Dlg Is Required for Short-Term Memory and Interacts with NMDAR in the Drosophila Brain
Abstract
1. Introduction
2. Results
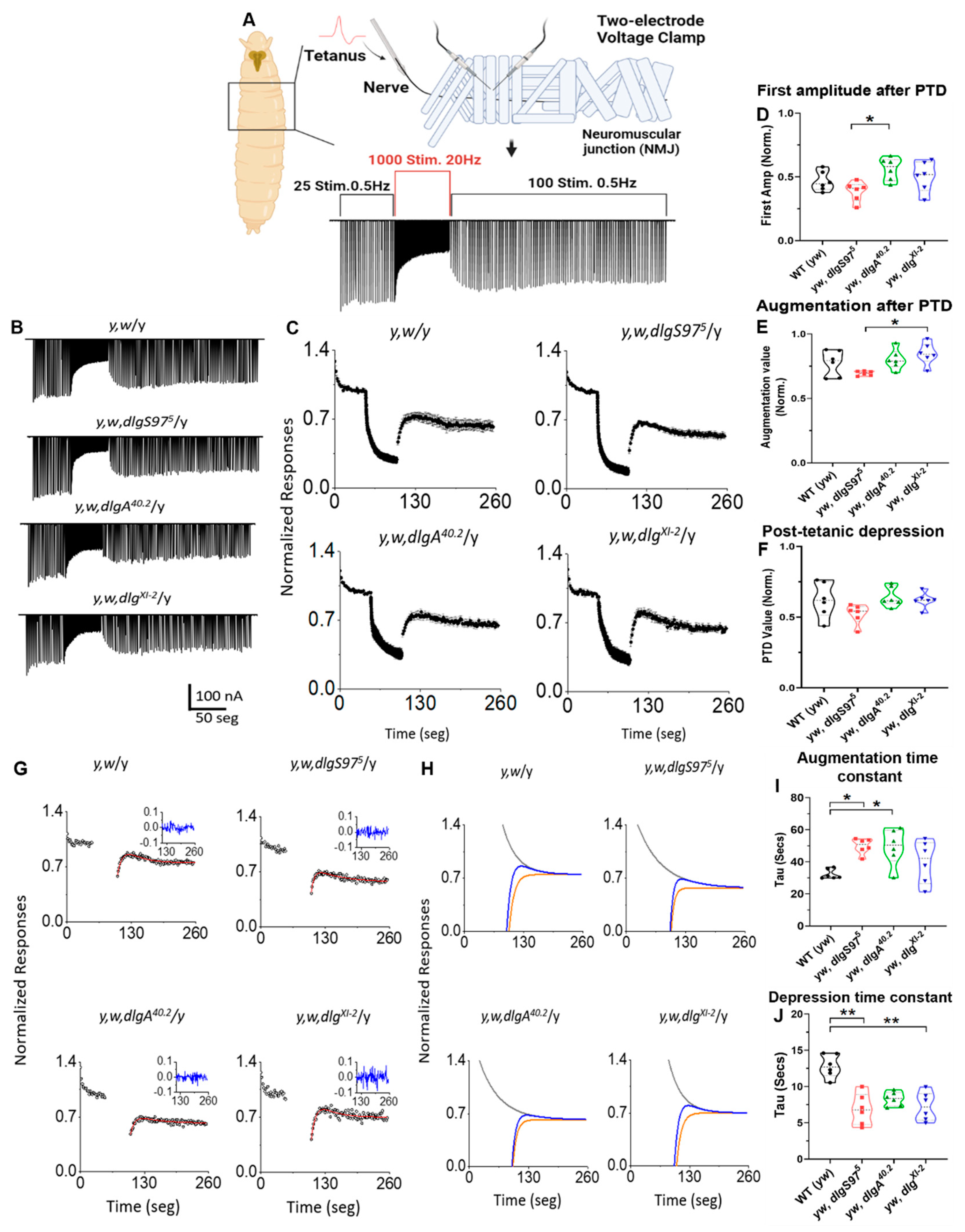
2.1. Dlg Modulates the Time Course of Augmentation and Depression
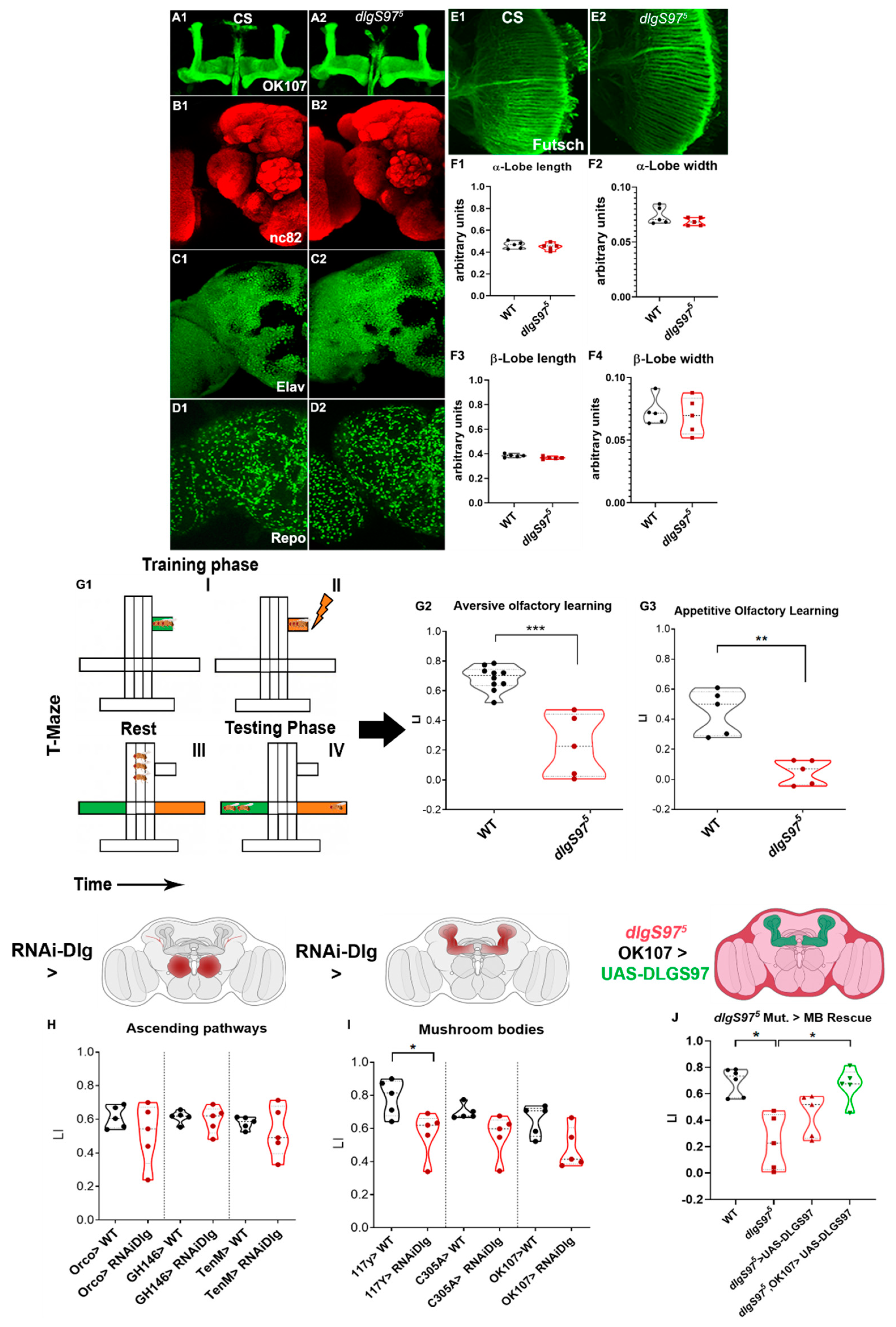
2.2. Dlg Mutants Show Altered Short-Term Memory
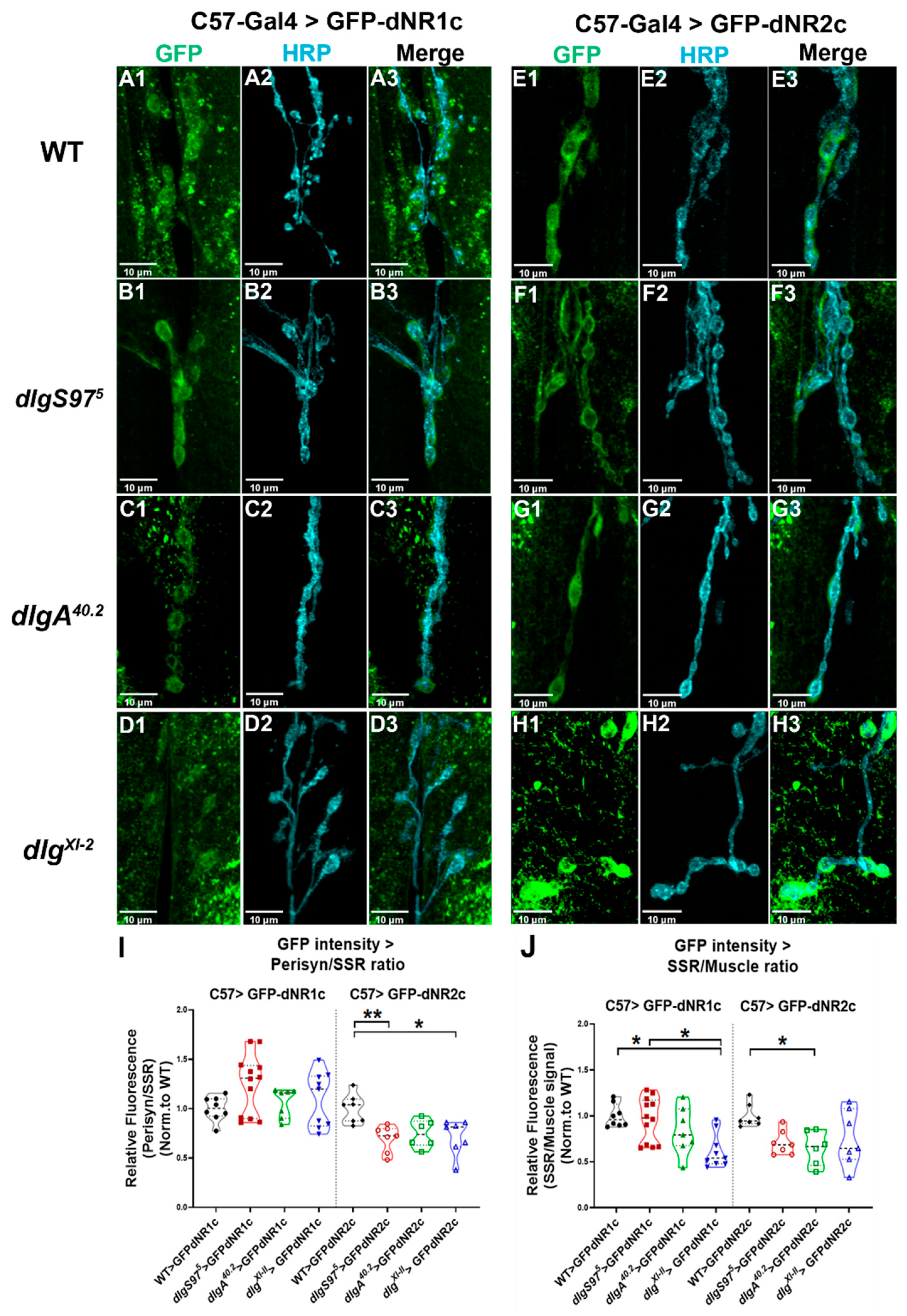
2.3. NMDA Receptors Associate with Dlg
3. Discussion
3.1. Short-Term Plasticity at the NMJ
3.2. Short-Term Memory Alterations Are Associated with Dlg Loss of Function
3.3. Dlg-NMDARs Association
4. Materials and Methods
4.1. Drosophila Stocks and Genetics
4.2. Generation of NMDAR CD8 Tails and Flies
4.3. Electrophysiology
4.4. Electrophysiological Data Acquisition and Analysis
4.5. Adult Learning and Short-Term Memory Paradigm
4.6. Immunoprecipitation Assays
4.7. Immunostaining
4.8. Image Acquisition and Analysis
4.9. Calcium Reporter (GCamp) Experiments
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, M.B. Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 2016, 8, a016824. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Ramaswami, M.; Boto, T.; Cervantes-Sandoval, I. The Making of Long-Lasting Memories: A Fruit Fly Perspective. Front. Behav. Neurosci. 2021, 15, 662129. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y. Memory Mechanisms: A common cascade for long-term memory. Curr. Biol. 1995, 5, 136–138. [Google Scholar] [CrossRef]
- Margulies, C.; Tully, T.; Dubnau, J. Deconstructing memory in Drosophila. Curr. Biol. 2005, 15, R700–R713. [Google Scholar] [CrossRef] [PubMed]
- Tully, T.; Quinn, W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A 1985, 157, 263–277. [Google Scholar] [CrossRef]
- Zars, T.; Fischer, M.; Schulz, R.; Heisenberg, M. Localization of a short-term memory in Drosophila. Science 2000, 288, 672–675. [Google Scholar] [CrossRef]
- Devineni, A.V.; Scaplen, K.M. Neural Circuits Underlying Behavioral Flexibility: Insights From Drosophila. Front. Behav. Neurosci. 2022, 15, 821680. [Google Scholar] [CrossRef]
