Abstract
SQUAMOSA Promoter-Binding Protein-Like (SPL) genes encode plant-specific transcription factors which bind to the SQUAMOSA promoter of the MADS-box genes to regulate its expression. It plays important regulatory roles in floral induction and development, fertility, light signals and hormonal transduction, and stress response in plants. In this study, 32 PySPL genes with complete SBP (squamosa promoter binding protein) conserved domain were identified from the genome of Prunus × yedoensis ‘Somei-yoshino’ and analyzed by bioinformatics. 32 PySPLs were distributed on 13 chromosomes, encoding 32 PySPL proteins with different physical and chemical properties. The phylogenetic tree constructed with Arabidopsis thaliana and Oryza sativa can be divided into 10 subtribes, indicating PySPLs of different clusters have different biological functions. The conserved motif prediction showed that the number and distribution of motifs on each PySPL is varied. The gene structure analysis revealed that PySPLs harbored exons ranging from 2 to 10. The predictive analysis of acting elements showed that the promoter of PySPLs contain a large number of light-responsive elements, as well as response elements related to hormone response, growth and development and stress response. The analysis of the PySPLs expressions in flower induction and flower organs based on qRT-PCR showed that PySPL06/22 may be the key genes of flower development, PySPL01/06 and PySPL22 may play a role in the development of sepal and pistil, respectively. The results provide a foundation for the study of SPL transcription factors of Prunus × yedoensis ‘Somei-yoshino’ and provide more reference information of the function of SPL gene in flowering.
1. Introduction
Transcription factor is a kind of DNA binding protein that binds to a specific sequence upstream of the 5′ end coding region to regulate its expression by altering the transcriptional level of the gene. According to the different region of the DNA-binding structure, transcription factors can be distinguished into families and subfamilies [1,2]. SQUAMOSA promoter-binding protein-like (SPL) transcription factor, or the SBP protein, is a kind of transcription factor ubiquitous in green plants with a highly conserved SBP domain. This domain contains two zinc-finger structures, C3H (C-C-C-H) and C2HC (C-C-H-C) [3], while the C-terminus contains a highly conserved bidirectional nuclear localization signal (NLS) [4]. This structural domain binds to the SQUAMOSA promoter of the MADS-box genes to regulate its expression [5]. SPL is a multigene family involved in plant growth, flower development, fertility, light signals and hormonal transduction and stress responses [6,7,8,9,10,11]. Since the discovery of the SPL genes from snapdragons, the SPL genes in Gossypium spp. [12], Populus trichocarpa [13], Betula luminifera [14], Ziziphus jujuba [15] and other plants have been identified successively.
SPL gene regulates the flowering time of plants. AtSPL3, AtSPL4 and AtSPL5 in A. thaliana are involved in the photoperiodic pathway through PNY and PNF to promote flowering [16]. Over expression of AtSPL3 and VpSBP11 with high homology to AtSPL3 in Vitis vinifera can activate the expression of FRUITFULL(FUL), APETALA1(AP1) and LEAFY(LFY) genes to advance flowering time in A. thaliana [17,18]. SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1(SOC1) and miR156-SPL3 regulatory modules interact in response to plant photoperiod and gibberellin signaling and thus participate in the control of flowering time in A. thaliana [19,20,21]. AtSPL9, AtSPL10 and AtSPL15 are involved in the regulation of flowering time via the age pathway in A. thaliana, while miR156/172 are the two miRNAs important in the age pathway. The miR156 delays flowering in A. thaliana by suppressing AtSPL15 activity [22], while AtSPL9 and AtSPL10 activate expression of miR172 to accelerate flowering [23]. miR156 in Prunus mume regulates the flowering process of P. mume by down- or up-regulating the expression of SPL genes [24].
SPL can also regulate floral transition, floral organ development and fertility. For example, PhSPLs in P. hybrida was found to be mainly expressed in axillary buds and inflorescence, where PhSPL9a and PhSPL9b facilitated the transition from vegetative growth to reproductive growth [25]. Over expression of AtSPL3 in A. thaliana under long daylight can cause abnormal inflorescence development [17], and OsSPL13, which is homologous to AtSPL3, also play a role in regulating flower development in Oryza sativa [26]. Over expression of AtSPL10 suppresses pedicel elongation in A. thaliana [27]. The miR156-mediated AtSPL2 in affects the development of petal and sepal, pollen production and viability in A. thaliana [28].
