Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia
Abstract
:1. Introduction
2. Results and Discussion
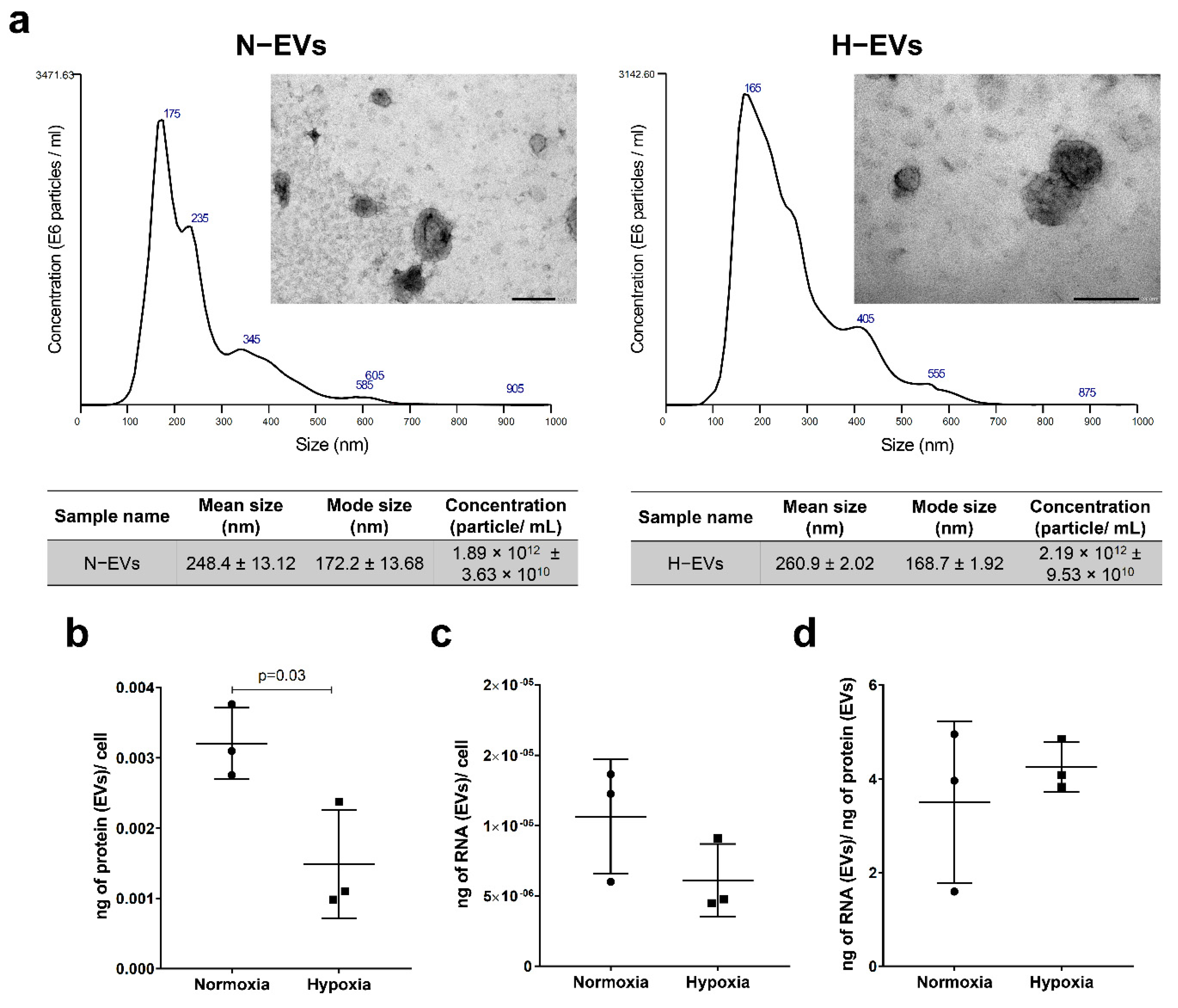
2.1. Protein and RNA Yield from EVs Isolated under Normoxic and Hypoxic Conditions
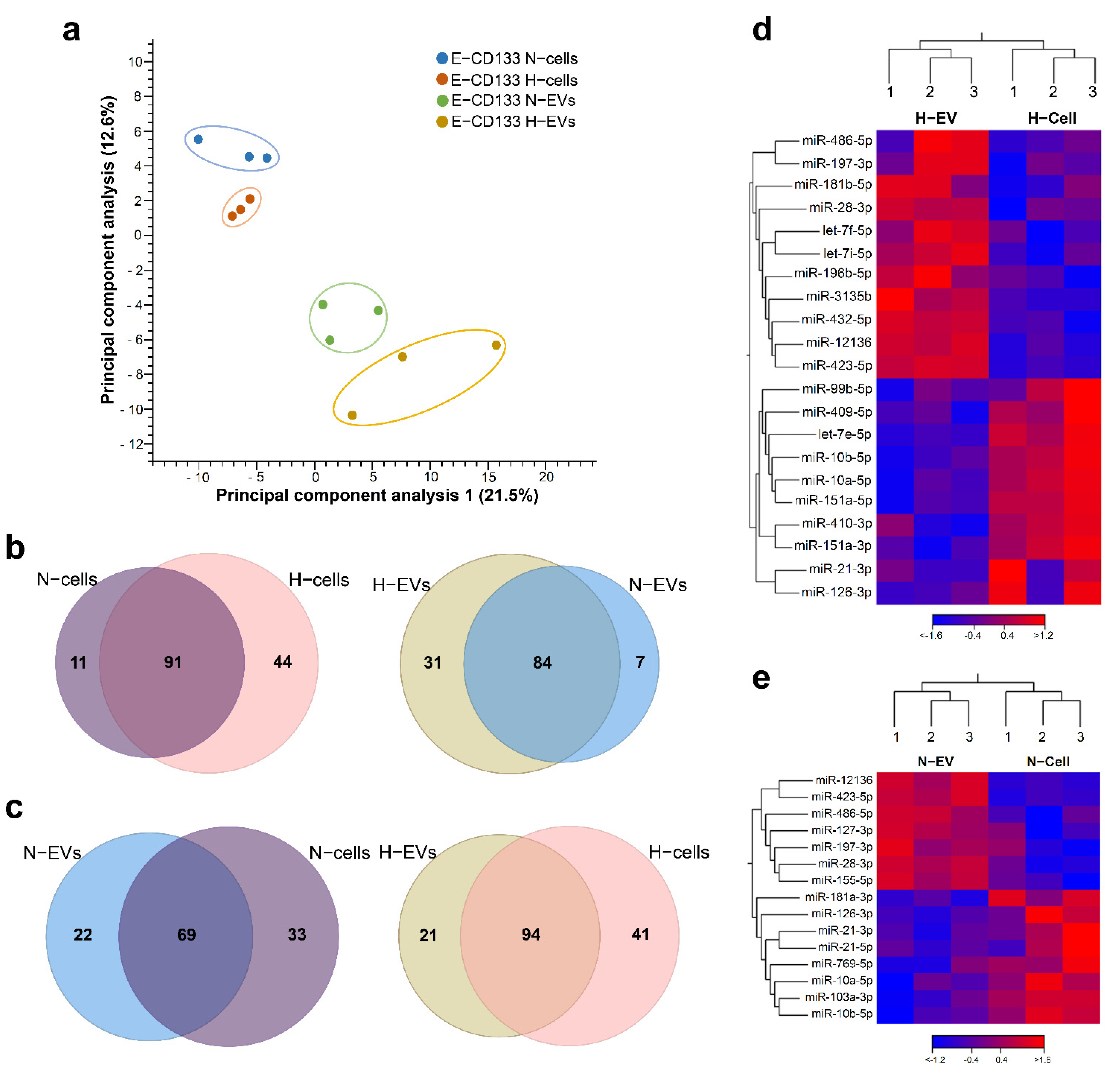
2.2. Hypoxia Treatment Changes the miRNA Content of E-CD133 Cells and EVs
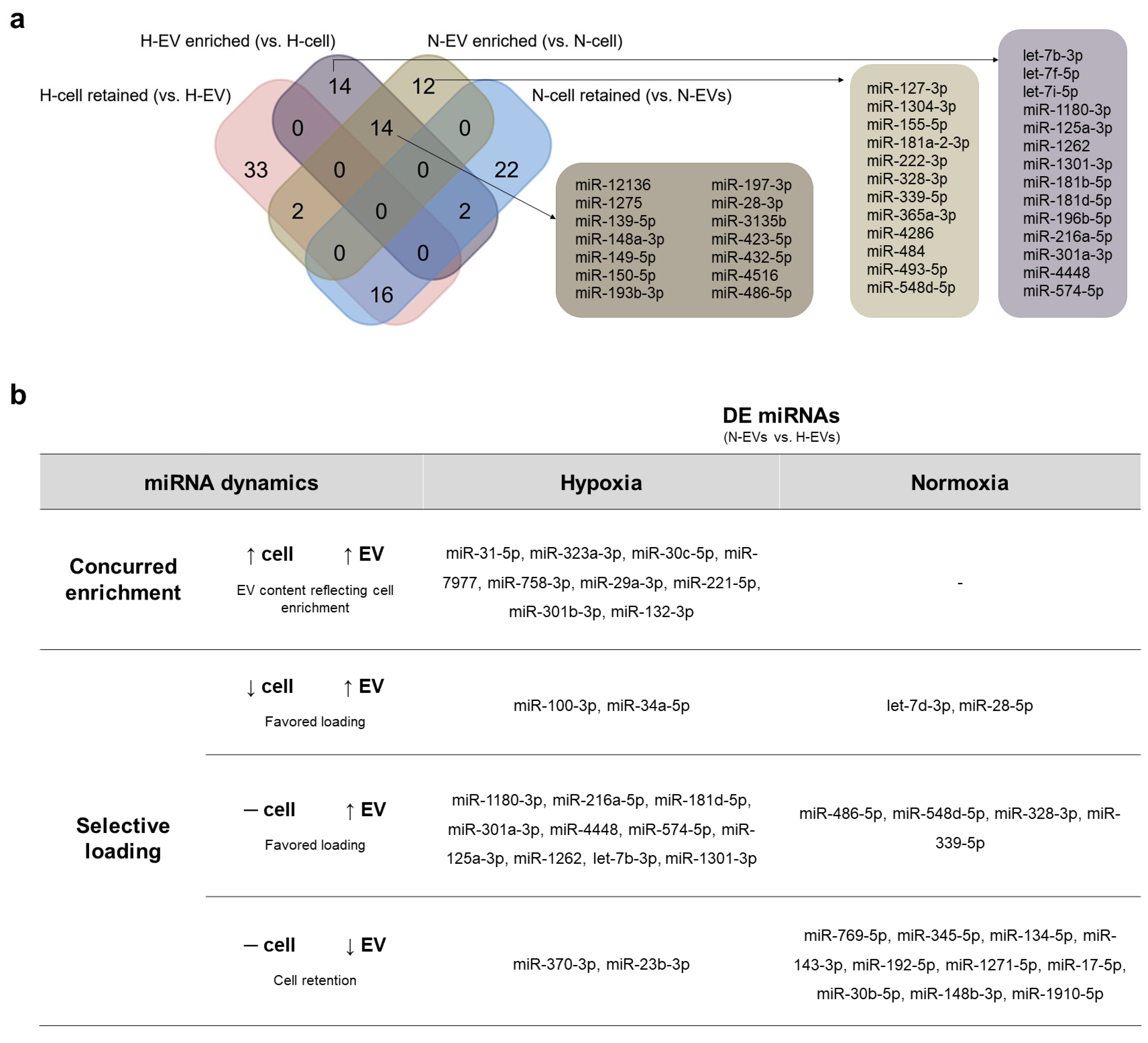
2.3. Favoring Loading in EVs or Cell Retention Concur to Differential N- and H-EVs Cargo, Indicating Selective miRNA Sorting to EVs
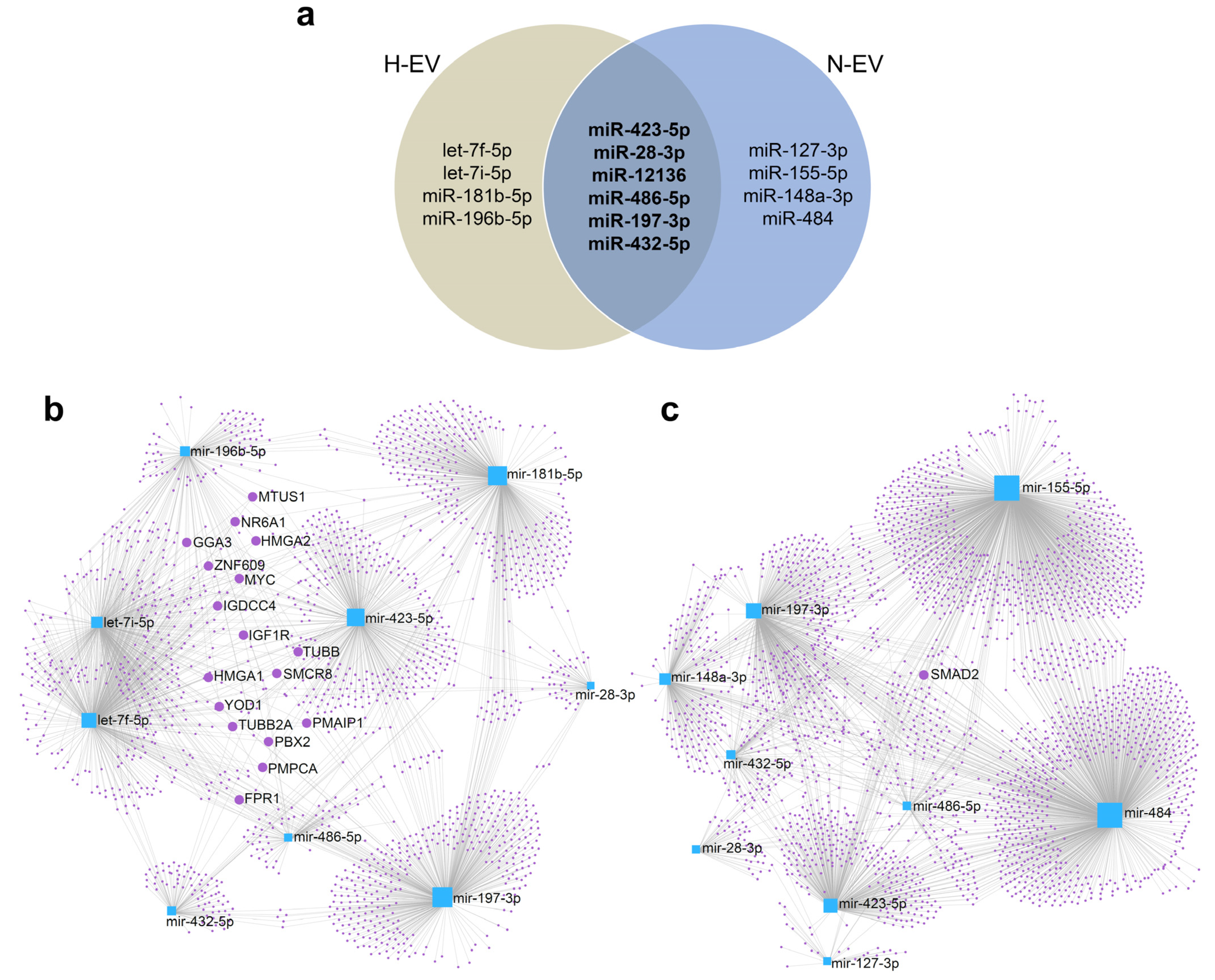
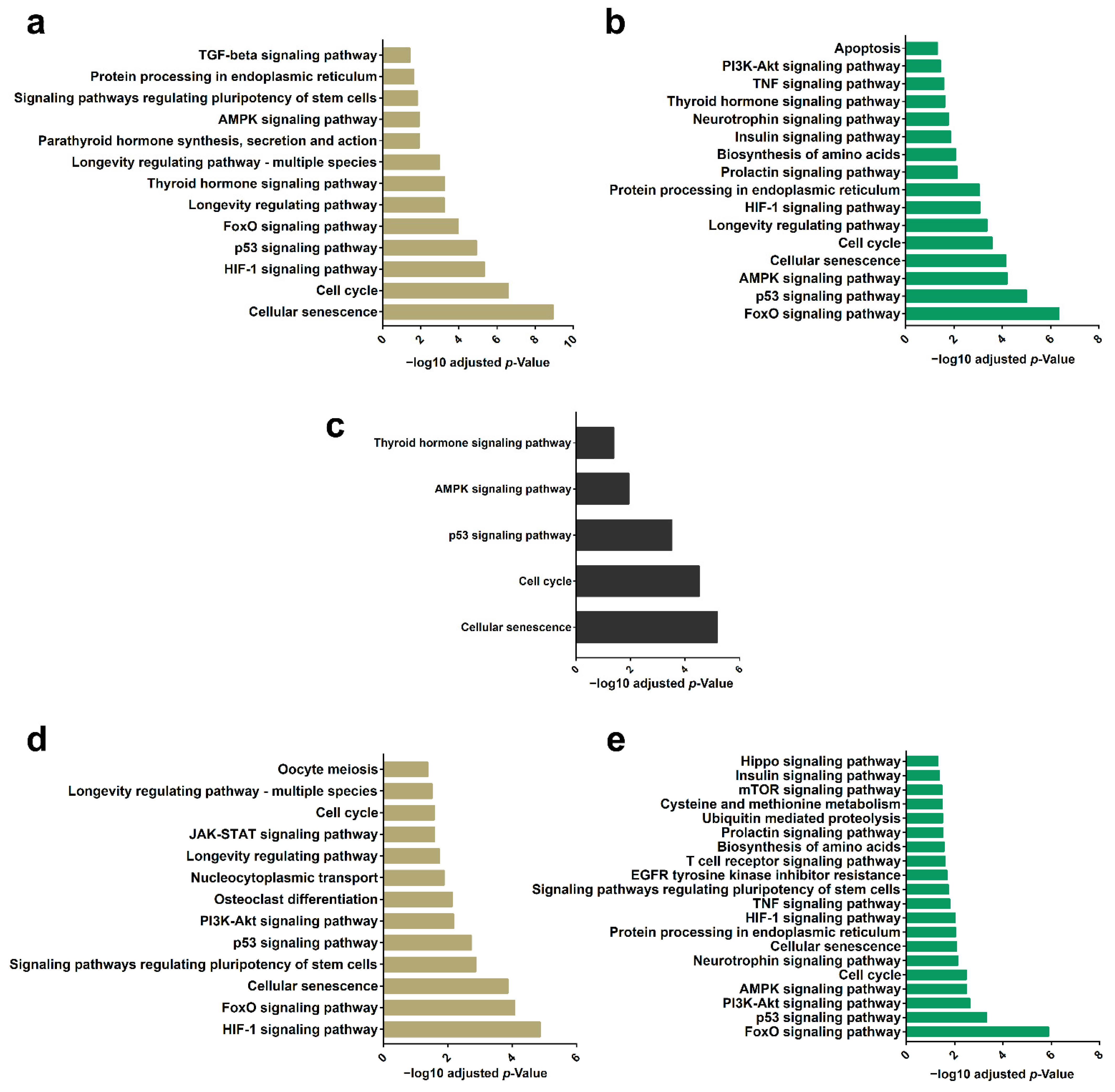
2.4. miRNA EV Cargo under Normoxia and Hypoxia Vary According to Cell Type
2.5. Differential Enrichment of miRNAs in EVs Highlights a Set of Target mRNAs and Modulation of Specific Signaling Pathways
3. Materials and Methods
3.1. CD133+ Cell Isolation and Expansion
3.2. Isolation of Extracellular Vesicles
3.3. Characterization of Extracellular Vesicles
3.4. Cell Culture under Hypoxic and Normoxic Conditions
3.5. RNA Isolation and Large-Scale Sequencing
3.6. Bioinformatic Analyses
3.7. Identification of miRNA Targets and Gene Ontology Analysis
3.8. Quantitative RT-PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Debbi, L.; Guo, S.; Safina, D.; Levenberg, S. Boosting Extracellular Vesicle Secretion. Biotechnol. Adv. 2022, 59, 107983. [Google Scholar] [CrossRef]
- Leitolis, A.; Suss, P.H.; Roderjan, J.G.; Angulski, A.B.B.; da Costa, F.D.A.; Stimamiglio, M.A.; Correa, A. Human Heart Explant-Derived Extracellular Vesicles: Characterization and Effects on the in Vitro Recellularization of Decellularized Heart Valves. Int. J. Mol. Sci. 2019, 20, 1279. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Reif, S. Extracellular Vesicles in Human Milk. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 209–215. [Google Scholar] [CrossRef]
- Blijdorp, C.J.; Tutakhel, O.A.Z.; Hartjes, T.A.; van den Bosch, T.P.P.; van Heugten, M.H.; Rigalli, J.P.; Willemsen, R.; Musterd-Bhaggoe, U.M.; Barros, E.R.; Carles-Fontana, R.; et al. Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles. J. Am. Soc. Nephrol. 2021, 32, 1210–1226. [Google Scholar] [CrossRef]
- Cabral, J.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Extracellular Vesicles as Modulators of Wound Healing. Adv. Drug Deliv. Rev. 2018, 129, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and Extracellular Vesicles: A Review on Methods, Vesicular Cargo and Functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Fernandes, H.; Zonnari, A.; Abreu, R.; Aday, S.; Barão, M.; Albino, I.; Lino, M.; Branco, A.; Seabra, C.; Barata, T.; et al. Extracellular Vesicles Enriched with an Endothelial Cell Pro-Survival MicroRNA Affects Skin Tissue Regeneration. Mol. Ther.-Nucleic Acids 2022, 28, 307–327. [Google Scholar] [CrossRef]