- Katz, B.; Miledi, R. The timing of calcium action during neuromuscular transmission. J. Physiol. 1967, 189, 535–544. [Google Scholar] [CrossRef]
- Südhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef]
- Guerrier, C.; Holcman, D. The first 100 nm inside the pre-synaptic terminal where calcium diffusion triggers vesicular release. Front. Synaptic Neurosci. 2018, 10, 23. [Google Scholar] [CrossRef]
- Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.; Escobedo, P.; Astorga, C.; Molina, C.; Sierralta, J. Role of the maguk protein family in synapse formation and function. Dev. Neurobiol. 2012, 72, 57–72. [Google Scholar] [CrossRef]
- Astorga, C.; Jorquera, R.A.; Ramírez, M.; Kohler, A.; López, E.; Delgado, R.; Córdova, A.; Olguín, P.; Sierralta, J. Presynaptic DLG regulates synaptic function through the localization of voltage-activated Ca2+Channels. Sci. Rep. 2016, 6, 32132. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Olguín, P.; Lafferte, G.; Thomas, U.; Ebitsch, S.; Gundelfinger, E.D.; Kukuljan, M.; Sierralta, J. Novel isoforms of dlg are fundamental for neuronal development in Drosophila. J. Neurosci. 2003, 23, 2093–2101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendoza-Topaz, C.; Urra, F.; Barría, R.; Albornoz, V.; Ugalde, D.; Thomas, U.; Gundelfinger, E.D.; Delgado, R.; Kukuljan, M.; Sanxaridis, P.D.; et al. DLGS97/SAP97 is developmentally upregulated and is required for complex adult behaviors and synapse morphology and function. J. Neurosci. 2008, 28, 304–314. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, Y.; Zhang, M. Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 2016, 17, 209–223. [Google Scholar] [CrossRef]
- Lloyd, T.E.; Verstreken, P.; Ostrin, E.J.; Phillippi, A.; Lichtarge, O.; Bellen, H.J. A genome-wide search for synaptic vesicle cycle proteins in Drosophila. Neuron 2000, 26, 45–50. [Google Scholar] [CrossRef]
