 32, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Berwick, D.C.; Javaheri, B.; Wetzel, A.; Hopkinson, M.; Nixon-Abell, J.; Grannò, S.; Pitsillides, A.A.; Harvey, K. Pathogenic LRRK2 variants are gain-of-function mutations that enhance LRRK2-mediated repression of β-catenin signaling. Mol. Neurodegener 2017, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Deng, J.; Pan, Q.; Zhan, Y.; Fan, J.B.; Zhang, K.; Zhang, Z. Targeted methylation sequencing reveals dysregulated Wnt signaling in Parkinson disease. J. Genet. Genomics 2016, 43, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.L.; Thompson, L.H. Modulating Wnt signaling to improve cell replacement therapy for Parkinson’s disease. J. Mol. Cell Biol. 2014, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.R.; Saltó, C.; Villaescusa, J.C.; Cajanek, L.; Yang, S.; Bryjova, L.; Nagy, I.I.; Vainio, S.J.; Ramirez, C.; Bryja, V.; et al. Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, E602–E610. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Maisel, M.; Wegner, F.; Liebau, S.; Kim, D.W.; Gerlach, M.; Schwarz, J.; Kim, K.S.; Storch, A. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells 2006, 24, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Suess, C.; Fauser, M.; Kanzler, S.; Witt, M.; Fabel, K.; Schwarz, J.; Höglinger, G.U.; Storch, A. Rostro-caudal gradual loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells 2009, 27, 928–941. [Google Scholar] [CrossRef]
- Arenas, E. Wnt signaling in midbrain dopaminergic neuron development and regenerative medicine for Parkinson’s disease. J. Mol. Cell Biol. 2014, 6, 42–53. [Google Scholar] [CrossRef]
- Joksimovic, M.; Awatramani, R. Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J. Mol. Cell Biol. 2014, 6, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Sariola, H.; Saarma, M. Novel functions and signalling pathways for GDNF. J. Cell Sci. 2003, 116, 3855–3862. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef]
- Kearns, C.M.; Gash, D.M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995, 672, 104–111. [Google Scholar] [CrossRef]
- Tomac, A.; Lindqvist, E.; Lin, L.F.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Heiss, J.D.; Lungu, C.; Hammoud, D.A.; Herscovitch, P.; Ehrlich, D.J.; Argersinger, D.P.; Sinharay, S.; Scott, G.; Wu, T.; Federoff, H.J.; et al. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov. Disord. 2019, 34, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R., Jr.; Lozano, A.M.; Penn, R.D.; Simpson, R.K., Jr.; Stacy, M.; et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef]
- Slevin, J.T.; Gash, D.M.; Smith, C.D.; Gerhardt, G.A.; Kryscio, R.; Chebrolu, H.; Walton, A.; Wagner, R.; Young, A.B. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: Response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg 2007, 106, 614–620. [Google Scholar] [CrossRef]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Whone, A.L.; Boca, M.; Luz, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 301–313. [Google Scholar] [CrossRef]
- Berlet, R.; Galang Cabantan, D.A.; Gonzales-Portillo, D.; Borlongan, C.V. Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson’s Disease, and Huntington’s Disease. Front. Cell Dev. Biol. 2022, 10, 798826. [Google Scholar] [CrossRef]
- Jacotte-Simancas, A.; Costa-Miserachs, D.; Coll-Andreu, M.; Torras-Garcia, M.; Borlongan, C.V.; Portell-Cortes, I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J. Neurotrauma 2015, 32, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, C.; Shear, A.; Heyck, M.; Sadanandan, N.; Zhang, H.; Gonzales-Portillo, B.; Cozene, B.; Sheyner, M.; Navarro-Torres, L.; Garcia-Sanchez, J.; et al. Inflammation-relevant microbiome signature of the stroke brain, gut, spleen, and thymus and the impact of exercise. J. Cereb. Blood Flow Metab. 2021, 41, 3200–3212. [Google Scholar] [CrossRef] [PubMed]
- Pianta, S.; Lee, J.Y.; Tuazon, J.P.; Castelli, V.; Mantohac, L.M.; Tajiri, N.; Borlongan, C.V. A Short Bout of Exercise Prior to Stroke Improves Functional Outcomes by Enhancing Angiogenesis. Neuromolecular Med. 2019, 21, 517–528. [Google Scholar] [CrossRef]