- Angulski, A.B.B.; Capriglione, L.G.; Batista, M.; Marcon, B.H.; Senegaglia, A.C.; Stimamiglio, M.A.; Correa, A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why Both Sources Are Advantageous for Regenerative Medicine. Stem Cell Rev. Rep. 2017, 13, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bussolati, B. Extracellular Vesicles in Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Lee, H. Extracellular Vesicles in Respiratory Disease. Adv. Clin. Chem. 2022, 108, 105–127. [Google Scholar] [CrossRef]
- Rudraprasad, D.; Rawat, A.; Joseph, J. Exosomes, Extracellular Vesicles and the Eye. Exp. Eye Res. 2022, 214, 108892. [Google Scholar] [CrossRef] [PubMed]
- Makhijani, P.; McGaha, T.L. Myeloid Responses to Extracellular Vesicles in Health and Disease. Front. Immunol. 2022, 13, 818538. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular Vesicles in Cardiovascular Disease: Biological Functions and Therapeutic Implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Endothelial Progenitor Cells Protect the Kidney from Ischemia-Reperfusion Injury by MicroRNA-Dependent Reprogramming of Resident Renal Cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Correa, A.; Ottoboni, G.S.; Senegaglia, A.C.; Capriglione, L.G.A.; Miyague, N.I.; Leite, L.M.B.; Jamur, V.R.; Rebelatto, C.L.K.; Olandoski, M.; Brofman, P.R.S. Expanded CD133+ Cells from Human Umbilical Cord Blood Improved Heart Function in Rats after Severe Myocardial Infarction. Stem Cells Int. 2018, 2018, 5412478. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Senegaglia, A.C.; Barboza, L.A.; Dallagiovanna, B.; Aita, C.A.M.; Hansen, P.; Rebelatto, C.L.K.; Aguiar, A.M.; Miyague, N.I.; Shigunov, P.; Barchiki, F.; et al. Are Purified or Expanded Cord Blood-Derived CD133+ Cells Better at Improving Cardiac Function? Exp. Biol. Med. 2010, 235, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b Promotes Neural Plasticity and Functional Recovery after Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular Vesicles Derived from Mesenchymal Stem/Stromal Cells: The Regenerative Impact in Liver Diseases. Hepatology 2022, 75, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, D.M.; Senegaglia, A.C.; de Moura, S.A.B.; Leitolis, A.; Capriglione, L.G.A.; Fracaro, L.; Boldrini Leite, L.M.; Utumi, P.H.; Fragoso, F.Y.I.; Meyer, F.; et al. Treatment of Chronic Kidney Disease with Extracellular Vesicles from Mesenchymal Stem Cells and CD133+ Expanded Cells: A Comparative Preclinical Analysis. Int. J. Mol. Sci. 2022, 23, 2521. [Google Scholar] [CrossRef]
- Valenzuela Alvarez, M.; Gutierrez, L.M.; Correa, A.; Lazarowski, A.; Bolontrade, M.F. Metastatic Niches and the Modulatory Contribution of Mesenchymal Stem Cells and Its Exosomes. Int. J. Mol. Sci. 2019, 20, 1946. [Google Scholar] [CrossRef]
- Rezaie, J.; Ahmadi, M.; Ravanbakhsh, R.; Mojarad, B.; Mahbubfam, S.; Shaban, S.A.; Shadi, K.; Berenjabad, N.J.; Etemadi, T. Tumor-Derived Extracellular Vesicles: The Metastatic Organotropism Drivers. Life Sci. 2022, 289, 120216. [Google Scholar] [CrossRef]
- Dierick, F.; Solinc, J.; Bignard, J.; Soubrier, F.; Nadaud, S. Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension. Cells 2021, 10, 1338. [Google Scholar] [CrossRef]