- Thomas, U.; Kobler, O.; Gundelfinger, E.D. The Drosophila larval neuromuscular junction as a model for scaffold complexes at glutamatergic synapses: Benefits and limitations. J. Neurogenet. 2010, 24, 109–119. [Google Scholar] [CrossRef]
- Guan, B.; Hartmann, B.; Kho, Y.H.; Gorczyca, M.; Budnik, V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr. Biol. 1996, 6, 695–706. [Google Scholar] [CrossRef]
- Woods, D.F.; Hough, C.; Peel, D.; Callaini, G.; Bryant, P.J. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 1996, 134, 1469–1482. [Google Scholar] [CrossRef]
- Kalkstein, J.M.; Magleby, K.L. Augmentation increases vesicular release probability in the presence of masking depression at the frog neuromuscular junction. J. Neurosci. 2004, 24, 11391–11403. [Google Scholar] [CrossRef] [PubMed]
- Fioravante, D.; Regehr, W.G. Short-term forms of presynaptic plasticity. Curr. Opin. Neurobiol. 2011, 21, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- Krashes, M.J.; Keene, A.C.; Leung, B.; Armstrong, J.D.; Waddell, S. Sequential Use of Mushroom Body Neuron Subsets during Drosophila Odor Memory Processing. Neuron 2007, 53, 103–115. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2000, 2, 219–232. [Google Scholar] [CrossRef]
- Driesen, N.R.; McCarthy, G.; Bhagwagar, Z.; Bloch, M.H.; Calhoun, V.D.; D’Souza, D.C.; Gueorguieva, R.; He, G.; Leung, H.C.; Ramani, R.; et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 2013, 38, 2613–2622. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Abraham, W.C. Glutamate receptors and synaptic plasticity: The impact of Evans and Watkins. Neuropharmacology 2022, 206, 108922. [Google Scholar] [CrossRef]
- Maiya, R.; Lee, S.; Berger, K.H.; Kong, E.C.; Slawson, J.B.; Griffith, L.C.; Takamiya, K.; Huganir, R.L.; Margolis, B.; Heberlein, U. DlgS97/SAP97, a Neuronal Isoform of Discs Large, Regulates Ethanol Tolerance. PLoS ONE 2012, 7, e48967. [Google Scholar] [CrossRef]