Flowering cherry (Prunus × yedoensis ‘Somei-yoshino’) is a famous early spring ornamental flowering tree with beautiful plant shape, brilliant flower color and early flowering period; it has a high ornamental value and is widely planted in the world. It has been shown that the expression of FLOWERING LOCUS T(FT) and gibberellin-mediated DELLA are increased during floral development before flowering in Prunus × yedoensis ‘Somei-yoshino’ [29], while the role of SPL in floral formation determination and floral development in this plant has rarely been reported. Based on the genomic data of Prunus × yedoensis ‘Somei-yoshino’ [29], 32 SPL genes were searched and identified. The chromosomal location, phylogenetic relationship, conserved domain, conserved motifs, gene structure and collinearity were carried out by bioinformatics methods. Finally, qRT-PCR was used to compare the expression levels of some PySPLs in different floral developmental stages and different floral organs, which provides some reference to further study the biological functions of SPL genes in floral transition and flower development in flowering cherry.
2. Results
2.1. Screening, Identification and Sequence Analysis of PySPLs
A total of 43 SPL genes were initially identified using BlastP with 16 Arabidopsis thaliana SPL protein sequences as the reference and screened out by Pfam (PF031100). Based on conserved structural domains followed by removal of redundancy, 32 SPL genes were finally obtained within the Prunus × yedoensis ‘Somei-yoshino’ genome, and respectively named PySPL 01~PySPL 32 according to their location on the chromosome (Table 1). Physicochemical properties analysis showed that the number of amino acids encoded by PySPLs was between 152 and 1070, molecular weight between 17,505.46~118,346.45 kD, theoretical pl between 5.75 and 10.12, and aliphatic index between 36.17 and 78.9. All members were unstable and hydrophilic in nature except PySPL11. All members were predicted to be localized in the nucleus, except PySPL04, PySPL19, PySPL20, PySPL22, PySPL23, and PySPL31 in the cytoplasm and nucleus.

Table 1.
Characteristics properties of PySPL in Prunus×yedoensis ‘Somei-yoshino’.
2.2. Chromosomal Localization of PySPLs
The chromosomal mapping results (Figure 1) showed that 32 PySPLs were distributed on all the 13 chromosomes except chromosomes SPA3/SPA8/SPE4/SPE6/SPE8. Six PySPLs were distributed on the chromosome SPA0, SPA7/SPE3/SPE7 distributed the least members (1).
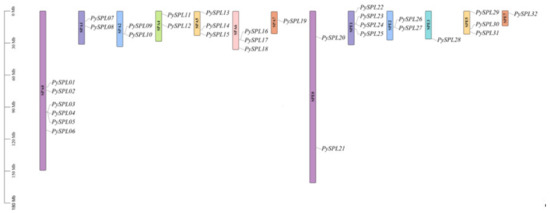
Figure 1.
Chromosomal localization of PySPLs in Prunus × yedoensis ‘Somei-yoshino’. The chromosome number is marked in the middle of each chromosome. The scale is in Mega-bases (Mb). Chromosome pairs are marked with the same color.
2.3. Phylogeny Analysis of PySPLs
A Neighbor-Joining (NJ) phylogenetic tree of the SPL protein was constructed by using the aligned protein sequence of all PySPLs, AtSPLs and OsSPLs (Figure 2, Supplementary Materials Tables S1 and S2). A total of 68 SPL proteins can be roughly divided into 10 clusters and 32 PySPLs are distributed in all 10 clusters. The cluster V contains the most members of PySPLs (5), cluster I, II, III, VI, VII, VIII contains the least members of PySPLs (2). Meanwhile, PySPLs proteins have higher similarity with AtSPLs proteins compared to OsSPLs proteins, which may be related to the fact that both Prunus × yedoensis ‘Somei-yoshino’ and A. thaliana are dicotyledons, while Oryza sativa is monocotyledonous.