- Koutna, I.; Peterkova, M.; Simara, P.; Stejskal, S.; Tesarova, L.; Kozubek, M. Proliferation and Differentiation Potential of CD133+ and CD34+ Populations from the Bone Marrow and Mobilized Peripheral Blood. Ann. Hematol. 2011, 90, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cannavicci, A.; Kutryk, M.J.B. Exploring Endothelial Colony-Forming Cells to Better Understand the Pathophysiology of Disease: An Updated Review. Stem Cells Int. 2022, 2022, 4460041. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Triampo, W. The blood circulating rare cell population. What is it and what is it good for? Cells 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Schilling, A.F. Hypoxia-Based Strategies for Angiogenic Induction: The Dawn of a New Era for Ischemia Therapy and Tissue Regeneration. Organogenesis 2013, 9, 261–272. [Google Scholar] [CrossRef]
- Burnley-Hall, N.; Willis, G.; Davis, J.; Rees, D.A.; James, P.E. Nitrite-Derived Nitric Oxide Reduces Hypoxia-Inducible Factor 1α-Mediated Extracellular Vesicle Production by Endothelial Cells. Nitric Oxide 2017, 63, 1–12. [Google Scholar] [CrossRef]
- de Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; van Balkom, B.W.M. Cellular Stress Conditions Are Reflected in the Protein and RNA Content of Endothelial Cell-Derived Exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- De Jong, O.G.; van Balkom, B.W.M.; Gremmels, H.; Verhaar, M.C. Exosomes from Hypoxic Endothelial Cells Have Increased Collagen Crosslinking Activity through Up-Regulation of Lysyl Oxidase-like 2. J. Cell. Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, H.; Zhang, M.; He, Y.; Li, X.; Xu, Y.; Liu, X. Hypoxia Induced Changes of Exosome Cargo and Subsequent Biological Effects. Front. Immunol. 2022, 13, 824188. [Google Scholar] [CrossRef]
- Viñas, J.L.; Burger, D.; Zimpelmann, J.; Haneef, R.; Knoll, W.; Campbell, P.; Gutsol, A.; Carter, A.; Allan, D.S.; Burns, K.D. Transfer of MicroRNA-486-5p from Human Endothelial Colony Forming Cell-Derived Exosomes Reduces Ischemic Kidney Injury. Kidney Int. 2016, 90, 1238–1250. [Google Scholar] [CrossRef]
- Ma, R.; Liang, Z.; Shi, X.; Xu, L.; Li, X.; Wu, J.; Zhao, L.; Liu, G. Exosomal MiR-486-5p Derived from Human Placental Microvascular Endothelial Cells Regulates Proliferation and Invasion of Trophoblasts via Targeting IGF1. Hum. Cell 2021, 34, 1310–1323. [Google Scholar] [CrossRef]
- ElKhouly, A.M.; Youness, R.A.; Gad, M.Z. MicroRNA-486-5p and MicroRNA-486-3p: Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions. Non-Coding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Y.; Lv, K.; Wang, Y.; Zhong, Z.; Xiao, C.; Zhu, K.; Ni, C.; Wang, K.; Kong, M.; et al. Small Extracellular Vesicles Containing MiR-486-5p Promote Angiogenesis after Myocardial Infarction in Mice and Nonhuman Primates. Sci. Transl. Med. 2021, 13, eabb0202. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Dellett, M.; Brown, E.D.; Guduric-Fuchs, J.; O’Connor, A.; Stitt, A.W.; Medina, R.J.; Simpson, D.A. MicroRNA-Containing Extracellular Vesicles Released from Endothelial Colony-Forming Cells Modulate Angiogenesis during Ischaemic Retinopathy. J. Cell. Mol. Med. 2017, 21, 3405–3419. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Zichittella, C.; Alessandro, R.; Conigliaro, A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells 2021, 10, 3355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of Cargo Sorting into Small Extracellular Vesicles. Bioengineered 2021, 12, 8186. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of Exosome Production and Cargo Sorting. Int. J. Biol. Sci. 2021, 17, 163. [Google Scholar] [CrossRef]
- Chen, S.; Xu, M.; Zhao, J.; Shen, J.; Li, J.; Liu, Y.; Cao, G.; Ma, J.; He, W.; Chen, X.; et al. MicroRNA-4516 Suppresses Pancreatic Cancer Development via Negatively Regulating Orthodenticle Homeobox 1. Int. J. Biol. Sci. 2020, 16, 2159–2169. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Yang, T.; Li, D.; Huang, Y.; Bai, G.; Li, Q. LINC00520 Up-Regulates SOX5 to Promote Cell Proliferation and Invasion by MiR-4516 in Human Hepatocellular Carcinoma. Biol. Chem. 2022, 403, 665–678. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, T.Y.; Tsai, K.J.; Lin, M.W.; Hsu, C.Y.; Wu, D.C.; Tsai, E.M.; Hsieh, T.H. NF-ΚB/MiR-18a-3p and MiR-4286/BZRAP1 Axis May Mediate Carcinogenesis in Helicobacter Pylori-Associated Gastric Cancer. Biomed. Pharmacother. 2020, 132, 110869. [Google Scholar] [CrossRef]
- Ling, C.; Wang, X.; Zhu, J.; Tang, H.; Du, W.; Zeng, Y.; Sun, L.; Huang, J.A.; Liu, Z. MicroRNA-4286 Promotes Cell Proliferation, Migration, and Invasion via PTEN Regulation of the PI3K/Akt Pathway in Non-Small Cell Lung Cancer. Cancer Med. 2019, 8, 3520–3531. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Huang, J.; Xu, Z.; Xing, C.; Yang, J.; Zhou, X. MiR-150-5p Inhibits Nasopharyngeal Cancer Genesis by Suppressing PYCR1. Am. J. Med. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zou, C.; Zhu, Y.; Luo, Y.; Chen, L.; Lei, Y.; Tang, K.; Sun, Y.; Zhang, W.; Li, S.; et al. HIF-1ɑ-Regulated MiR-1275 Maintains Stem Cell-like Phenotypes and Promotes the Progression of LUAD by Simultaneously Activating Wnt/β-Catenin and Notch Signaling. Theranostics 2020, 10, 2553–2570. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, K.; Liu, P.; Luo, P.; Zhu, D.; Yin, J.; Yang, Q.; Huang, Y.; Gao, J.; Ai, Z.; et al. MiR-4286 Functions in Osteogenesis and Angiogenesis via Targeting Histone Deacetylase 3 and Alleviates Alcohol-Induced Bone Loss in Mice. Cell Prolif. 2021, 54, e13054. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, H.; Chen, J.; Wang, Z.; Yin, R. LncRNA MIAT Can Regulate the Proliferation, Apoptosis, and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells by Targeting MiR-150-5p. Bioengineered 2022, 13, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Zhang, L.; Zhou, Y.; Ji, X.; Liu, J.; Liu, D.; Yin, P.; Peng, Y.; Hao, M.; et al. Synergistic Effects of BMP9 and MiR-548d-5p on Promoting Osteogenic Differentiation of Mesenchymal Stem Cells. Biomed. Res. Int. 2015, 2015, 309747. [Google Scholar] [CrossRef] [PubMed]
