Multiple Cell Cultures for MRI Analysis
Abstract
:1. Introduction
1.1. Nodular Cell Culture
1.2. The 3D Culture Matrices Based on a Hydrogel
2. Natural Matrices
2.1. Culture Based on Matrigel™ or Cultrex®
2.2. Collagen-Based Matrices
2.3. Matrices Derived from Fibroblasts
2.4. Matrices Based on Calcium Alginate
2.5. Matrices Based on Fibrinogen
2.6. Matrices Based on Hyaluronic Acid
2.7. Gelatin-Based Matrices
2.8. Chitosan-Based Matrices
2.9. Alginate-Based Matrices
2.10. Matrix Based on Silk Fibroin
3. Synthetic Matrices
3.1. Poly (Lactic-Co-Glycolic Acid) PLGA Matrices
3.2. Synthetic Peptides
3.3. Dies Based on Electrospun Poly (ε-Caprolactone) PCL Scaffolding
3.4. Matrices Based on Poly (Ethylene Glycol) (PEG)
4. The 3D Cell Culture
4.1. Hollow Fiber Bioreactors
4.2. Stirred-Tank Bioreactors (STBR)
4.3. Rotary Cell Culture System (RCCS) Bioreactors
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hong, S.; Rhee, S.; Jung, K.O. In vivo molecular and single cell imaging. BMB Rep. 2022, 55, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, P.; Gong, T.; Zhou, Y.; Wang, A.; Tang, X.; Song, X.; Fan, Y. Reporter Genes for Brain Imaging Using MRI, SPECT and PET. Int. J. Mol. Sci. 2022, 23, 8443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, C.; Ke, X.; Zhou, J. Treatment Response and Prognosis Evaluation in High-Grade Glioma: An Imaging Review Based on MRI. J. Magn. Reson. Imaging 2022, 56, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Moonshi, S.S.; Wu, Y.; Ta, H.T. Visualizing stem cells in vivo using magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, 1760. [Google Scholar]
- Sato, N.; Choyke, P.L. Whole-Body Imaging to Assess Cell-Based Immunotherapy: Preclinical Studies with an Update on Clinical Translation. Mol. Imaging Biol. 2022, 24, 235–248. [Google Scholar] [CrossRef]
- Li, P.; Wang, D.; Hu, J.; Yang, X. The role of imaging in targeted delivery of nanomedicine for cancer therapy. Adv. Drug Deliv. Rev. 2022, 189, 114447. [Google Scholar] [CrossRef]
- Hu, H.; Quintana, J.; Weissleder, R.; Parangi, S.; Miller, M. Deciphering albumin-directed drug delivery by imaging. Adv. Drug Deliv. Rev. 2022, 185, 114237. [Google Scholar] [CrossRef]
- Miniksar, Ö.H.; Yıldız Miniksar, D.; Honca, M.; Onat, T.; Gocmen, A.Y.; Öz, H. The Effect of Preoperative Anxiety on Fetal Cord Blood Tumor Necrosis Factor-Alpha, Interleukin-6, and Neonatal Outcomes in Pregnant Women. Psychiatr. Danub. 2021, 33, 321–326. [Google Scholar]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive Aptasensor Coupled with Microfluidic Enrichment for Real-Time Detection of Trace SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 2812–2819. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, T.; Shung, K.K.; Zhu, B. Recent Advancements in Ultrasound Transducer: From Material Strategies to Biomedical Applications. BME Frontiers 2022, 2022, 9764501. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Liang, H.; Wu, Z.; Li, J.; Ou-Yang, J.; Yang, X.; Peng, Y.B.; Zhu, B. Transcranial Focused Ultrasound Stimulation of Periaqueductal Gray for Analgesia; IEEE Transactions on Biomedical Engineering: Piscataway, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wan, Y.; Guan, D.; Ni, S.; Wang, L.; Zhang, Z.; Liu, J.; Liang, C.; Yu, Y.; Lu, A.; et al. A Loop-Based and AGO-Incorporated Virtual Screening Model Targeting AGO-Mediated miRNA-mRNA Interactions for Drug Discovery to Rescue Bone Phenotype in Genetically Modified Mice. Adv. Sci. 2020, 7, 1903451. [Google Scholar]
- Zheng, J.; Long, X.; Chen, H.; Ji, Z.; Shu, B.; Yue, R.; Liao, Y.; Ma, S.; Qiao, K.; Liu, Y.; et al. Photoclick Reaction Constructs Glutathione-Responsive Theranostic System for Anti-Tuberculosis. Front. Mol. Biosci. 2022, 9, 845179. [Google Scholar] [CrossRef] [PubMed]
- Hegre, O.D.; Marshall, S.; Schulte, B.A.; Hickey, G.E.; Williams, F.; Sorenson, R.L.; Serie, J.R. Nonenzymic in vitro isolation of perinatal islets of Langerhans. In Vitro 1983, 19, 611–620. [Google Scholar] [CrossRef]
- Cukierman, E. Cell migration analyses within fibroblast-derived 3-D matrices. Methods Mol. Biol. 2005, 294, 79–93. [Google Scholar] [PubMed]
- Xu, S.; Gao, J. Invasiveness and metastasis of tumor spheroid aggregates of human giant cell carcinoma (lung clone strain PLA801-95D) in vitro and in vivo. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1991, 13, 353–358. [Google Scholar] [PubMed]
- Kleinman, H.K.; McGarvey, M.L.; Hassell, J.R.; Star, V.L.; Cannon, F.B.; Laurie, G.W.; Martin, G.R. Basement membrane complexes with biological activity. Biochemistry 1986, 25, 312–318. [Google Scholar] [CrossRef]
