Prefrontal Cortical Control of Activity in Nucleus Accumbens Core Is Weakened by High-Fat Diet and Prevented by Co-Treatment with N-Acetylcysteine: Implications for the Development of Obesity
Abstract
1. Introduction
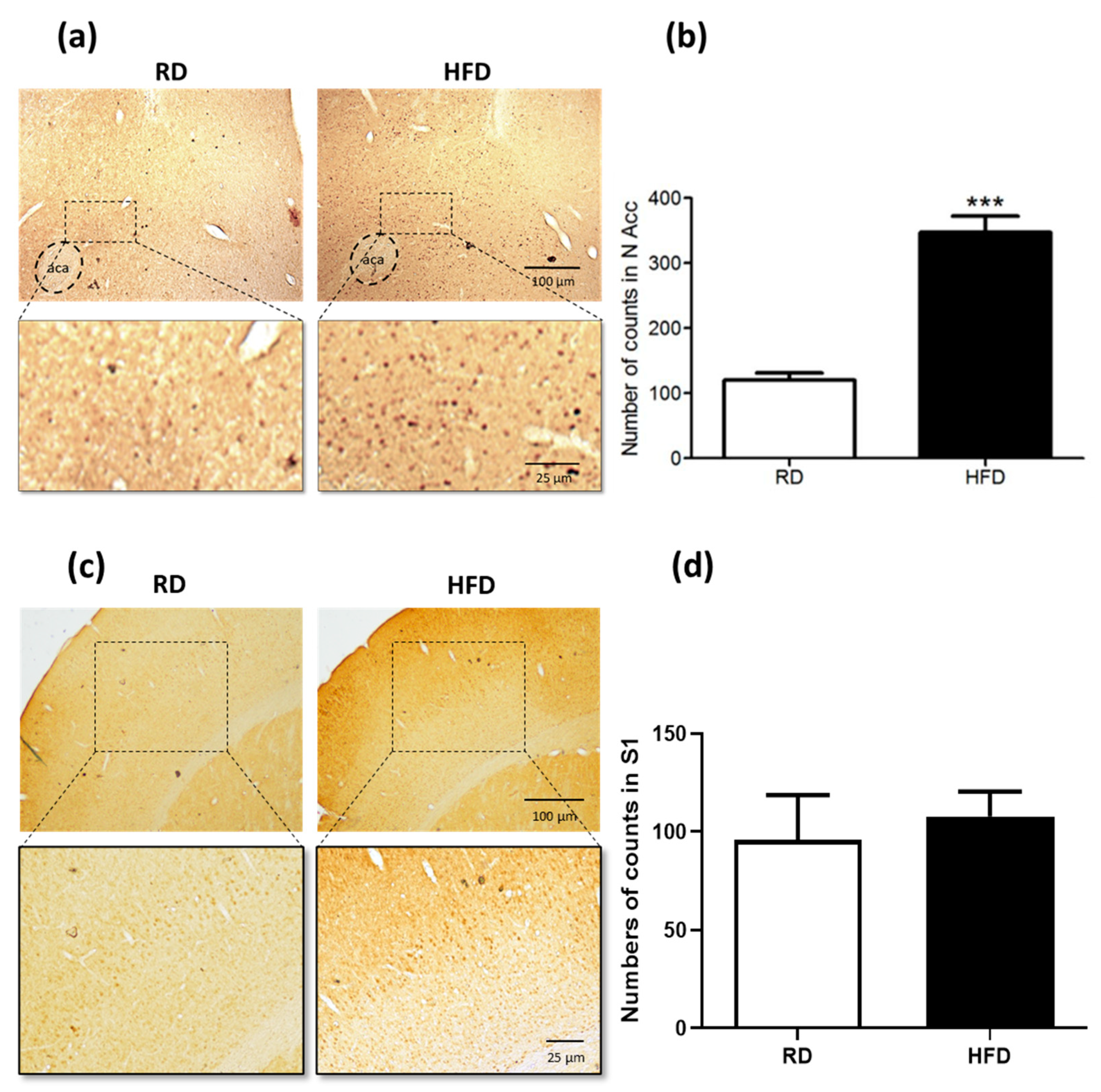
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Experiment 1: FosB/ΔFosB Immunohistochemistry in NAcc and S1 Cortex
4.3. Experiment 2: In Vivo Assessment of LTD in the NAcc and Effect of N-Acetylcysteine
4.4. Experiment 3: Effect of Diet and N-Acetylcysteine on Body Weight Gain
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, P.J. Common cellular and molecular mechanisms in obesity and drug addiction. Nat. Rev. Neurosci. 2011, 12, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.; Schmidt, L.A.; Brindis, C.D. The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.M.; Kenny, P.J. Utility of ‘substance use disorder’ as a heuristic for understanding overeating and obesity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 118, 110580. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Bocarsly, M.E.; Rada, P.; Kim, A.; Hoebel, B.G. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol. Behav. 2008, 94, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.T.; Sweigart, K.L.; Lakoski, J.M.; Norgren, R.; Hajnal, A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1260–R1268. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, F.H.; Roberts, D.C.; Corwin, R.L. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol. Biochem. Behav. 2006, 84, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, S.L.; Bale, T.L. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry 2007, 61, 1021–1029. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Sugar and fat bingeing have notable differences in addictive-like behavior. J. Nutr. 2009, 139, 623–628. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Buttigieg, A.; Flores, O.; Hernández, A.; Sáez-Briones, P.; Burgos, H.; Morgan, C. Preference for high-fat diet is developed by young Swiss CD1 mice after short-term feeding and is prevented by NMDA receptor antagonists. Neurobiol. Learn. Mem. 2014, 107, 13–18. [Google Scholar] [CrossRef]
- Liang, N.C.; Hajnal, A.; Norgren, R. Sham feeding corn oil increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1236–R1239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Kourrich, S.; Rothwell, P.E.; Klug, J.R.; Thomas, M.J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007, 27, 7921–7928. [Google Scholar] [CrossRef]
- Martin, M.; Chen, B.T.; Hopf, F.W.; Bowers, M.S.; Bonci, A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006, 9, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Pacchioni, A.; Moran, M.; Olive, M.F.; Gass, J.T.; Lavin, A.; Kalivas, P.W. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kasanetz, F.; Deroche-Gamonet, V.; Berson, N.; Balado, E.; Lafourcade, M.; Manzoni, O.; Piazza, P.V. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 2010, 328, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.K.; Zaman, S.; Damez-Werno, D.M.; Koo, J.W.; Bagot, R.C.; DiNieri, J.A.; Nugent, A.; Finkel, E.; Chaudhury, D.; Chandra, R.; et al. FosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci. 2013, 33, 18381–18395. [Google Scholar] [CrossRef]