- Xiu, C.; Zheng, H.; Jiang, M.; Li, J.; Zhou, Y.; Mu, L.; Liu, W. MSCs-Derived MiR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN. Int. J. Stem Cells 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Bi, T.; Yang, S. Exosomal MicroRNA-150-5p from Bone Marrow Mesenchymal Stromal Cells Mitigates Cerebral Ischemia/Reperfusion Injury via Targeting Toll-like Receptor 5. Bioengineered 2022, 13, 3030–3043. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Long, K.; Qiu, W.; Wang, Y.; Hu, Z.; Liu, C.; Luo, Y.; Jiang, A.; Jin, L.; et al. Overexpression of Exosomal Cardioprotective MiRNAs Mitigates Hypoxia-Induced H9c2 Cells Apoptosis. Int. J. Mol. Sci. 2017, 18, 711. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Qu, Z.; Hao, J.; Zhang, W. Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membranes 2022, 12, 225. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial Reparative Functions of Exosomes from Mesenchymal Stem Cells Are Enhanced by Hypoxia Treatment of the Cells via Transferring MicroRNA-210 in an NSMase2-Dependent Way. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Rayabaram, J.; Ijee, S.; Bagchi, A.; Chaudhury, A.D.; Roy, D.; Chambayil, K.; Singh, J.; Nakamura, Y.; Velayudhan, S.R. Comprehensive Analysis of MicroRNAs in Human Adult Erythropoiesis. Cells 2021, 10, 3018. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xiong, H.; Chen, J.; Yang, X.; Liu, Y.; Guo, J.; Jiang, T.; Xu, Z.; Yuan, M.; Liu, Y.; et al. The Whole Profiling and Competing Endogenous RNA Network Analyses of Noncoding RNAs in Adipose-Derived Stem Cells from Diabetic, Old, and Young Patients. Stem Cell Res. Ther. 2021, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, M.Á.; Pulido, M.; Marinaro, F.; Álvarez, V.; Báez-Díaz, C.; Blanco, V.; Silla-Castro, J.C.; Sanchez-Cabo, F.; Sánchez-Margallo, F.M.; Crisóstomo, V.; et al. Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction. Biomedicines 2022, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, Y.; Zhu, X.; Saadiq, I.M.; Jordan, K.L.; Eirin, A.; Lerman, L.O. Metabolic Syndrome Increases Senescence-Associated Micro-RNAs in Extracellular Vesicles Derived from Swine and Human Mesenchymal Stem/Stromal Cells. Cell Commun. Signal. 2020, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.N.; Zain, S.M.; Kamarulzaman, M.H.; Low, T.Y.; Chilian, W.M.; Pan, Y.; Ting, K.N.; Hamid, A.; Kadir, A.A.; Pung, Y.F. Intracellular and Exosomal MicroRNAome Profiling of Human Vascular Smooth Muscle Cells during Replicative Senescence. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H770–H783. [Google Scholar] [CrossRef]
- Schiele, M.A.; Lipovsek, J.; Schlosser, P.; Soutschek, M.; Schratt, G.; Zaudig, M.; Berberich, G.; Köttgen, A.; Domschke, K. Epigenome-Wide DNA Methylation in Obsessive-Compulsive Disorder. Transl. Psychiatry 2022, 12, 221. [Google Scholar] [CrossRef]
- Yue, W.; Cheng, W.; Liu, Z.; Tang, Y.; Lu, T.; Zhang, D.; Tang, M.; Huang, Y. Genome-Wide DNA Methylation Analysis in Obsessive-Compulsive Disorder Patients. Sci. Rep. 2016, 6, 31333. [Google Scholar] [CrossRef]