- Bilozur, M.; Hay, E.D. Neural crest cell migration in 3D extracellular matrix utilizes laminin, fibronectin or collagen. Dev. Biol. 1988, 125, 19–33. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr.; Dannenberg, A.M., Jr.; Pula, P.J.; Selzer, M.G.; Ruppert, C.L.; Higuchi, K.; Kajiki, A.; Nakamura, M.; Dahms, N.M.; Kerr, J.S.; et al. Extracellular collagenase, proteoglycanase and products of their activity, released in organ culture by intact dermal inflammatory lesions produced by sulfur mustard. J. Investig. Dermatol. 1990, 95, 717–726. [Google Scholar] [CrossRef]
- Decker, M.L.; Behnke-Barclay, M.; Cook, M.G.; La Pres, J.J.; Clark, W.A.; Decker, R.S. Cell shape and organization of the contractile apparatus in cultured adult cardiac myocytes. J. Mol. Cell Cardiol. 1991, 23, 817–832. [Google Scholar] [CrossRef]
- Talbot, N.C.; Rexroad, C.E., Jr.; Powell, A.M.; Pursel, V.G.; Caperna, T.J.; Ogg, S.L.; Nel, N.D. A continuous culture of pluripotent fetal hepatocytes derived from the 8-day epiblast of the pig. In Vitro Cell Dev. Biol. Anim. 1994, 30, 843–850. [Google Scholar] [CrossRef]
- Hanthamrongwit, M.; Reid, W.H.; Grant, M.H. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: The effect of cross-linking agents and diamines. Biomaterials 1996, 17, 775–780. [Google Scholar] [CrossRef]
- Schor, S.L.; Ellis, I.; Dolman, C.; Banyard, J.; Humphries, M.J.; Mosher, D.F.; Grey, A.M.; Mould, A.P.; Sottile, J.; Schor, A.M. Substratum-dependent stimulation of fibroblast migration by the gelatin-binding domain of fibronectin. J. Cell Sci. 1996, 109, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Dillon, G.P.; Yu, X.; Sridharan, A.; Ranieri, J.P.; Bellamkonda, R.V. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J. Biomater. Sci. Polym. Ed. 1998, 9, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Selden, C.; Shariat, A.; McCloskey, P.; Ryder, T.; Roberts, E.; Hodgson, H. Three-dimensional in vitro cell culture leads to a marked upregulation of cell function in human hepatocyte cell lines--an important tool for the development of a bioartificial liver machine. Ann. N. Y. Acad. Sci. 1999, 875, 353–363. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005, 26, 4442–4452. [Google Scholar] [CrossRef]
- Rivard, C.H.; Chaput, C.; Rhalmi, S.; Selmani, A. Polyesters biosynthétiques absorbables et régénération tissulaire. Etude de la prolifération tridimensionnelle de chondrocytes et ostéoblastes ovins [Bio-absorbable synthetic polyesters and tissue regeneration. A study of three-dimensional proliferation of ovine chondrocytes and osteoblasts]. Ann. Chir. 1996, 50, 651–658. [Google Scholar]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef]
- Schantz, J.T.; Teoh, S.H.; Lim, T.C.; Endres, M.; Lam, C.X.; Hutmacher, D.W. Repair of calvarial defects with customized tissue-engineered bone grafts I. Evaluation of osteogenesis in a three-dimensional culture system. Tissue Eng. 2003, 9, S113–S126. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef]
- Shatford, R.A.; Nyberg, S.L.; Meier, S.J.; White, J.G.; Payne, W.D.; Hu, W.S.; Cerra, F.B. Hepatocyte function in a hollow fiber bioreactor: A potential bioartificial liver. J. Surg. Res. 1992, 53, 549–557. [Google Scholar] [CrossRef]
- Margaritis, A.; Bajpai, P. Continuous ethanol production from Jerusalem artichoke tubers. I. Use of free cells of Kluyveromyces marxianus. Biotechnol. Bioeng. 1982, 24, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Mitteregger, R.; Vogt, G.; Rossmanith, E.; Falkenhagen, D. Rotary cell culture system (RCCS): A new method for cultivating hepatocytes on microcarriers. Int. J. Artif. Organs 1999, 22, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Foxall, R.; Narang, P.; Glaysher, B.; Hub, E.; Teal, E.; Coles, M.C.; Ashton-Key, M.; Beers, S.A.; Cragg, M.S. Developing a 3D B Cell Lymphoma Culture System to Model Antibody Therapy. Front. Immunol. 2020, 11, 605231. [Google Scholar] [CrossRef]
- Haro, M.; Orsulic, S. A Paradoxical Correlation of Cancer-Associated Fibroblasts With Survival Outcomes in B-Cell Lymphomas and Carcinomas. Front. Cell Dev. Biol. 2018, 6, 98. [Google Scholar] [CrossRef]