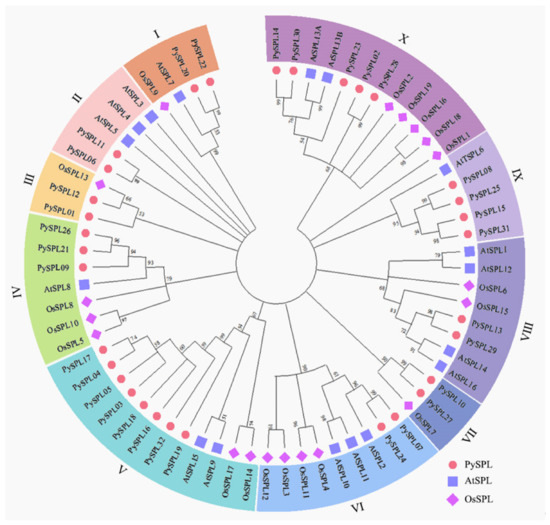
Figure 2.
Phylogenetic tree of SPL in Prunus × yedoensis ‘Somei-yoshino’, Arabidopsis thaliana and Oryza sativa. The full-length amino acid sequences of 32 PySPLs, 17 AtSPLs and 19 OsSPLs was used to construct a phylogenetic tree with 1000 bootstrap replicates by MEGA11. PySPLs were divided into subfamilies according to the branch relationships.
2.4. Multiple Sequence Alignment and Conserved Domain Visualization of PySPLs
Multiple sequence alignment and conserved domain visualization of the PySPLs showed that all PySPLs protein contain highly conserved SBP domains (Figure 3), including two zinc-finger structures (Cys-Cys-Cys-His, Cys-Cys-His-Cys) and one bidirectional nuclear localization signal (NLS).
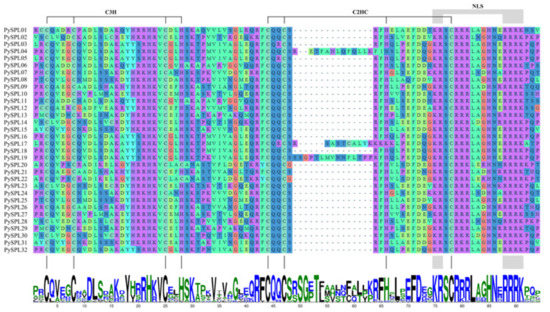
Figure 3.
Alignment and conserved domains logo of conserved domains sequence of PySPLs protein.C3H and C2HC were two zinc-finger structures, NLS was bidirectional nuclear localization signal, which were corresponding to the sequence logos of conserved domains.
2.5. Conserved Motifs and Gene Structure Analysis of PySPLs
A phylogenetic tree of 32 PySPLs was constructed to further analyze their evolutionary relationships (Figure 4A). Conserved motifs of all 32 PySPLs were analyzed by the online software MEME. The results showed that Motif 1 and Motif 2 are included in all PySPLs member, are functional conserved motifs of PySPLs and contain the SBP domain. Meanwhile, PySPLs with closer evolutionary relationships contain approximately the same conserved motif. Almost all PySPLs contained more than two conserved motifs except PySPL01, PySPL12, and PySPL17 (Figure 4B). The number of exons and introns contained by PySPLs varied widely (Figure 4C). PySPL20 and PySPL22 had the longest gene sequences and contained the largest number of exons (10), while PySPL01, PySPL06, PySPL11, PySPL12 and PySPL17 contained the fewest number of exons (2). A total of 20 PySPLs have 3′-UTR and 5′-UTR; 11 members lack 3′-UTR and 5′-UTR; one member (PySPL19) lacks 5′-UTR. PySPLs with closer evolutionary relationships have similar gene structures.

Figure 4.
Phylogenetic relationship, conserved protein motif, and gene structure analysis of PySPLs. (A) A phylogenetic tree harboring amino acid sequence 32 of PySPLs by Neighbor-Joining method with 1000 bootstrap replicates. (B) Conserved motifs of 32 PySPLs arranged according to evolutionary relationships. Motifs of different colors have their corresponding amino acid sequences. (C) Gene structure of 32 PySPLs arranged according to evolutionary relationships, the exons, introns and UTRs are indicated by different colored boxes and lines.
2.6. Cis-Acting Elements Analysis of PySPLs Promoter
2000 bp upstream sequences of the start codon of 32 PySPLs contains a large number of light response elements, phytohormone (ABA, GA, Auxin, SA, Meja) response elements and some specific response elements including meristem expression, low-temperature induction, drought induction, anaerobic induction, circadian control, defense and stress response (Figure 5). Light response elements are distributed among all PySPLs and accounted for the largest number of total elements in each PySPL, which indicating that the expression of PySPLs is likely to be influenced by light; second, all PySPLs promoter regions contain anaerobic induction response elements except PySPL12.Phytochrome response element and seed-specific response element are only distributed in PySPL14/30 and PySPL06/11 promoter region, respectively. The above results indicated that PySPLs is regulated differently, suggesting the functions of PySPLs were diverse.