- Wu, C.L.; Xia, S.; Fu, T.F.; Wang, H.; Chen, Y.H.; Leong, D.; Chiang, A.S.; Tully, T. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat. Neurosci. 2007, 10, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Kobler, O.; Kittel, R.J.; Wichmann, C.; Sierralta, J.; Sigrist, S.J.; Gundelfinger, E.D.; Knust, E.; Thomas, U. A perisynaptic ménage à trois between Dlg, DLin-7, and Metro controls proper organization of Drosophila synaptic junctions. J. Neurosci. 2010, 30, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, F.J.; Bokhari, A.; Rogero, O.; Gorczyca, M.; Zhang, J.; Kim, E.; Sheng, M.; Budnik, V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J. Neurosci. 1997, 17, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Davis, G.W. Transsynaptic control of presynaptic Ca2+ influx achieves homeostatic potentiation of neurotransmitter release. Curr. Biol. 2012, 22, 1102–1108. [Google Scholar] [CrossRef]
- Gaviño, M.A.; Ford, K.J.; Archila, S.; Davis, G.W. Homeostatic synaptic depression is achieved through a regulated decrease in presynaptic calcium channel abundance. Elife 2015, 2015, e05473. [Google Scholar] [CrossRef]
- Schwarz, Y.; Oleinikov, K.; Schindeldecker, B.; Wyatt, A.; Weißgerber, P.; Flockerzi, V.; Boehm, U.; Freichel, M.; Bruns, D. TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses. PLoS Biol. 2019, 17, e3000445. [Google Scholar] [CrossRef]
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464. [Google Scholar] [CrossRef]
- Lee, S.H.; Lutz, D.; Mossalam, M.; Bolshakov, V.Y.; Frotscher, M.; Shen, J. Presenilins regulate synaptic plasticity and mitochondrial calcium homeostasis in the hippocampal mossy fiber pathway. Mol. Neurodegener. 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Chen, C.C.; Shen, J.W.; Chung, N.C.; Min, M.Y.; Cheng, S.J.; Liu, I.Y. Retrieval of context-associated memory is dependent on the Ca v3.2 T-type calcium channel. PLoS ONE 2012, 7, e29384. [Google Scholar] [CrossRef]