- Apollonio, B.; Jarvis, P.; Phillips, B.; Kuhnl, A.; Salisbury, J.; Zacharioudakis, G.; Sutton, L.A.; Rosenquist, R.; Jarrett, R.; Amini, R.M.; et al. Diffuse Large B-Cell Lymphoma Remodels the Fibroblastic Reticular Network that Acquires Aberrant Immunosuppressive Capabilities; Implications for the Regulation of Anti-Tumor Immunity in the Immuno-Oncology Era. Blood 2018, 132, 675. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kunou, S.; Shimada, K.; Tsunoda, M.; Aoki, T.; Iriyama, C.; Tomita, A.; Nakamura, S.; Hayakawa, F.; Kiyoi, H. Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. 2019, 110, 269–278. [Google Scholar] [CrossRef]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef]
- Dolznig, H.; Rupp, C.; Puri, C.; Haslinger, C.; Schweifer, N.; Wieser, E.; Kerjaschki, D.; Garin-Chesa, P. Modeling Colon Adenocarcinomas in Vitro. A 3D Co-Culture System Induces Cancer-Relevant Pathways upon Tumor Cell and Stromal Fibroblast Interaction. Am. J. Pathol. 2011, 179, 487–501. [Google Scholar] [CrossRef]
- Erez, N.; Glanz, S.; Raz, Y.; Avivi, C.; Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013, 437, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Laidmäe, I.; Ērglis, K.; Cēbers, A.; Janmey, P.A.; Uibo, R. Salmon fibrinogen and chitosan scaffold for tissue engineering: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater. 2018, 7, e1800225. [Google Scholar] [CrossRef]
- Lv, D.; Yu, S.C.; Ping, Y.F.; Wu, H.; Zhao, X.; Zhang, H.; Cui, Y.; Chen, B.; Zhang, X.; Dai, J.; et al. A three-dimensional collagen scaffold cell culture system for screening anti-glioma therapeutics. Oncotarget 2016, 7, 56904–56914. [Google Scholar] [CrossRef]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-printing cell-laden Matrigel–agarose constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Lu, T.J.; Chiu, F.Y.; Chiu, H.Y.; Chang, M.C.; Hung, S.C. Chondrogenic Differentiation of Mesenchymal Stem Cells in Three-Dimensional Chitosan Film Culture. Cell Transpl. 2017, 26, 417–427. [Google Scholar] [CrossRef]
- Andersen, T.; Markussen, C.; Dornish, M.; Heier-Baardson, H.; Melvik, J.E.; Alsberg, E.; Christensen, B.E. In Situ Gelation for Cell Immobilization and Culture in Alginate Foam Scaffolds. Tissue Eng. Part A. 2014, 20, 600–610. [Google Scholar]
- Ghuman, H.; Mauney, C.; Donnelly, J.; Massensini, A.R.; Badylak, S.F.; Modo, M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018, 80, 66–84. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Infanger, D.W.; Lynch, M.E.; Fischbach, C. Engineered culture models for studies of tumor-microenvironment interactions. Annu. Rev. Biomed. Eng. 2013, 15, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gurski, L.A.; Zhang, C.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012, 33, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Marchini, A.; Favoino, C.; Gelain, F. Multi-Functionalized Self-Assembling Peptides as Reproducible 3D Cell Culture Systems Enabling Differentiation and Survival of Various Human Neural Stem Cell Lines. Front. Neurosci. 2020, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Benoit, D.S.W. Degradable Poly(ethylene glycol) (PEG)-based Hydrogels for Spatiotemporal Control of siRNA/Nanoparticle Delivery. J. Control Release 2018, 287, 58–66. [Google Scholar] [CrossRef]
- Kuriakose, A.E.; Hu, W.; Nguyen, K.T.; Menon, J.U. Scaffold-based lung tumor culture on porous PLGA microparticle substrates. PLoS ONE 2019, 14, e0217640. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef]
- Ovadia, E.M.; Colby, D.W.; Kloxin, A.M. Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells. Biomater. Sci. 2018, 6, 1358–1370. [Google Scholar] [CrossRef]
- Price, K.J.; Tsykin, A.; Giles, K.M.; Sladic, R.T.; Epis, M.R.; Ganss, R.; Goodall, G.J.; Leedman, P.J. Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem. Biophys. Res. Commun. 2012, 427, 343–348. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Hermenean, A.; Ciceu, A.; Herman, H.; Ionita, D.; Dinischiotu, A. Influence of Matrigel on Single- and Multiple-Spheroid Cultures in Breast Cancer Research. SLAS Discov. 2019, 24, 563–578. [Google Scholar] [CrossRef]
- Shirk, A.J.; Kuver, R. Epidermal growth factor mediates detachment from and invasion through collagen I and Matrigel in Capan-1 pancreatic cancer cells. BMC Gastroenterol. 2005, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wessels, D.J.; Pradhan, N.; Park, Y.N.; Klepitsch, M.A.; Lusche, D.F.; Daniels, K.J.; Conway, K.D.; Voss, E.R.; Hegde, S.V.; Conway, T.P.; et al. Reciprocal signaling and direct physical interactions between fibroblasts and breast cancer cells in a 3D environment. PLoS ONE 2019, 14, e0218854. [Google Scholar] [CrossRef] [PubMed]