- Louafi, F.; Martinez-Nunez, R.T.; Sanchez-Elsner, T. MicroRNA-155 Targets SMAD2 and Modulates the Response of Macrophages to Transforming Growth Factor-β. J. Biol. Chem. 2010, 285, 41328–41336. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, J.; Yu, J.; Liao, J.; Liu, S.; Cai, N.; Liang, H.; Chen, X.; Ding, Z.; Zhang, B. MicroRNA-148a-3p Inhibits Progression of Hepatocelluar Carcimoma by Repressing SMAD2 Expression in an Ago2 Dependent Manner. J. Exp. Clin. Cancer Res. 2020, 39, 150. [Google Scholar] [CrossRef]
- Chen, P.C.; Kuo, Y.C.; Chuong, C.M.; Huang, Y.H. Niche Modulation of IGF-1R Signaling: Its Role in Stem Cell Pluripotency, Cancer Reprogramming, and Therapeutic Applications. Front. Cell Dev. Biol. 2021, 8, 625943. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Ma, S.; Li, Y.; Yu, Y.; Zhou, Y.X.; Lu, Y.D.; Jin, L.; Wang, Z.L.; Yu, J.H. Hsa-Let-7c Controls the Committed Differentiation of IGF-1-Treated Mesenchymal Stem Cells Derived from Dental Pulps by Targeting IGF-1R via the MAPK Pathways. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating Exosomes Suppress the Induction of Regulatory T Cells via Let-7i in Multiple Sclerosis. Nat. Commun. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Wang, X.F.; Qian, X.; Tao, T.; Wang, L.; Chen, Q.D.; Wang, X.R.; Cao, L.; Wang, Y.Y.; Zhang, J.X.; et al. MiRNA-181b Suppresses IGF-1R and Functions as a Tumor Suppressor Gene in Gliomas. RNA 2013, 19, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Piscitelli, S.; Passaro, F.; Russo, T. HMGA Proteins in Stemness and Differentiation of Embryonic and Adult Stem Cells. Int. J. Mol. Sci. 2020, 21, 362. [Google Scholar] [CrossRef]
- Kaur, H.; Ali, S.Z.; Huey, L.; Hütt-Cabezas, M.; Taylor, I.; Mao, X.G.; Weingart, M.; Chu, Q.; Rodriguez, F.J.; Eberhart, C.G.; et al. The Transcriptional Modulator HMGA2 Promotes Stemness and Tumorigenicity in Glioblastoma. Cancer Lett. 2016, 377, 55–64. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Ditzel, H.J.; Duijf, P.H.G.; Khaze, V.; Gjerstorff, M.F.; Baradaran, B. HMGA2 as a Critical Regulator in Cancer Development. Genes 2021, 12, 269. [Google Scholar] [CrossRef]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. MiRNAs Regulate the HIF Switch during Hypoxia: A Novel Therapeutic Target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and Regulation of Apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO Transcription Factors in Aging and Age-Related Metabolic and Neurodegenerative Diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Chen, J.; Chen, L.; Wang, R.; Chu, X. MicroRNAs as Regulators and Mediators of Forkhead Box Transcription Factors Function in Human Cancers. Oncotarget 2017, 8, 12433. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kondo, E.; Takeuchi, M.; Harashima, A.; Otani, T.; Tsuji-Takayama, K.; Yamasaki, F.; Kumon, H.; Kibata, M.; Nakamura, S. MiR-155, a Modulator of FOXO3a Protein Expression, Is Underexpressed and Cannot Be Upregulated by Stimulation of HOZOT, a Line of Multifunctional Treg. PLoS ONE 2011, 6, e16841. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-Kinase/Akt Signaling by Muscle-Enriched MicroRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Rogers, R.G.; Ciullo, A.; Marbán, E.; Ibrahim, A.G. Extracellular Vesicles as Therapeutic Agents for Cardiac Fibrosis. Front. Physiol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Reed, S.L.; Escayg, A. Extracellular Vesicles in the Treatment of Neurological Disorders. Neurobiol. Dis. 2021, 157, 105445. [Google Scholar] [CrossRef]