- An, L.; Zhang, T. Comparison Impairments of Spatial Cognition and Hippocampal Synaptic Plasticity between Prenatal and Postnatal Melamine Exposure in Male Adult Rats. Neurotox. Res. 2016, 29, 218–229. [Google Scholar] [CrossRef]
- Bröker-Lai, J.; Kollewe, A.; Schindeldecker, B.; Pohle, J.; Nguyen Chi, V.; Mathar, I.; Guzman, R.; Schwarz, Y.; Lai, A.; Weißgerber, P.; et al. Heteromeric channels formed by TRPC 1, TRPC 4 and TRPC 5 define hippocampal synaptic transmission and working memory. EMBO J. 2017, 36, 2770–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, C.F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science 1991, 251, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Grübel, K.; Busch, S.; Friedrich, A.B.; Siwanowicz, I.; Tanimoto, H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009, 23, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.F.; Pologruto, T.A.; Hannan, F.; Hakker, I.; Svoboda, K.; Zhong, Y. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J. Neurosci. 2004, 24, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Owald, D.; Chandra, V.; Talbot, C.; Huetteroth, W.; Waddell, S. Neural correlates of water reward in thirsty Drosophila. Nat. Neurosci. 2014, 17, 1536–1542. [Google Scholar] [CrossRef]
- Fiala, A. Olfaction and olfactory learning in Drosophila: Recent progress. Curr. Opin. Neurobiol. 2007, 17, 720–726. [Google Scholar] [CrossRef]
- Cognigni, P.; Felsenberg, J.; Waddell, S. Do the right thing: Neural network mechanisms of memory formation, expression and update in Drosophila. Curr. Opin. Neurobiol. 2018, 49, 51–58. [Google Scholar] [CrossRef]
- Aso, Y.; Hattori, D.; Yu, Y.; Johnston, R.M.; Iyer, N.A.; Ngo, T.T.B.; Dionne, H.; Abbott, L.F.; Axel, R.; Tanimoto, H.; et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 2014, 3, e04577. [Google Scholar] [CrossRef]
- Raji, J.I.; Potter, C.J. The number of neurons in Drosophila and mosquito brains. PLoS ONE 2021, 16, e0250381. [Google Scholar] [CrossRef]