- Watson, N.; Ji, X.; Yasuhara, T.; Date, I.; Kaneko, Y.; Tajiri, N.; Borlongan, C.V. No pain, no gain: Lack of exercise obstructs neurogenesis. Cell Transplant. 2015, 24, 591–597. [Google Scholar] [CrossRef]
- Yasuhara, T.; Hara, K.; Maki, M.; Matsukawa, N.; Fujino, H.; Date, I.; Borlongan, C.V. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience 2007, 149, 182–191. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; Stewart, L.K.; Timmerman, K.L.; Coen, P.M. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 388–393. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, H.; Wan, M.; Qiu, P.; Xia, R.; He, J.; Tao, J.; Chen, L.; Zheng, G. The Effects of Aerobic Exercise on Oxidative Stress in Older Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 701151. [Google Scholar] [CrossRef]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Parissis, J.; Kroupis, C.; Georgiadis, M.; Karatzas, D.; Karavolias, G.; Koniavitou, K.; Coats, A.J.; Kremastinos, D.T. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur. Heart J. 2001, 22, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Parissis, J.; Karatzas, D.; Kroupis, C.; Georgiadis, M.; Karavolias, G.; Paraskevaidis, J.; Koniavitou, K.; Coats, A.J.; Kremastinos, D.T. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J. Am. Coll Cardiol. 2002, 39, 653–663. [Google Scholar] [CrossRef]
- Murphy, E.A.; Enos, R.T.; Velázquez, K.T. Influence of Exercise on Inflammation in Cancer: Direct Effect or Innocent Bystander? Exerc. Sport Sci. Rev. 2015, 43, 134–142. [Google Scholar] [CrossRef]
- Daou, H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef]
- Seo, D.Y.; Heo, J.W.; Ko, J.R.; Kwak, H.B. Exercise and Neuroinflammation in Health and Disease. Int. Neurourol. J. 2019, 23, S82–S92. [Google Scholar] [CrossRef]
- Jawed, Y.; Beli, E.; March, K.; Kaleth, A.; Loghmani, M.T. Whole-Body Vibration Training Increases Stem/Progenitor Cell Circulation Levels and May Attenuate Inflammation. Mil. Med. 2020, 185, 404–412. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H.; McAlpine, C.S.; Cremer, S.; Hoyer, F.F.; Ji, F.; et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef]
- Niemiro, G.M.; Allen, J.M.; Mailing, L.J.; Khan, N.A.; Holscher, H.D.; Woods, J.A.; De Lisio, M. Effects of endurance exercise training on inflammatory circulating progenitor cell content in lean and obese adults. J. Physiol. 2018, 596, 2811–2822. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Real, C.C.; Garcia, P.C.; Britto, L.R.G. Treadmill Exercise Prevents Increase of Neuroinflammation Markers Involved in the Dopaminergic Damage of the 6-OHDA Parkinson’s Disease Model. J. Mol. Neurosci. 2017, 63, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Jang, Y.C.; Hwang, D.J.; Um, H.S.; Lee, N.H.; Jung, J.H.; Cho, J.Y. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-κB signaling pathway. Neuroscience 2017, 356, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol Pharmacol 2014, 65, 441–448. [Google Scholar]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Luciano, T.; Trom, C.B.; Silva, L.A.; Quevedo, J.; Souza, C.T.; Lira, F.S.; Pinho, R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 2012, 227, 305–312. [Google Scholar] [CrossRef]
- Kronenberg, G.; Reuter, K.; Steiner, B.; Brandt, M.D.; Jessberger, S.; Yamaguchi, M.; Kempermann, G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003, 467, 455–463. [Google Scholar] [CrossRef]
- Steiner, B.; Zurborg, S.; Hörster, H.; Fabel, K.; Kempermann, G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience 2008, 154, 521–529. [Google Scholar] [CrossRef]
- Pérez-Domínguez, M.; Tovar, Y.R.L.B.; Zepeda, A. Neuroinflammation and physical exercise as modulators of adult hippocampal neural precursor cell behavior. Rev. Neurosci. 2018, 29, 1–20. [Google Scholar] [CrossRef]
- Wu, C.W.; Chen, Y.C.; Yu, L.; Chen, H.I.; Jen, C.J.; Huang, A.M.; Tsai, H.J.; Chang, Y.T.; Kuo, Y.M. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J. Neurochem. 2007, 103, 2471–2481. [Google Scholar] [CrossRef]
- Zigmond, M.J.; Cameron, J.L.; Leak, R.K.; Mirnics, K.; Russell, V.A.; Smeyne, R.J.; Smith, A.D. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 2009, 15 (Suppl. S3), S42–S45. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Neurogenic effects of rotarod walking exercise in subventricular zone, subgranular zone, and substantia nigra in MPTP-induced Parkinson’s disease mice. Sci. Rep. 2022, 12, 10544. [Google Scholar] [CrossRef] [PubMed]