- Szwedowicz, U.; Łapińska, Z.; Gajewska-Naryniecka, A.; Choromańska, A. Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules 2022, 27, 1303. [Google Scholar] [CrossRef]
- Angulski, A.B.B.; Capriglione, L.G.A.; Barchiki, F.; Brofman, P.; Stimamiglio, M.A.; Senegaglia, A.C.; Correa, A. Systemic Infusion of Expanded CD133 + Cells and Expanded CD133 + Cell-Derived EVs for the Treatment of Ischemic Cardiomyopathy in a Rat Model of AMI. Stem Cells Int. 2019, 2019, 4802578. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. MiRNet 2.0: Network-Based Visual Analytics for MiRNA Functional Analysis and Systems Biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
| Enrichment | miRNA | nº Targets | KEGG Pathway | Adjusted p-Value |
|---|---|---|---|---|
| E-CD133 EV enriched (hypoxia only) | let-7f-5p | 397 | KEGG:04115—p53 signaling pathway | 0.003691 |
| KEGG:04630—JAK-STAT signaling pathway | 0.007606 | |||
| let-7i-5p | 334 | KEGG:04550—Signaling pathways regulating pluripotency of stem cells | 0.00683 | |
| KEGG:04630—JAK-STAT signaling pathway | 0.020841 | |||
| KEGG:04115—p53 signaling pathway | 0.038995 | |||
| miR-181b-5p | 375 | KEGG:03013—Nucleocytoplasmic transport | 0.015346 | |
| KEGG:04140—Autophagy—animal | 0.033046 | |||
| miR-196b-5p | 150 | KEGG:04725—Cholinergic synapse | 0.000757 | |
| KEGG:04261—Adrenergic signaling in cardiomyocytes | 0.000777 | |||
| KEGG:04915—Estrogen signaling pathway | 0.0031 | |||
| KEGG:04921—Oxytocin signaling pathway | 0.007137 | |||
| KEGG:04912—GnRH signaling pathway | 0.018242 | |||
| KEGG:04210—Apoptosis | 0.020724 | |||
| KEGG:04371—Apelin signaling pathway | 0.023727 | |||
| KEGG:04010—MAPK signaling pathway | 0.030375 | |||
| KEGG:04720—Long-term potentiation | 0.032404 | |||
| KEGG:04922—Glucagon signaling pathway | 0.039067 | |||
| KEGG:04066—HIF-1 signaling pathway | 0.043144 | |||
| KEGG:04218—Cellular senescence | 0.049952 | |||
| E-CD133 EV enriched (normoxia only) | miR-155-5p | 904 | KEGG:04917—Prolactin signaling pathway | 3.01 × 10−5 |
| KEGG:04668—TNF signaling pathway | 3.07 × 10−5 | |||
| KEGG:04068—FoxO signaling pathway | 0.000133 | |||
| KEGG:04660—T cell receptor signaling pathway | 0.00054 | |||
| KEGG:04550—Signaling pathways regulating pluripotency of stem cells | 0.000632 | |||
| KEGG:04218—Cellular senescence | 0.000873 | |||
| KEGG:04657—IL-17 signaling pathway | 0.006472 | |||
| KEGG:04620—Toll-like receptor signaling pathway | 0.006688 | |||
| KEGG:04380—Osteoclast differentiation | 0.009192 | |||
| KEGG:04066—HIF-1 signaling pathway | 0.015699 | |||
| KEGG:04152—AMPK signaling pathway | 0.016785 | |||
| KEGG:04151—PI3K-Akt signaling pathway | 0.026386 | |||