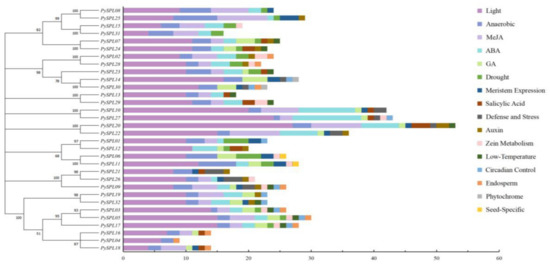
Figure 5.
Cis-acting elements analysis of PySPLs promoters by Plantcare. Different colored boxes represent different cis-acting elements. The horizontal coordinate represents the number of cis-acting elements.
2.7. Collinearity Analysis of PySPLs
According to the collinearity analysis results (Figure 6), the PySPL gene family is formed mainly due to the segmental duplication of chromosomes. A total of 31 genes in the PySPLs family except PySPL01 were co-linearly related (26 pairs), and all of them were due to segmental duplications. There are cases where one PySPL gene is co-linearly related to multiple PySPLs, such as PySPL08 being co-linearly related to PySPL15, PySPL25 and PySPL31. PySPL14 is co-linearly related to PySPL23, PySPL28 and PySPL30. PySPL01 did not have a collinearity relationship. Presumably it appeared before the differentiation of cherry species.
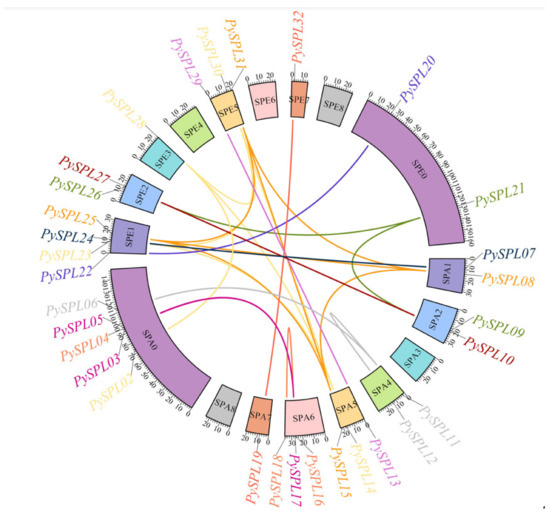
Figure 6.
Collinearity analysis of PySPLs in Prunus × yedoensis ’Somei-yoshino’. The chromosome number is marked in the middle of each chromosome. The scale bar of chromosome length is Mb, genes with segmental duplication relationships has the same color markers.
2.8. Expression Analysis of PySPLs in Flowering Induction
The morphology of the samples in each period (Figure 7) showed that, compared with the dormant period (DP), the flower buds of Prunus × yedoensis ‘Somei-Yoshino’ continued to expand, and the flower primordium continued to grow within one month before flowering. At 20 days before flowering (24DBF), it was clearly observed that each flower bract was within the bud and the anthers and ovary were within the flower bract. Ten days before flowering (14DBF), the sepals, petals, stamens and pistils were clearly visible in form and color, thus indicating that the development of floral organs was basically completed ten days before flowering. Meanwhile, the pedicel and calyx tube elongate significantly ten days before flowering. A total of 12 PySPLs containing representative conserved motif distributions and gene structures were selected for qRT-PCR based on their evolutionary developmental relationships (Figure 8). In contrast to dormant periods (DP), the relative expression level of some PySPLs was reduced significantly, including PySPL02, PySPL08, PySPL10 and PySPL31, which may have a negative regulatory effect on flower induction. The relative expression level of PySPL01 and PySPL14 was not significantly different in the early stage and decreased at the basic completion of floral organ development (14DBF). PySPL06 had the highest relative expression at mid-flower development (24DBF) and then decreased, considering that PySPL06 is associated with flower organ development in Prunus × yedoensis ‘Somei-yoshino’. PySPL26 had the highest relative expression level in the progress of flower primordium (34DBF and 24DBF) while the relative expression level of PySPL07, PySPL22, PySPL29 and PySPL32 changed at random in all periods (3DBF).