- Frazzitta, G.; Maestri, R.; Bertotti, G.; Riboldazzi, G.; Boveri, N.; Perini, M.; Uccellini, D.; Turla, M.; Comi, C.; Pezzoli, G.; et al. Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil. Neural. Repair. 2015, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- De Lisio, M.; Baker, J.M.; Parise, G. Exercise promotes bone marrow cell survival and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp. Hematol. 2013, 41, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.; Asher, A.; Bailey, C.; Fu, J.B. The role of physical rehabilitation in stem cell transplantation patients. Support. Care Cancer 2015, 23, 2447–2460. [Google Scholar] [CrossRef]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation 2015, 12, 9. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Masino, S.A.; Rho, J.M. Mechanisms of Ketogenic Diet Action. In Jasper’s Basic Mechanisms of the Epilepsies; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Park Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Quan, Z.; Wong, W.; Guo, J.; Zhang, R.; Yang, Q.; Dai, R.; McGeer, P.L.; Qing, H. Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease. Neurochem. Res. 2016, 41, 2788–2796. [Google Scholar] [CrossRef]
- Chen, M.; Wang, T.; Yue, F.; Li, X.; Wang, P.; Li, Y.; Chan, P.; Yu, S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 2015, 286, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Batista-Nascimento, L.; Pimentel, C.; Menezes, R.A.; Rodrigues-Pousada, C. Iron and neurodegeneration: From cellular homeostasis to disease. Oxid. Med. Cell Longev. 2012, 2012, 128647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Liang, Q.; Sun, Q.; Chen, C.; Zhang, Y.; Ding, Y.; Zhou, P. Metal chelator EGCG attenuates Fe(III)-induced conformational transition of α-synuclein and protects AS-PC12 cells against Fe(III)-induced death. J. Neurochem. 2017, 143, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Karthikeyan, A.; Dheen, S.T. Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromolecular Med. 2016, 18, 453–464. [Google Scholar] [CrossRef]
- Duan, W.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999, 57, 195–206. [Google Scholar] [CrossRef]
- Mattson, M.P.; Duan, W.; Guo, Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: Cellular and molecular mechanisms. J. Neurochem. 2003, 84, 417–431. [Google Scholar] [CrossRef]
- Berglöf, E.; Small, B.J.; Bickford, P.C.; Strömberg, I. Beneficial effects of antioxidant-enriched diet for tyrosine hydroxylase-positive neurons in ventral mesencephalic tissue in oculo grafts. J. Comp. Neurol. 2009, 515, 72–82. [Google Scholar] [CrossRef]
- Bittencourt, A.; Brum, P.O.; Ribeiro, C.T.; Gasparotto, J.; Bortolin, R.C.; de Vargas, A.R.; Heimfarth, L.; de Almeida, R.F.; Moreira, J.C.F.; de Oliveira, J.; et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr. Neurosci. 2022, 25, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Wei, W.Y.; Tsai, K.J.; Wang, L.C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2019, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.Y.; Yeo, X.Y.; Bae, H.G.; Lee, D.P.S.; Ho, R.C.; Kim, J.E.; Jo, D.G.; Jung, S. Association of Gut Microbiome Dysbiosis with Neurodegeneration: Can Gut Microbe-Modifying Diet Prevent or Alleviate the Symptoms of Neurodegenerative Diseases? Life 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Milan Manani, S.; Virzì, G.M.; Giuliani, A.; Baretta, M.; Corradi, V.; De Cal, M.; Biasi, C.; Crepaldi, C.; Ronco, C. Lipopolysaccharide Evaluation in Peritoneal Dialysis Patients with Peritonitis. Blood Purif. 2020, 49, 434–439. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef] [PubMed]
- Iba, M.; Kim, C.; Sallin, M.; Kwon, S.; Verma, A.; Overk, C.; Rissman, R.A.; Sen, R.; Sen, J.M.; Masliah, E. Neuroinflammation is associated with infiltration of T cells in Lewy body disease and α-synuclein transgenic models. J. Neuroinflammation 2020, 17, 214. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Cao, B.B.; Qiu, Y.H.; Peng, Y.P. Th17 Cells Induce Dopaminergic Neuronal Death via LFA-1/ICAM-1 Interaction in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol 2017, 54, 7762–7776. [Google Scholar] [CrossRef]