| KEGG:04659—Th17 cell differentiation | 0.032336 | |||
| KEGG:04120—Ubiquitin-mediated proteolysis | 0.045985 | |||
| KEGG:04510—Focal adhesion | 0.049545 | |||
| miR-148a-3p | 213 | KEGG:04390—Hippo signaling pathway | 0.000604 | |
| KEGG:04550—Signaling pathways regulating pluripotency of stem cells | 0.001656 | |||
| KEGG:04068—FoxO signaling pathway | 0.005113 | |||
| KEGG:04151—PI3K-Akt signaling pathway | 0.005314 | |||
| KEGG:04218—Cellular senescence | 0.019607 | |||
| KEGG:04110—Cell cycle | 0.023304 | |||
| KEGG:04612—Antigen processing and presentation | 0.02659 | |||
| KEGG:04310—Wnt signaling pathway | 0.032609 | |||
| KEGG:04115—p53 signaling pathway | 0.036182 | |||
| miR-484 | 891 | KEGG:01230—Biosynthesis of amino acids | 0.011065 | |
| KEGG:03010—Ribosome | 0.033447 | |||
| E-CD133 EV enriched (both hypoxia and normoxia) | miR-486-5p | 67 | KEGG:04218—Cellular senescence | 0.001365 |
| miR-423-5p | 343 | KEGG:04110—Cell cycle | 0.010135 | |
| KEGG:01230—Biosynthesis of amino acids | 0.042544 | |||
| miR-432-5p | 94 | KEGG:04911—Insulin secretion | 0.047795 |
| Name | Forward Primer Sequence |
|---|---|
| hsa-miR-100-5p | AACCCGTAGATCCGAACTTGTG |
| hsa-miR-10b-5p | TACCCTGTAGAACCGAATTTGTG |
| hsa-miR-99b-5p | CACCCGTAGAACCGACCTTGCG |
| hsa-let-7f-5p | TGAGGTAGTAGATTGTATAGTT |
| hsa-miR-12136 | GAAAAAGTCATGGAGGCC |
| hsa-miR-127-3p | TCGGATCCGTCTGAGCTTGGCT |
| hsa-miR-486-5p | TCCTGTACTGAGCTGCCCCGAG |
| hsa-miR-3135b | GGCTGGAGCGAGTGCAGTGGTG |
| hsa-miR-484 | TCAGGCTCAGTCCCCTCCCGAT |
| hsa-miR-423-5p | TGAGGGGCAGAGAGCGAGACTTT |
| hsa-miR-181b-5p | AACATTCATTGCTGTCGGTGGGT |
| hsa-miR-339-5p | TCCCTGTCCTCCAGGAGCTCACG |
| hsa-miR-548d-5p | AAAAGTAATTGTGGTTTTTGCC |
| cel-miR-39-3p | TCACCGGGTGTAAATCAGCTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robert, A.W.; Marcon, B.H.; Angulski, A.B.B.; Martins, S.d.T.; Leitolis, A.; Stimamiglio, M.A.; Senegaglia, A.C.; Correa, A.; Alves, L.R. Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia. Int. J. Mol. Sci. 2022, 23, 10066. https://doi.org/10.3390/ijms231710066
Robert AW, Marcon BH, Angulski ABB, Martins SdT, Leitolis A, Stimamiglio MA, Senegaglia AC, Correa A, Alves LR. Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia. International Journal of Molecular Sciences. 2022; 23(17):10066. https://doi.org/10.3390/ijms231710066
Chicago/Turabian StyleRobert, Anny Waloski, Bruna Hilzendeger Marcon, Addeli Bez Batti Angulski, Sharon de Toledo Martins, Amanda Leitolis, Marco Augusto Stimamiglio, Alexandra Cristina Senegaglia, Alejandro Correa, and Lysangela Ronalte Alves. 2022. "Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia" International Journal of Molecular Sciences 23, no. 17: 10066. https://doi.org/10.3390/ijms231710066
APA StyleRobert, A. W., Marcon, B. H., Angulski, A. B. B., Martins, S. d. T., Leitolis, A., Stimamiglio, M. A., Senegaglia, A. C., Correa, A., & Alves, L. R. (2022). Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia. International Journal of Molecular Sciences, 23(17), 10066. https://doi.org/10.3390/ijms231710066