Figure 7.
Flower buds of Prunus × yedoensis ‘Somei-yoshino’ for qRT-PCR. DP:dormant period, 34DBF:34 day before flowering, 24DBF:24 day before flowering, 14DBF:14 day before flowering,3DBF:3 day before flowering. (a–d) are the internal morphology of flower bud samples DP, 34DBF, 24DBF, 14DBF, 3DBF respectively.
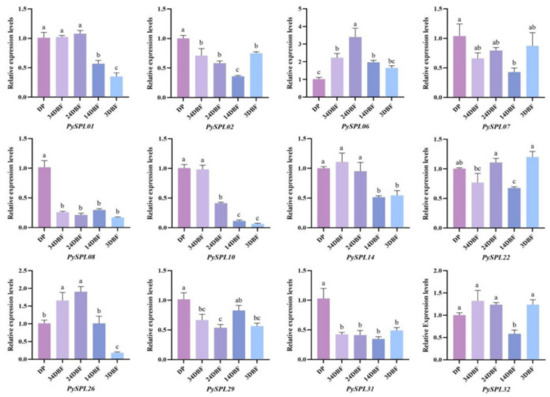
Figure 8.
Expression of PySPLs in flower buds at different flower developmental stages in Prunus × yedoensis ’Somei-yoshino’ by qRT-PCR. DP:dormant period,34DBF:34 day before flowering, 24DBF:24 day before flowering,14DBF:14 day before flowering, 3DBF:3 day before flowering. Each value is shown as average ± standard deviation from three biological replicate sampling. p < 0.05 for significance. a, b, c and d represent the level of significant difference between different samples, the difference between samples with the same letter is not significant; the greater the difference of letters, the more significant the difference between samples.
In addition, the relative expression of 12 PySPLs genes in different flower organs which were collected at the blooming stage (0DBF) was analyzed with the dormant flower buds (DP) as a control and the ACTIN gene as the internal reference by qRT-PCR (Figure 9). PySPL01, PySP06, PySPL07 and PySP31 had the highest relative expression in sepals, PySPL22 and PySPL29 had the highest relative expression in pistils and PySPL14 was expressed in all organs with insignificant differences. The expressions of PySPL08, PySPL10, PySPL26 were barely detected while it is detectable during flowering development. The expression of PySPL32 was not detected while its expression in 3DBF is almost the same as DP (Figure 7).
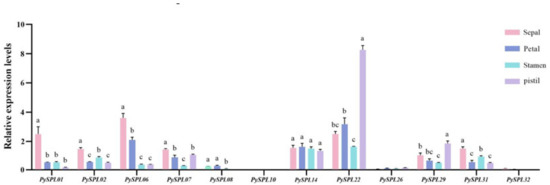
Figure 9.
Expression of PySPLs in different floral organs in Prunus × yedoensis ‘Somei-yoshino’. All samples were collected at blooming stage (0DBF). Each value is shown as average ± standard deviation from three biological replicate samplings. p < 0.05 for significance. a, b, c and d represent the level of significant difference between different samples, the difference between samples with the same letter is not significant; the greater the difference of letters, the more significant the difference between samples.
3. Discussion
With the continuous development of genomics, more and more attention has been paid to whole-genome identification of transcription factors and their gene expression profiles. SPL is a plant-specific transcription factor, and the number of its members is independent of genome size [30]. Genome-wide identification of the SPL gene family has been reported in many species and is responsible for diverse physiological activities such as floral development, stress response and phytohormone transduction. However, no systematic study has been performed in flowering cherry. This study comprehensively identified the SPL genes of Prunus × yedoensis ‘Somei-yoshino’ and carried out a systematically analysis on them.