- Fromigué, O.; Louis, K.; Wu, E.; Belhacène, N.; Loubat, A.; Shipp, M.; Auberger, P.; Mari, B. Active stromelysin-3 (MMP-11) increases MCF-7 survival in three-dimensional Matrigel culture via activation of p42/p44 MAP-kinase. Int. J. Cancer 2003, 106, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kasper, G.; Reule, M.; Tschirschmann, M.; Dankert, N.; Stout-Weider, K.; Lauster, R.; Schrock, E.; Mennerich, D.; Duda, G.N.; Lehmann, K.E. Stromelysin-3 over-expression enhances tumourigenesis in MCF-7 and MDA-MB-231 breast cancer cell lines: Involvement of the IGF-1 signalling pathway. BMC Cancer 2007, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Rolfe, B.E.; Hayward, I.P.; Campbell, G.R.; Campbell, J.H. Reorganization of structural proteins in vascular smooth muscle cells grown in collagen gel and basement membrane matrices (Matrigel): A comparison with their in situ counterparts. J. Struct. Biol. 2001, 133, 43–54. [Google Scholar] [CrossRef]
- Mondrinos, M.J.; Koutzaki, S.; Jiwanmall, E.; Li, M.; Dechadarevian, J.P.; Lelkes, P.I.; Finck, C.M. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006, 12, 717–728. [Google Scholar] [CrossRef]
- Li, W.; Machule, D.; Gao, C.; DenBesten, P.K. Growth of ameloblast-lineage cells in a three-dimensional Matrigel environment. Eur. J. Oral Sci. 2006, 1, 159–165. [Google Scholar] [CrossRef]
- Abilez, O.; Benharash, P.; Mehrotra, M.; Miyamoto, E.; Gale, A.; Picquet, J.; Xu, C.; Zarins, C. A novel culture system shows that stem cells can be grown in 3D and under physiologic pulsatile conditions for tissue engineering of vascular grafts. J. Surg. Res. 2006, 132, 170–178. [Google Scholar] [CrossRef]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, Z.F.; Meng, Y.; Zhao, Y.N.; Han, J.; Su, G.N.; Chen, B.; Dai, J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Jabaji, Z.; Brinkley, G.J.; Khalil, H.A.; Sears, C.M.; Lei, N.Y.; Lewis, M.; Stelzner, M.; Martín, M.G.; Dunn, J.C.Y. Type I Collagen as an Extracellular Matrix for the In Vitro Growth of Human Small Intestinal Epithelium. PLoS ONE 2014, 9, e107814. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Gallo, N.; Vergara, D.; Salvatore, L.; Nobile, C.; Ragusa, A.; Gaballo, A. Investigation on the Composition of Agarose-Collagen I Blended Hydrogels as Matrices for the Growth of Spheroids from Breast Cancer Cell Lines. Pharmaceutics 2021, 13, 963. [Google Scholar] [CrossRef]
- Rossi, L.; Reverberi, D.; Podestá, G.; Lastraioli, S.; Corvó, R. Co-culture with human fibroblasts increases the radiosensitivity of MCF-7 mammary carcinoma cells in collagen gels. Int. J. Cancer 2000, 85, 667–673. [Google Scholar] [CrossRef]
- Chen, W.C.; Yao, C.L.; Chu, I.M.; Wei, Y.H. Compare the effects of chondrogenesis by culture of human mesenchymal stem cells with various type of the chondroitin sulfate C. J. Biosci. Bioeng. 2011, 111, 226–231. [Google Scholar] [CrossRef]
- Cattin, S.; Ramont, L.; Rüegg, C. Characterization and In Vivo Validation of a Three-Dimensional Multi-Cellular Culture Model to Study Heterotypic Interactions in Colorectal Cancer Cell Growth, Invasion and Metastasis. Front. Bioeng. Biotechnol. 2018, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, B.; Hu, Q.; Qin, Y.; Xu, W.; Liu, W.; Yu, X.; Xu, J. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics 2018, 8, 5072–5087. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef]
- Liao, D.; Luo, Y.; Markowitz, D.; Xiang, R.; Reisfeld, R.A. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE 2009, 4, e7965. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1 + TAMs. Sci. Rep. 2019, 9, 3172. [Google Scholar] [CrossRef]
- Yasuda, K.; Torigoe, T.; Mariya, T.; Asano, T.; Kuroda, T.; Matsuzaki, J.; Ikeda, K.; Yamauchi, M.; Emori, M.; Asanuma, H.; et al. Fibroblasts induce expression of FGF4 in ovarian cancer stem-like cells/cancer-initiating cells and upregulate their tumor initiation capacity. Lab. Investig. 2014, 94, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Lisanti, M.P.; Sotgia, F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 2014, 25, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Saito, A.; Mikami, Y.; Ohshima, M.; Morishita, Y.; Nakajima, J.; Kohyamam, T.; Nagase, T. Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem. Biophys. Res. Commun. 2012, 423, 158–163. [Google Scholar] [CrossRef]
- Nakamura, H.; Sugano, M.; Miyashita, T.; Hashimoto, H.; Ochiai, A.; Suzuki, K.; Tsuboi, M.; Ishii, G. Organoid culture containing cancer cells and stromal cells reveals that podoplanin-positive cancer-associated fibroblasts enhance proliferation of lung cancer cells. Lung Cancer 2019, 134, 100–107. [Google Scholar] [CrossRef]