- Xia, S.; Miyashita, T.; Fu, T.F.; Lin, W.Y.; Wu, C.L.; Pyzocha, L.; Lin, I.R.; Saitoe, M.; Tully, T.; Chiang, A.S. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr. Biol. 2005, 15, 603–615. [Google Scholar] [CrossRef]
- Albornoz, V.; Mendoza-Topaz, C.; Oliva, C.; Tello, J.; Olguín, P.; Sierralta, J. Temporal and spatial expression of Drosophila DLGS97 during neural development. Gene Expr. Patterns 2008, 8, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Keene, A.C.; Waddell, S. Drosophila olfactory memory: Single genes to complex neural circuits. Nat. Rev. Neurosci. 2007, 8, 341–354. [Google Scholar] [CrossRef] [PubMed]
- De Belle, J.S.; Heisenberg, M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 1994, 263, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.B.; Roberts, I.J.H.; Armstrong, J.D.; Kaiser, K.; Forte, M.; Tully, T.; O’Kane, C.J. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science 1996, 274, 2104–2107. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L. Physiology and biochemistry of Drosophila learning mutants. Physiol. Rev. 1996, 76, 299–317. [Google Scholar] [CrossRef]
- Heisenberg, M. What do the mushroom bodies do for the insect brain? An introduction. Learn. Mem. 1998, 5, 1–10. [Google Scholar] [CrossRef]
- Wolf, R.; Wittig, T.; Liu, L.; Wustmann, G.; Eyding, D.; Heisenberg, M. Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learn. Mem. 1998, 5, 166–178. [Google Scholar] [CrossRef]
- Kiragasi, B.; Wondolowski, J.; Li, Y.; Dickman, D.K. A Presynaptic Glutamate Receptor Subunit Confers Robustness to Neurotransmission and Homeostatic Potentiation. Cell Rep. 2017, 19, 2694–2706. [Google Scholar] [CrossRef]
- Kiragasi, B.; Goel, P.; Perry, S.; Han, Y.; Li, X.; Dickman, D. The auxiliary glutamate receptor subunit dSol-1 promotes presynaptic neurotransmitter release and homeostatic potentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 25830–25839. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J. Physiol. 1976, 262, 189–214. [Google Scholar] [CrossRef]