PySPLs were distributed on different chromosomes in Prunus × yedoensis ‘Somei-yoshino’ with widely different physicochemical properties, and the distribution was not significantly associated with gene similarity or covariance. The phylogenetic tree constructed by PySPLs, AtSPLs and OsSPL could be divided into 10 subclades, among which eight subclades contained members of AtSPLs, which is the same distribution as the phylogenetic relationship of AtSPLs [31,32]. It indicates that most of the PySPLs members had high homology with AtSPLs. The conserved motifs and gene structures of members in the same subclade are roughly the same, and most of them were co-linearly related, PySPL09, PySPL21 and PySPL26 were distributed at different positions on different chromosomes with same conserved motif (Motif1/2/5/9/10) and similar gene structures. Meanwhile, different subclade members contained different conserved motifs, which may determine the diversity of functions of PySPLs. PySPL06 and PySPL11 clustered with AtSPL3, AtSPL4 and AtSPL5 in the same subclade, indicating their higher homology relationships, suggesting that PySPL06, PySPL11 may be related to flowering regulation of Prunus × yedoensis ‘Somei-yoshino’.
Gene duplication is the basis of the functional differentiation of homologous genes, is the main pathway for the generation of new functional genes and plays a pivotal role in the evolution of species [33]. Gene duplication mainly includes segmental duplication, whole-gene duplication, tandem duplication and replicative transposition [34]. Collinearity analysis indicated that there was a large number of segmental replications in the PySPLs, suggesting that segmental replication is the main way in which the PySPLs family evolved and expanded. The differences in the number of coding regions of PySPLs may be related to the loss and mutation of segmental replication during evolution [35,36].
Promoter is a DNA region located upstream of the transcription start site that can be recognized and bound by RNA polymerase. It has a transcription start site and is enriched with conserved sequences of TA (TATA region) as well as CCAAT sequences (CAAT region) and other key elements that can improve the initiation efficiency. Promoter also has some specific elements such as light-responsive cis-elements and G-boxes that can bind to specific trans-acting elements to initiate target genes and express them at specific intensities in specific times and spaces [37]. 29% of the specific elements contained in the promoter region of PySPLs, were associated with hormone response, which also indicates a strong association of SPL genes with plant phytohormone response; 45% were associated with light response and the FT/SOC1-mediated photoperiodic pathway can promote flowering in Arabidopsis thaliana by binding to the AtSPL3, AtSPL4 and AtSPL5 promoter region, which dependent on PENNYWISE (PNY) and POUND-FOOLISH (PNF) to induce its expression [16,38,39,40]. The expression of FT in Prunus × yedoensis ‘Somei-yoshino’ flower buds increased and then decreased between the end of dormancy and flowering [29], and the expression trend was basically the same as that of PySPL06, which has high homology with AtSPL3, AtSPL4 and AtSPL5, suggesting that the large number of light response elements contained in the promoter region of PySPLs may be related to FT-mediated photoperiod-induced flowering in Prunus × yedoensis ‘Somei-yoshino’ (Supplementary Materials Figure S1) [16,17,18,19,20,21].
SPL transcription factors have an important role in plant flowering, and they regulate the floral transition various Flowering induction pathway in plants [41,42,43]. The development of floral organs was largely completed by 10 days before flowering. During the previous time, the expression of most PySPLs gradually decreased, among which PySPL06 and PySPL22 had a significant increase in the middle and then decreased. NCBI-BlastP compared the three genes with Arabidopsis thaliana and found that PySPL06 had the highest homology with AtSPL3, which was the same as the conclusion of previous studies [17], indicating that PySPL06 may be involved in the regulation of flower organs development in flowering cherry; PySPL22 had the highest homology with AtSPL7 and was very prominently expressed in the pistil, which is presumed to be involved in pistil development. Furthermore, miR156 can delay plant flowering by suppressing SPL expression [22], however, the expression of some PySPLs gradually decreased during the flowering induction process, such as PySPL08, PySPL10 and PySPL31, which is in contrast to previous studies and needs further investigation.
The extent of flower organ growth depends on cell proliferation and cell expansion, and these two processes coordinate with each other to determine the size of the entire floral organ of the plant [44,45]. Over expression of miR156 increases cell size and decreases cell number, whereas over expression of AtSPL3, AtSPL4, AtSPL5 and AtSPL15 decreases cell size and increases cell number [46]. To further understand the role of these PySPLs in floral organ development, the expression of these PySPLs in different floral organs was analyzed. The final development of sepal may be related to PySPL01 and PySPL06, PySPL22 may be strongly associated with pistil development. Expression of PySPL14 is associated with the entire floral organ. The expression of PySPL26 was very low in floral organs at anthesis while it was highly expressed in the middle of flower development, probably because its expression has significantly decreased before the onset of anthesis, so its function on floral organ development needs to be further explored.