- Mertsalmi, T.H.; Pekkonen, E.; Scheperjans, F. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov. Disord. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, M.; Chen, H.; Tang, W.; Nie, Y.; Zhou, Y. Fecal microbiota transplantation to treat Parkinson’s disease with constipation: A case report. Medicine (Baltimore) 2019, 98, e16163. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Scheperjans, F.; Pekkonen, E.; Kaakkola, S.; Auvinen, P. Linking Smoking, Coffee, Urate, and Parkinson’s Disease—A Role for Gut Microbiota? J. Parkinsons Dis. 2015, 5, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva. Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar] [PubMed]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef] [Green Version]
| Citation | Model | Cell type | Dosage | Findings |
|---|---|---|---|---|
| Haney et al. (2013) [94] | 6-OHDA mice | Modified macrophages with antioxidant plasmid | 5 × 106 cells/mouse in 100 µL PBS | Macrophages released exosomes containing antioxidant genetic material prompting neurons to increase protein synthesis. Mice had improved motor function. |
| Ugen et al. (2015) [56] | A53T α-synuclein overexpressing cells and mice | Bone marrow-derived dendritic cells | 106 cells | α-synuclein sensitized DCs induced α-synuclein antibodies, improved motor function in mice, and had lower pro-inflammatory cytokine levels. |
| Oh et al. (2017) [95] | A53T α-synuclein overexpressing cells and mice | Mesenchymal stem cells | 1 × 106 cells in 200 µL saline in tail | Eukaryotic elongation factor 1A-2 from MSCs increased neuronal survival by improving axonal transport and monitoring α-synuclein pathological phosphorylation |
| Kojima et al. (2018) [96] | 6-OHDA mice | Catalase mRNA in designer exosomes | Four hundred microliters of the cell/Matrigel mixture | Reduced neuroinflammation and neurotoxicity in Parkinson’s mice. |
| L’Episcopo et al. (2018) [97] | MPTP mice | Neural stem cells | 100 × 103 cells | NSCs that became astrocytic expressed Wnt1 and prompted Wnt/ β-catenin signaling in substantia nigra pars compacta midbrain dopaminergic neurons and microglia. This allowed for dopaminergic neuron rescue and decreased microglial inflammation. |
| Lee et al. (2019a) [98] | MPTP mice | Human umbilical cord blood stem cells | 500 µL cord blood plasma | Mice showed improved motor and GI function, ameliorated dopamine cell loss, and reduced neurological and GI inflammation. |
| Lee et al. (2019b) [99] | 6-OHDA mice | Human umbilical cord blood stem cells | Three separate doses of 4 × 106 cells | Mice showed improved motor and GI function, ameliorated dopamine cell loss, and reduced neurological and GI inflammation. |
| Serapide et al. (2020) [100] | MPTP Mice | Engrafted astrocytes | 150 × 103 ventral midbrain-Astrocytes | Grafted astrocytes can rescue dying dopaminergic neurons, likely via antioxidant and anti-inflammatory Nrf2/ARE/Wnt/β-catenin signaling. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, J.; Lockard, G.; Monsour, M.; Alayli, A.; Choudhary, H.; Borlongan, C.V. Sequestration of Inflammation in Parkinson’s Disease via Stem Cell Therapy. Int. J. Mol. Sci. 2022, 23, 10138. https://doi.org/10.3390/ijms231710138
Gordon J, Lockard G, Monsour M, Alayli A, Choudhary H, Borlongan CV. Sequestration of Inflammation in Parkinson’s Disease via Stem Cell Therapy. International Journal of Molecular Sciences. 2022; 23(17):10138. https://doi.org/10.3390/ijms231710138
Chicago/Turabian StyleGordon, Jonah, Gavin Lockard, Molly Monsour, Adam Alayli, Hassan Choudhary, and Cesario V. Borlongan. 2022. "Sequestration of Inflammation in Parkinson’s Disease via Stem Cell Therapy" International Journal of Molecular Sciences 23, no. 17: 10138. https://doi.org/10.3390/ijms231710138
APA StyleGordon, J., Lockard, G., Monsour, M., Alayli, A., Choudhary, H., & Borlongan, C. V. (2022). Sequestration of Inflammation in Parkinson’s Disease via Stem Cell Therapy. International Journal of Molecular Sciences, 23(17), 10138. https://doi.org/10.3390/ijms231710138