- Nayar, S.; Campos, J.; Smith, C.G.; Iannizzotto, V.; Gardner, D.H.; Mourcin, F.; Roulois, D.; Turner, J.; Sylvestre, M.; Asam, S.; et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc. Natl. Acad. Sci. USA 2019, 116, 13490–13497. [Google Scholar] [CrossRef]
- Cribaro, G.P.; Saavedra-López, E.; Romarate, L.; Mitxitorena, I.; Díaz, L.R.; Casanova, P.V.; Roig-Martínez, M.; Gallego, J.M.; Perez-Vallés, A.; Barcia, C. Three-dimensional vascular microenvironment landscape in human glioblastoma. Acta Neuropathol. Commun. 2021, 9, 24. [Google Scholar] [CrossRef]
- Chhetri, A.; Rispoli, J.V.; Lelièvre, S.A. 3D Cell Culture for the Study of Microenvironment-Mediated Mechanostimuli to the Cell Nucleus: An Important Step for Cancer Research. Front. Mol. Biosci. 2021, 8, 628386. [Google Scholar] [CrossRef]
- Zhao, S.; Nan, L.; Wang, Y.; Wei, L.; Mo, S. Effects of Smad4 on the expression of caspase-3 and Bcl-2 in human gingival fibroblasts cultured on 3D PLGA scaffolds induced by compressive force. Int. J. Mol. Med. 2021, 47, 04858. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Donati, I.; Paoletti, S. Material Properties of Alginates. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 13, pp. 1–53. [Google Scholar]
- Kuo, C.K.; Ma, P.X. Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Reis, R.L. Biomaterials for 3D Tumor Modeling. In Technology & Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–772. ISBN 978-0-12-818128-7. [Google Scholar]
- Fischbach, C.; Kong, H.J.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404. [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Ke, C.J.; Yen, K.C.; Hsieh, H.C.; Sun, J.S.; Lin, F.H. 3D Porous Calcium-Alginate Scaffolds Cell Culture System Improved Human Osteoblast Cell Clusters for Cell Therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef]
- Cavo, M.; Fato, M.; Peñuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef]
- Karimpoor, M.; Yebra-Fernandez, E.; Parhizkar, M.; Orlu, M.; Craig, D.; Khorashad, J.S.; Edirisinghe, M. Alginate foam-based three-dimensional culture to investigate drug sensitivity in primary leukaemia cells. J. R. Soc. Interface 2018, 15, 20170928. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef]
- Sidhu, K.; Kim, J.; Chayosumrit, M.; Dean, S.; Sachdev, P. Alginate Microcapsule as a 3D Platform for Propagation and Differentiation of Human Embryonic Stem Cells (hESC) to Different Lineages. J. Vis. Exp. 2012, 61, 3608. [Google Scholar] [CrossRef]
- Xu, F.; Xu, L.; Wang, Q.; Ye, Z.; Zhou, Y.; Tan, W.S. 3D Dynamic Culture of Rabbit Articular Chondrocytes Encapsulated in Alginate Gel Beads Using Spinner Flasks for Cartilage Tissue Regeneration. Biomed. Res. Int. 2014, 2014, 539789. [Google Scholar] [CrossRef] [PubMed]
- Dikovsky, D.; Bianco-Peled, H.; Seliktar, D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials 2006, 27, 1496–1506. [Google Scholar] [CrossRef]
- Kim, P.D.; Peyton, S.R.; VanStrien, A.J.; Putnam, A.J. The influence of ascorbic acid, TGF-beta1, and cell-mediated remodeling on the bulk mechanical properties of 3-D PEG-fibrinogen constructs. Biomaterials 2009, 30, 3854–3864. [Google Scholar] [CrossRef]
- Shachaf, Y.; Gonen-Wadmany, M.; Seliktar, D. The biocompatibility of PluronicF127 fibrinogen-based hydrogels. Biomaterials 2010, 31, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J. Biomed. Mater. Res. A 2017, 105, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Yates, T.J.; Lopez, L.E.; Cerwinka, W.H.; Bakkar, A.; Lokeshwar, V.B. Targeting hyaluronidase for cancer therapy: Antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011, 71, 4085–4095. [Google Scholar] [CrossRef]
- Nakod, P.S.; Kim, Y.; Rao, S.S. Three-dimensional biomimetic hyaluronic acid hydrogels to investigate glioblastoma stem cell behaviors. Biotechnol. Bioeng. 2019, 117, 511–522. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, M.S.; Park, I.C.; Kang, H.S.; Yoo, H.; Park, S.H.; Rhee, C.H.; Hong, S.I.; Lee, S.H. PTEN suppresses hyaluronic acid-induced matrix metalloproteinase-9 expression in U87MG glioblastoma cells through focal adhesion kinase dephosphorylation. Cancer Res. 2002, 62, 6318–6322. [Google Scholar]