- Brown, R.M.; Kupchik, Y.M.; Spencer, S.; Garcia-Keller, C.; Spanswick, D.C.; Lawrence, A.J.; Simonds, S.E.; Schwartz, D.J.; Jordan, K.A.; Jhou, T.C.; et al. Addiction-like synaptic impairments in diet-induced obesity. Biol. Psychiatry 2017, 81, 797–806. [Google Scholar] [CrossRef]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef]
- Shen, H.; Kalivas, P.W. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin-self administration. Int. J. Neuropsychopharmacol. 2013, 16, 1165–1167. [Google Scholar] [CrossRef]
- Gipson, C.D.; Reissner, K.J.; Kupchik, Y.M.; Smith, A.C.; Stankeviciute, N.; Hensley-Simon, M.E.; Kalivas, P.W. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 9124–9129. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.; Behrens, T.E. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008, 11, 389–397. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Baler, R.D.; Volkow, N.D. Drug addiction: The neurobiology of disrupted self-control. Trends Mol. Med. 2006, 12, 559–566. [Google Scholar] [CrossRef]
- Garofalo, S.; Timmermann, C.; Battaglia, S.; Maier, M.E.; di Pellegrino, G. Mediofrontal negativity signals unexpected timing of salient outcomes. J. Cogn. Neurosci. 2017, 29, 718–727. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, 7, e14122. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef]
- Gutman, A.L.; Ewald, V.A.; Cosme, C.V.; Worth, W.R.; LaLumiere, R.T. The infralimbic and prelimbic cortices contribute to the inhibitory control of cocaine-seeking behavior during a discriminative stimulus task in rats. Addict. Biol. 2017, 22, 1719–1730. [Google Scholar] [CrossRef]
- Chen, B.T.; Yau, H.J.; Hatch, C.; Kusumoto-Yoshida, I.; Cho, S.L.; Hopf, F.W.; Bonci, A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 2013, 496, 359–362. [Google Scholar] [CrossRef]
- Domingo-Rodriguez, L.; Ruiz de Azua, I.; Dominguez, E.; Senabre, E.; Serra, I.; Kummer, S.; Navandar, M.; Baddenhausen, S.; Hofmann, C.; Andero, R.; et al. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat. Commun. 2020, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castaño, A.; Rivero-Santana, A.; Perestelo-Pérez, L.; Duarte-Díaz, A.; Toledo-Chávarri, A.; Ramos-García, V.; Álvarez-Pérez, Y.; Cudeiro-Mazaira, J.; Padrón-González, I.; Serrano-Pérez, P. Transcranial magnetic stimulation for the treatment of cocaine addiction: A systematic review. J. Clin. Med. 2021, 10, 5595. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Lafenêtre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Smith, K.E.; Luo, S.; Mason, T.B. A systematic review of neural correlates of dysregulated eating associated with obesity risk in youth. Neurosci. Biobehav. Rev. 2021, 124, 245–266. [Google Scholar] [CrossRef]
- Kidd, C.; Loxton, N.J. A narrative review of reward sensitivity, rash impulsivity, and food addiction in adolescents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110265. [Google Scholar] [CrossRef]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Reichel, C.M.; Moussawi, K.; Do, P.H.; Kalivas, P.W.; See, R.E. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J. Pharmacol. Exp. Ther. 2011, 337, 487–493. [Google Scholar] [CrossRef]
- Nocito Echevarria, M.A.; Andrade Reis, T.; Ruffo Capatti, G.; Siciliano Soares, V.; da Silveira, D.X.; Fidalgo, T.M. N-acetylcysteine for treating cocaine addiction—A systematic review. Psychiatry Res. 2017, 251, 197–203. [Google Scholar] [CrossRef]