4. Materials and Methods
4.1. Experimental Materials
Samples of flower buds and flower organs were collected in Beijing from January to April 2022 (Figure 7). All samples were frozen immediately in liquid nitrogen and stored at −80 °C for gene expression pattern analysis.
The genome data of Prunus × yedoensis ‘Somei-yoshino’ was downloaded from GDR (https://www.rosaceae.org/,accessed on 3 March 2022) [47], the genome-wide data of Arabidopsis thaliana, and gene sequences of AtSPL family of Arabidopsis thaliana were downloaded from the Arabidopsis thaliana database TAIR (https://www.arabidopsis.org/, accessed on 3 March 2022), the gene sequences and protein sequences of OsSPLs family were downloaded from the Oryza sativa Genome database RGAP (http://rice.uga.edu/, accessed on 3 March 2022).
4.2. Identification and Chromosomal Location of PySPLs
The protein sequences of 16 AtSPLs were used as a reference in Genome protein database of Prunus × yedoensis ‘Somei-yoshino’ (https://www.rosaceae.org/, accessed on 5 March 2022) for BlastP Searches. Meanwhile, SBP DNA-binding domain (PF031100) from Pfam database (http://pfam.xfam.org/search, accessed on 6 March 2022) was used to identify PySPLs by Hmmer Search. Shared candidate PySPL sequences of two results were confirmed with the NCBI CDD (https://www.ncbi.nlm.nih.gov/, accessed on 9 March 2022) and SMART program (http://smart.embl-heidelberg.de/, accessed on 9 March 2022) for complete domain analysis [48,49]. Redundant candidates were removed, and the members of the PySPLs gene family were finally determined. The protein sequence was submitted to the ExPASy server (https://web.expasy.org/protparam/, accessed on 10 March 2022) to analyze its physicochemical properties and Cell-Ploc2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/, accessed on 10 March 2022) was used to predict subcellular localization [50]. The location of PySPLs on chromosome was analyzed and mapping by TBtools software [51].
4.3. Phylogenetic Analysis of PySPLs
The protein sequences of Prunus × yedoensis ‘Somei-yoshino’, Arabidopsis thaliana and Oryza sativa were aligned by the MUSCLE software with the default parameters. The NJ phylogenetic tree was constructed using MEGA11 [52] with 1000 bootstrap replicates 50% partial deletion in Gaps/Missing data treatment. iTOL was used to modify the NJ phylogenetic tree for better visualization [53].
4.4. Conserved Domains, Conserved Motifs and Gene Structure Analysis of PySPLs
The MEGA11 software was used to multiple sequence alignment analysis based on the amino acid sequence of PySPLs by the neighbor-joining method with 1000 bootstrap replicates and the conserved domain sequences were submitted to the Weblogo3 (http://weblogo.threeplusone.com/, accessed on 13 March 2022) for analysis and visualization [54]. MEME online software (https://meme-suite.org/meme/meme/, accessed on 13 March 2022) was used to confirm conserved motifs in PySPLs protein sequences [55]. Finally, based on the Prunus × yedoensis ‘Somei-yoshino’ genome database, the TBtools software was used to analyze the gene structure of all PySPLs [51].
4.5. Cis-acting Elements Analysis of PySPLs Promoter
Based on the genomic data of Prunus × yedoensis ‘Somei-yoshino’, promoter sequences (2000 bp upstream sequences of the start codon) of PySPLs were extracted using the TBtools software and submitted to Plantcare (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 16 March 2022) to predict the function and number of cis-acting elements. Excel was used to analyze the data and draw the analysis graph [56].
4.6. Syntenic Analysis of PySPLs
The duplication pattern of PySPLs was analyzed by using One Step MCScanx program of TBtools software and visualized by the Advance Circos package of TBtools [51].