- Harris, A.F.; Lacombe, J.; Liyanage, S.; Han, M.Y.; Wallace, E.; Karsunky, S.; Abidi, N.; Zenhausern, F. Supercritical carbon dioxide decellularization of plant material to generate 3D biocompatible scaffolds. Sci. Rep. 2021, 11, 3643. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of breast cancer stem cells with fibrous scaffolds. Integr. Biol. 2013, 5, 768–777. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Gu, K.; Bavoux, M.M.; Flores-Torres, S.; Kort-Mascort, J.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Ehrlicher, A.J.; et al. Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication 2019, 12, 015024. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Bioprintable Alginate/Gelatin Hydrogel 3D In Vitro Model Systems Induce Cell Spheroid Formation. J. Vis. Exp. 2018, 137, 57826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers 2018, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Characterization and evaluation of chitosan matrix for in vitro growth of MCF-7 breast cancer cell lines. Biomaterials 2004, 25, 5147–5154. [Google Scholar] [CrossRef]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef]
- Mohseni, M.; Shojaei, S.; Mehravi, B.; Mohammadi, E. Natural polymeric nanoparticles as a non-invasive probe for mesenchymal stem cell labelling. Artif. Cells Nanomed. Biotechnol. 2020, 48, 770–776. [Google Scholar] [CrossRef]
- Huang, Y.; Siewe, M.; Madihally, S.V. Effect of spatial architecture on cellular colonization. Biotechnol. Bioeng. 2006, 93, 64–75. [Google Scholar] [CrossRef]
- Lan, S.F.; Starly, B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol. Appl. Pharmacol. 2011, 256, 62–72. [Google Scholar] [CrossRef]
- Yu, L.; Ni, C.; Grist, S.M.; Bayly, C.; Cheung, K.C. Alginate core-shell beads for simplified three-dimensional tumor spheroid culture and drug screening. Biomed. Microdevices 2015, 17, 33. [Google Scholar] [CrossRef]
- Lee, S.M.; Han, N.; Lee, R.; Choi, I.H.; Park, Y.B.; Shin, J.S.; Yoo, K.H. Real-time monitoring of 3D cell culture using a 3D capacitance biosensor. Biosens. Bioelectron. 2016, 77, 56–61. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Dixon, T.A.; Cohen, E.; Huang, W.; Omenetto, F.G.; Kaplan, D.L. 3D Freeform Printing of Silk Fibroin. Acta Biomater. 2018, 71, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Kundu, S.C. A Non-Mulberry Silk Fibroin Protein Based 3D In Vitro Tumor Model for Evaluation of Anticancer Drug Activity. Adv. Funct. Mater. 2012, 22, 4778–4788. [Google Scholar] [CrossRef]
- Dondajewska, E.; Juzwa, W.; Mackiewicz, A.; Dams-Kozlowska, H. Heterotypic breast cancer model based on a silk fibroin scaffold to study the tumor microenvironment. Oncotarget 2018, 9, 4935–4950. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; Naskar, D.; Kinnear, B.F.; Grenik, E.; Dye, D.E.; Grounds, M.D.; Kundu, S.C.; Coombe, D.R. Silk fibroin scaffolds with muscle-like elasticity support in vitro differentiation of human skeletal muscle cells. J. Tissue Eng. Regen. Med. 2017, 11, 3178–3192. [Google Scholar] [CrossRef]
- Ashari, N.; Pang, H.W.; Simon, T.; Xiong, Y.; Coburn, J.M.; Bromberg, J.S.; Kaplan, D.L.; McLenithan, J.; Fontaine, M.J. Silk fibroin preserves beta cell function under inflammatory stress while stimulating islet cell surface GLUT2 expression. Cell Immunol. 2018, 329, 10–16. [Google Scholar] [CrossRef]
- Lovett, M.L.; Cannizzaro, C.; Daheron, L.; Messmer, B.; Vunjak-Novakovic, G.; Kaplana, D.L. Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007, 28, 5271–5279. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Wang, S.; Sun, X.; Zhang, X.; Chen, J.; Kaplan, D.L.; Jiang, X. Apatite-coated Silk Fibroin Scaffolds to Healing Mandibular Border Defects in Canines. Bone 2009, 45, 517–527. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Xie, Q.; Sun, H.; Huang, Y.; Zhang, D.D.; Yu, Z.; Bi, X.; Chen, J.; Wang, J.; et al. Electrospun silk fibroin/poly(lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. [Google Scholar]
- Niu, L.; Shi, M.; Feng, Y.; Sun, X.; Wang, Y.; Cheng, Z.; Li, M. The Interactions of Quantum Dot-Labeled Silk Fibroin Micro/Nanoparticles with Cells. Materials 2020, 13, 3372. [Google Scholar] [CrossRef]
- Mishra, A.; Mukhopadhyay, S.K.; Dey, S. Evaluation of Cyclosaplin Efficacy Using a Silk Based 3D Tumor Model. Biomolecules 2019, 9, 123. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials 2011, 32, 5773–5781. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering Adipose-like Tissue in vitro and in vivo Utilizing Human Bone Marrow and Adipose-derived Mesenchymal Stem Cells with Silk Fibroin 3D Scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Nguyen, Q.T.; Chen, A.C.; Sah, R.L.; Kundua, S.C. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 2011, 32, 8927–8937. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of Composite Scaffold Containing Silk Fibroin, Chitosan, and Gelatin for 3D Cell Culture and Bone Tissue Regeneration. Med. Sci. Monit. 