- Hurley, M.M.; Resch, J.M.; Maunze, B.; Frenkel, M.M.; Baker, D.A.; Choi, S. N-acetylcysteine decreases binge eating in a rodent model. Int. J. Obes. 2016, 40, 1183–1186. [Google Scholar] [CrossRef]
- Teegarden, S.L.; Nestler, E.J.; Bale, T.L. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol. Psychiatry 2008, 64, 941–950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nestler, E.J.; Barrot, M.; Self, D.W. ΔFosB: A sustained molecular switch for addiction. Proc. Natl. Acad. Sci. USA 2001, 98, 11042–11046. [Google Scholar] [CrossRef] [PubMed]
- Vaquer-Alicea, A.D.C.; Vázquez-Torres, R.; Devarie-Hornedo, M.; Vicenty-Padilla, J.C.; Santos-Vera, B.; María-Ríos, C.; Vélez-Hernández, M.E.; Sacktor, T.; Jiménez-Rivera, C.A. aPKC-mediated persistent increase in AMPA/NMDA ratio in the VTA participates in the neuroadaptive signal necessary to induce NAc synaptic plasticity after cocaine administration. Neuroscience 2018, 392, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yeh, C.M.; Wu, M.Y.; Chang, A.Y.; Chan, J.Y.; Chan, S.H.; Hsu, K.S. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J. Neurosci. 2011, 31, 4194–4203. [Google Scholar] [CrossRef]
- Renteria, R.; Jeanes, Z.M.; Morrisett, R.A. Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPV1 receptors. Alcohol Clin. Exp. Res. 2014, 38, 2763–2769. [Google Scholar] [CrossRef]
- Jeanes, Z.M.; Buske, T.R.; Morrisett, R.A. Cell type-specific synaptic encoding of ethanol exposure in the nucleus accumbens shell. Neuroscience 2014, 277, 184–195. [Google Scholar] [CrossRef]
- Hope, B.T. Cocaine and the AP-1 Transcription Factor Complex. Ann. N. Y. Acad. Sci. 1998, 844, 1–6. [Google Scholar] [CrossRef]
- Lafragette, A.; Bardo, M.T.; Lardeux, V.; Solinas, M.; Thiriet, N. Reduction of cocaine-induced locomotor effects by enriched environment is associated with cell-specific accumulation of FosB in striatal and cortical subregions. Int. J. Neuropsychopharmacol. 2017, 20, 237–246. [Google Scholar] [CrossRef]
- Thomas, M.J.; Beurrier, C.; Bonci, A.; Malenka, R.C. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001, 4, 1217–1223. [Google Scholar] [CrossRef]
- Counotte, D.S.; Schiefer, C.; Shaham, Y.; O’Donnell, P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology 2014, 231, 1675–1684. [Google Scholar] [CrossRef]
- Fritz, B.M.; Muñoz, B.; Yin, F.; Bauchle, C.; Atwood, B.K. A high-fat, high-sugar ‘western’ diet alters dorsal striatal glutamate, opioid, and dopamine transmission in mice. Neuroscience 2017, 372, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, D.; Kalivas, P. Metaplasticity at the addicted tetrapartite synapse: A denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol. Learn. Mem. 2018, 154, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. N-acetylcysteine protects mice from high fat diet-induced metabolic disorders. Pharm. Res. 2016, 33, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Amen, S.L.; Piacentine, L.B.; Ahmad, M.E.; Li, S.J.; Mantsch, J.R.; Risinger, R.C.; Baker, D.A. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 2011, 36, 871–878. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Hong, X.; Lu, S.; Tang, S.; Shen, Y.; Feng, M.; Guo, P.; Fang, Y. A case of trichotillomania with binge eating disorder: Combined with N-acetylcysteine synergistic therapy. Ann. Gen. Psychiatry 2021, 20, 46. [Google Scholar] [CrossRef]