- Quinn, W.G.; Harris, W.A.; Benzer, S. Conditioned behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1974, 71, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Waddell, S. Drosophila aversive olfactory conditioning. Cold Spring Harb. Protoc. 2011, 6, pdb-prot5608. [Google Scholar] [CrossRef] [PubMed]
- Caipo, L.; González-Ramírez, M.C.; Guzmán-Palma, P.; Contreras, E.G.; Palominos, T.; Fuenzalida-Uribe, N.; Hassan, B.A.; Campusano, J.M.; Sierralta, J.; Oliva, C. Slit neuronal secretion coordinates optic lobe morphogenesis in Drosophila. Dev. Biol. 2020, 458, 32–42. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertin, F.; Moya-Alvarado, G.; Quiroz-Manríquez, E.; Ibacache, A.; Köhler-Solis, A.; Oliva, C.; Sierralta, J. Dlg Is Required for Short-Term Memory and Interacts with NMDAR in the Drosophila Brain. Int. J. Mol. Sci. 2022, 23, 9187. https://doi.org/10.3390/ijms23169187
Bertin F, Moya-Alvarado G, Quiroz-Manríquez E, Ibacache A, Köhler-Solis A, Oliva C, Sierralta J. Dlg Is Required for Short-Term Memory and Interacts with NMDAR in the Drosophila Brain. International Journal of Molecular Sciences. 2022; 23(16):9187. https://doi.org/10.3390/ijms23169187
Chicago/Turabian StyleBertin, Francisca, Guillermo Moya-Alvarado, Eduardo Quiroz-Manríquez, Andrés Ibacache, Andrés Köhler-Solis, Carlos Oliva, and Jimena Sierralta. 2022. "Dlg Is Required for Short-Term Memory and Interacts with NMDAR in the Drosophila Brain" International Journal of Molecular Sciences 23, no. 16: 9187. https://doi.org/10.3390/ijms23169187
APA StyleBertin, F., Moya-Alvarado, G., Quiroz-Manríquez, E., Ibacache, A., Köhler-Solis, A., Oliva, C., & Sierralta, J. (2022). Dlg Is Required for Short-Term Memory and Interacts with NMDAR in the Drosophila Brain. International Journal of Molecular Sciences, 23(16), 9187. https://doi.org/10.3390/ijms23169187