2017, 23, 5311–5320. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Yi, S.; Yang, F.; Jie, C.; Zhang, G. A novel strategy to the formulation of carmustine and bioactive nanoparticles co-loaded PLGA biocomposite spheres for targeting drug delivery to glioma treatment and nursing care. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3438–3447. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tao, R.; Wu, W.; Cao, H.; Xin, J.; Li, J.; Guo, J.; Jiang, L.; Gao, C.; Demetriou, A.A.; et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev. 2010, 19, 1427–1436. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panda, A.K.; Labhasetwar, V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules 2005, 6, 1132–1139. [Google Scholar] [CrossRef]
- Priwitaningrum, D.L.; Blondé, J.G.; Sridhar, A.; van Baarlen, J.; Hennink, W.E.; Storm, G.; Le Gac, S.; Prakash, J. Tumor stroma-containing 3D spheroid arrays: A tool to study nanoparticle penetration. J. Control Release 2016, 244, 257–268. [Google Scholar] [CrossRef]
- Yang, Y.; Basu, S.; Tomasko, D.L.; Lee, L.J.; Yang, S.T. Fabrication of well-defined PLGA scaffolds using novel microembossing and carbon dioxide bonding. Biomaterials 2005, 26, 2585–2594. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, X. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell–scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int. J. Nanomed. 2011, 6, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ding, Y.; Sun, X.S.; Nguyen, T.A. Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS ONE 2013, 8, e59482. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.S.; Lamhamedi-Cherradi, S.E.; Burdett, E.; Ramamoorthy, V.; Lazar, A.J.; Kasper, F.K.; Farach-Carson, M.C.; Vishwamitra, D.; Demicco, E.G.; Menegaz, B.A.; et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc. Natl. Acad. Sci. USA 2013, 110, 6500–6505. [Google Scholar] [CrossRef]
- Worthington, P.; Pochan, D.J.; Langhans, S.A. Peptide Hydrogels—Versatile Matrices for 3D Cell Culture in Cancer Medicine. Front. Oncol. 2015, 5, 92. [Google Scholar] [CrossRef]
- Jin, J.; Sui, B.; Gou, J.; Liu, J.; Tang, X.; Xu, H.; Zhang, Y.; Jin, X. PSMA Ligand Conjugated PCL-PEG Polymeric Micelles Targeted to Prostate Cancer Cells. PLoS ONE 2014, 9, e112200. [Google Scholar] [CrossRef]
- Chambers, K.F.; Mosaad, E.M.; Russell, P.J.; Clements, J.A.; Doran, M.R. 3D Cultures of Prostate Cancer Cells Cultured in a Novel High-Throughput Culture Platform Are More Resistant to Chemotherapeutics Compared to Cells Cultured in Monolayer. PLoS ONE 2014, 9, e111029. [Google Scholar]
- Zhao, Y.; Bunch, T.D.; Isom, S.C. Effects of electrical biostimulation and silver ions on porcine fibroblast cells. PLoS ONE 2021, 16, e0246847. [Google Scholar] [CrossRef]
- Hamedani, Y.; Chakraborty, S.; Sabarwal, A.; Pal, S.; Bhowmick, S.; Balan, M. Novel Honokiol-eluting PLGA-based scaffold effectively restricts the growth of renal cancer cells. PLoS ONE 2020, 15, e0243837. [Google Scholar] [CrossRef]
- Zheng, C.S.; Attarilar, S.; Li, K.; Wang, C.; Liu, J.; Wang, L.; Yang, J.; Tang, Y. 3D-printed HA15-loaded β-Tricalcium Phosphate/Poly (Lactic-co-glycolic acid) Bone Tissue Scaffold Promotes Bone Regeneration in Rabbit Radial Defects. Int. J. Bioprint. 2021, 7, 317. [Google Scholar] [CrossRef]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef]
- Lin, C.C.; Korc, M. Designer hydrogels: Shedding light on the physical chemistry of the pancreatic cancer microenvironment. Cancer Lett. 2018, 436, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Balion, Z.; Sipailaite, E.; Stasyte, G.; Vailionyte, A.; Mazetyte-Godiene, A.; Seskeviciute, I.; Bernotiene, R.; Phopase, J.; Jekabsone, A. Investigation of Cancer Cell Migration and Proliferation on Synthetic Extracellular Matrix Peptide Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 773. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Sen, A.; Bae, S.; Lee, J.S.; Webb, K. PEG-diacrylate/hyaluronic acid semi-interpenetrating network compositions for 3D cell spreading and migration. Acta Biomater. 2015, 14, 43–52. [Google Scholar] [CrossRef]