- Kiliç, F.; Keleş, S. Repetitive behaviors treated with N-acetylcysteine: Case series. Clin. Neuropharmacol. 2019, 42, 139–141. [Google Scholar] [CrossRef]
- Wells, J.C. The evolution of human adiposity and obesity: Where did it all go wrong? Dis. Model. Mech. 2012, 5, 595–607. [Google Scholar] [CrossRef]
- Schulte, E.M.; Tuttle, H.M.; Gearhardt, A.N. Belief in food addiction and obesity-related policy support. PLoS ONE 2016, 11, e0147557. [Google Scholar] [CrossRef]
- Roberts-Wolfe, D.J.; Kalivas, P.W. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol. Disord. Drug Targets 2015, 14, 745–756. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine in substance use disorder: A lesson from preclinical and clinical research. Pharmacol. Rep. 2021, 73, 1205–1219. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; pp. 1–220. ISBN 978-0-309-38629-6. [Google Scholar]
- Rothwell, N.J.; Stock, M.J. The development of obesity in animals: The role of dietary factors. Clin. Endocrinol. Metab. 1984, 13, 437–449. [Google Scholar] [CrossRef]
- Pagliassotti, M.J.; Gayles, E.C.; Hill, J.O. Fat and energy balance. Ann. N. Y. Acad. Sci. 1997, 827, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.K. Fat nutrition and diet in childhood. Am. J. Cardiol. 1973, 31, 581–587. [Google Scholar] [CrossRef]
- Sims, E.A. Storage and expenditure of energy in obesity and their implications for management. Med. Clin. N. Am. 1989, 73, 97–110. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, C.; Sáez-Briones, P.; Barra, R.; Reyes, A.; Zepeda-Morales, K.; Constandil, L.; Ríos, M.; Ramírez, P.; Burgos, H.; Hernández, A. Prefrontal Cortical Control of Activity in Nucleus Accumbens Core Is Weakened by High-Fat Diet and Prevented by Co-Treatment with N-Acetylcysteine: Implications for the Development of Obesity. Int. J. Mol. Sci. 2022, 23, 10089. https://doi.org/10.3390/ijms231710089
Morgan C, Sáez-Briones P, Barra R, Reyes A, Zepeda-Morales K, Constandil L, Ríos M, Ramírez P, Burgos H, Hernández A. Prefrontal Cortical Control of Activity in Nucleus Accumbens Core Is Weakened by High-Fat Diet and Prevented by Co-Treatment with N-Acetylcysteine: Implications for the Development of Obesity. International Journal of Molecular Sciences. 2022; 23(17):10089. https://doi.org/10.3390/ijms231710089
Chicago/Turabian StyleMorgan, Carlos, Patricio Sáez-Briones, Rafael Barra, Andrea Reyes, Katherine Zepeda-Morales, Luis Constandil, Miguel Ríos, Paulina Ramírez, Héctor Burgos, and Alejandro Hernández. 2022. "Prefrontal Cortical Control of Activity in Nucleus Accumbens Core Is Weakened by High-Fat Diet and Prevented by Co-Treatment with N-Acetylcysteine: Implications for the Development of Obesity" International Journal of Molecular Sciences 23, no. 17: 10089. https://doi.org/10.3390/ijms231710089
APA StyleMorgan, C., Sáez-Briones, P., Barra, R., Reyes, A., Zepeda-Morales, K., Constandil, L., Ríos, M., Ramírez, P., Burgos, H., & Hernández, A. (2022). Prefrontal Cortical Control of Activity in Nucleus Accumbens Core Is Weakened by High-Fat Diet and Prevented by Co-Treatment with N-Acetylcysteine: Implications for the Development of Obesity. International Journal of Molecular Sciences, 23(17), 10089. https://doi.org/10.3390/ijms231710089