- McKee, C.; Brown, C.; Bakshi, S.; Walker, K.; Govind, C.K.; Chaudhry, G.R. Transcriptomic Analysis of Naïve Human Embryonic Stem Cells Cultured in Three-Dimensional PEG Scaffolds. Biomolecules 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Stowers, R.; Lou, J.; Xia, Y.; Chaudhuril, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019, 200, 15–24. [Google Scholar] [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; DeForest, C.A.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. Biodegradable PEG-Based Amphiphilic Block Copolymers for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.H. Enhanced Chondrogenic Differentiation of Dental Pulp Stem Cells Using Nanopatterned PEG-GelMA-HA Hydrogels. Tissue Eng. Part A. 2014, 20, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuña, R.; Quirós, M.; Huang, S.; Siuda, D.; Spence, J.R.; Nusrat, A.; García, A.J. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018, 13, 2102–2119. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef] [PubMed]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; St-Denis Bissonnette, F.; Johnston, M.J.W.; et al. Hollow-fiber bioreactor production of extracellular vesicles from human bone marrow mesenchymal stromal cells yields nanovesicles that mirrors the immuno-modulatory antigenic signature of the producer cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Tai, Y.L.; Lin, C.J.; Li, T.K.; Shen, T.L.; Hsieh, J.T.; Chen, B.P.C. The role of extracellular vesicles in prostate cancer with clinical applications. Endocr. Relat. Cancer 2020, 27, R133–R144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granrose, D.; Zurawski, J.; Heaton, W.; Tandeski, T.; Dulatov, G.; Highsmith, A.A.; Conen, M.; Clark, G.; Jones, A.; Loftus, H.; et al. Transition from static culture to stirred tank bioreactor for the allogeneic production of therapeutic discogenic cell spheres. Stem Cell Res. Ther. 2021, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Manstein, F.; Ullmann, K.; Kropp, C.; Halloin, C.; Triebert, W.; Franke, A.; Farr, C.M.; Sahabian, A.; Haase, A.; Breitkreuz, Y.; et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med. 2021, 10, 1063–1080. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, W.; Yan, H.; Yue, L.; Zhang, Y.; Han, F.; Chen, X.; Li, Y. Rotary culture promotes the proliferation of MCF-7 cells encapsulated in three-dimensional collagen-alginate hydrogels via activation of the ERK1/2-MAPK pathway. Biomed. Mater. 2012, 7, 015003. [Google Scholar] [CrossRef]
- Fournier, R.; Harrison, R.E. Methods for studying MLO-Y4 osteocytes in collagen-hydroxyapatite scaffolds in the rotary cell culture system. Connect Tissue Res. 2021, 62, 436–453. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Zou, Y.; Zhao, Y.; Han, J.; Xu, B.; Chen, B.; Xiao, Z.; Song, H.; Shi, Y.; et al. The Rotary Cell Culture System increases NTRK3 expression and promotes neuronal differentiation and migratory ability of neural stem cells cultured on collagen sponge. Stem Cell Res. Ther. 2021, 12, 298. [Google Scholar] [CrossRef] [PubMed]

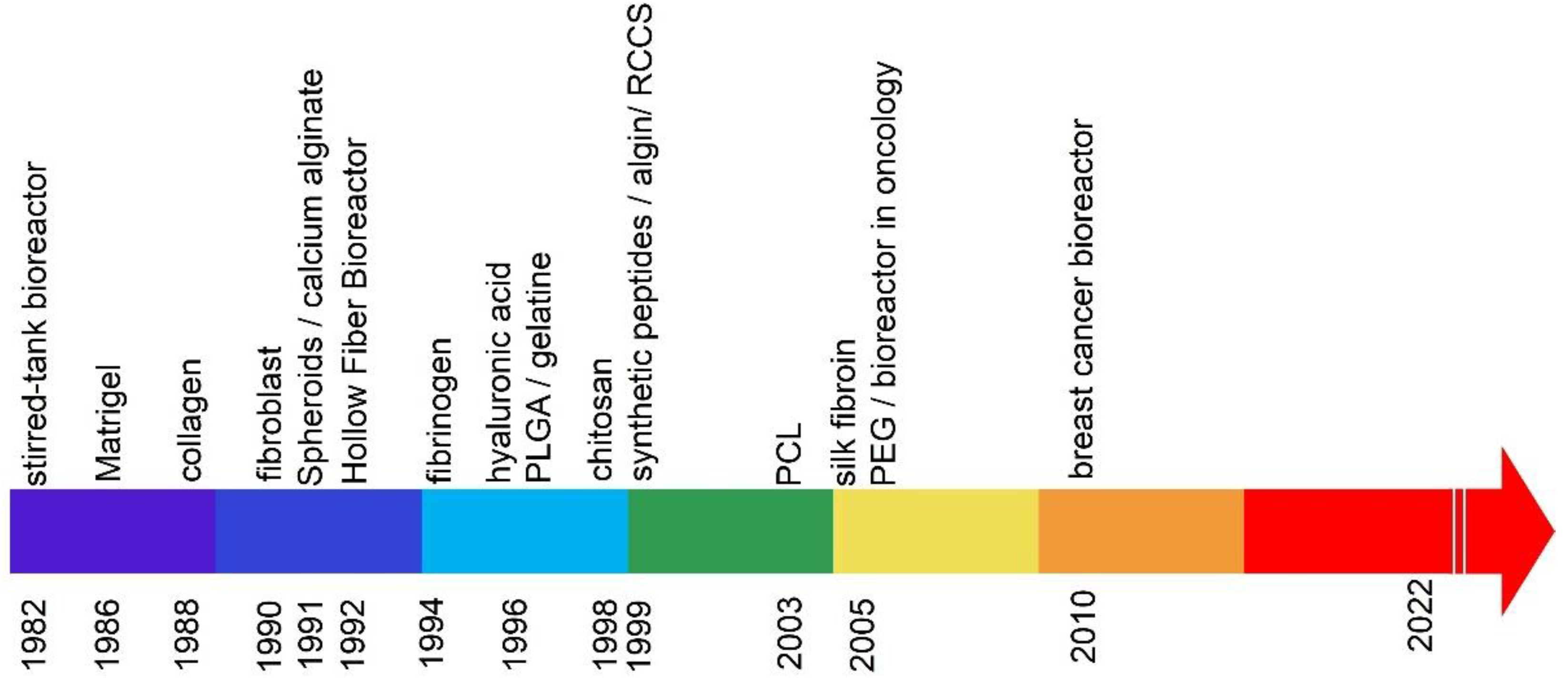
| Cell Culture Method | No. | Type of Cell Culture | References | |
|---|---|---|---|---|
| 3D cell culture | 1 | Spheroidal | [16] | |
| 3D culture matrices based on a hydrogel | ||||
| 2 | Natural Polymers | Matrigel | [17] | |
| 3 | Collagen | [18] | ||
| 4 | Fibroblast | [19] | ||
| 5 | Calcium alginate | [20] | ||
| 6 | Fibrinogen | [21] | ||
| 7 | Hyaluronic acid | [22] | ||
| 8 | Gelatine | [23] | ||
| 9 | Chitosan | [24] | ||
| 10 | Algin | [25] | ||
| 11 | Silk fibroin | [26] | ||
| 12 | Synthetic Polymers | Poly (lactic-co-glycolic acid) (PLGA) | [27] | |
| 13 | Synthetic peptides | [28] | ||
| 14 | Scaffolding made of electro-spun poly (ε-caprolactone) (PCL) | [29] | ||
| 15 | Poly (ethylene glycol) (PEG), | [30] | ||
| 16 | 3D bioreactors | Hollow fiber bioreactor | [31] | |
| 17 | Stirred-tank | [32] | ||
| 18 | Rotary cell culture system (RCCS) | [33] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bober, Z.; Aebisher, D.; Olek, M.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Multiple Cell Cultures for MRI Analysis. Int. J. Mol. Sci. 2022, 23, 10109. https://doi.org/10.3390/ijms231710109
Bober Z, Aebisher D, Olek M, Kawczyk-Krupka A, Bartusik-Aebisher D. Multiple Cell Cultures for MRI Analysis. International Journal of Molecular Sciences. 2022; 23(17):10109. https://doi.org/10.3390/ijms231710109
Chicago/Turabian StyleBober, Zuzanna, David Aebisher, Marcin Olek, Aleksandra Kawczyk-Krupka, and Dorota Bartusik-Aebisher. 2022. "Multiple Cell Cultures for MRI Analysis" International Journal of Molecular Sciences 23, no. 17: 10109. https://doi.org/10.3390/ijms231710109
APA StyleBober, Z., Aebisher, D., Olek, M., Kawczyk-Krupka, A., & Bartusik-Aebisher, D. (2022). Multiple Cell Cultures for MRI Analysis. International Journal of Molecular Sciences, 23(17), 10109. https://doi.org/10.3390/ijms231710109