4.7. qRT-PCR
To know the expression of PySPLs in the flowering induction of Prunus × yedoensis ‘Somei-Yoshino’, based on the phylogenetic relationship, conserved motif and gene structure of all PySPLs, 12 PySPLs were screened out, and primers were designed using Primer premier 5.0 according to CDS sequences (Supplementary Materials Table S3), and all primers were checked by primer-Blast of NCBI and primer check of TBtools. The ACTIN gene was used as the internal reference. Total RNA was extracted using Rapid Plant RNA Extraction Kit and reverse transcribed into cDNA by Reverse Transcription Kit, all reagents were provided by Aidlab Biotechnologies Co., Ltd. (Beijing, China). qRT-PCR was performed on qTOWER2.2 provided by Analytik Jena AG (Beijing, China) with Takara’s SYBR® Premix Ex TaqTM II provided by Takara (Beijing, China). Each sample was repeated three times. The relative expression of genes was calculated by the 2−ΔΔCt method (1):
melting curves and standard curves were checked to ensure amplification efficiency. Data processing was performed with Excel, graphs were drawn by GraphPad Prism 9 and significance analysis was performed with ANOVA by SPSS 26, p < 0.05 for significance.
Relative expression level = 2−ΔΔCt
ΔΔCt = ΔCt (test sample) − ΔCt (standard sample)
ΔCt = Ct (Target gene) − Ct (Internal reference gene)
ΔΔCt = ΔCt (test sample) − ΔCt (standard sample)
ΔCt = Ct (Target gene) − Ct (Internal reference gene)
5. Conclusions
In this study, we identified and analyzed 32 PySPLs in the flowering cherry variety Prunus × yedoensis ‘Somei-yoshino’ by bioinformatics and conducted a preliminary investigation on its role in the floral transition and flower development of Prunus × yedoensis ‘Somei-yoshino’, which provided a genetic resource and a certain basis for further exploring the role of SPL transcription factors in the flowering regulation of flowering cherry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231710052/s1.
Author Contributions
L.G. and T.L. conceptualized and designed the experiment; L.G. and T.L. performed the laboratory experiments and statical analysis; L.G. and T.L. wrote the original draft, revised the manuscript; Y.L. funding acquisition and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Center of China State Forestry and Grassland Administration (Grant number: 2016-LY-107) and China National Natural Science Foundation (Grant number: 31672190).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luscombe, N.M.; Austin, S.E.; Berman, H.M.; Thornton, J.M. An overview of the structures of protein-DNA complexes. Genome Biol. 2000, 1, reviews001.1. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kigawa, T.; Inou, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Nunokawa, E.; et al. A novel zinc-binding motif revealed by solution structures of DNA-binding do-mains of Arabidopsis SBP-family transcription factors. J. Mol. Biol. 2004, 337, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Jach, G.; Saedler, H.; Huijser, P. Functional dissection of the plant-specific sbp-domain: Overlap of the DNA-binding and nuclear localization domains. J. Mol. Biol. 2005, 352, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Saedler, H.; Huijser, P. A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene squamosa. Mol. Gen. Genet. MGG 1996, 250, 7–16. [Google Scholar] [CrossRef]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef]
- Hou, H.M.; Yan, Q.; Wang, X.P.; Xu, H. A sbp-box gene vpsbp5 from Chinese wild vitis species responds to erysiphe necator and defense signaling molecules. Plant Mol. Biol. Report. 2013, 31, 1261–1270. [Google Scholar] [CrossRef]
- Chao, L.; Liu, Y.; Chen, D.; Xue, X.; Mao, Y.; Chen, X. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermo tolerance at reproductive stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef]
- Martin, R.C.; Asahina, M.; Liu, P.P.; Kristof, J.R.; Coppersmith, J.L.; Pluskota, W.E.; Bassel, G.W.; Goloviznina, N.A.; Nguyen, T.T.; Martinez-Andújar, C.; et al. The microRNA156 and micro-RNA172 gene regulation cascades at post-germinative stages in Arabidopsis. Seed Sci. Res. 2010, 20, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.; Salinas, M.; Höhmann, S.; Berndtgen, R.; Huijser, P. miR156-targeted and nontargeted SBP-box transcription factors act in concert to secure male fertility in Arabidopsis. Plant Cell 2010, 22, 3935–3950. [Google Scholar] [CrossRef]
- Cai, C.; Guo, W.; Zhang, B. Genome-wide identification and characterization of SPL transcription factor family and their evolution and expression profiling analysis in cotton. Sci. Rep. 2018, 8, 762. [Google Scholar] [CrossRef]
- Li, C.; Lu, S. Molecular characterization of the SPL gene family in Populus trichocarpa. BMC Plant Biol. 2014, 14, 131. [Google Scholar] [CrossRef]
- Li, X.; Lin, E.; Huang, H.; Niu, M.; Tong, Z.; Zhang, J. Molecular characterization of SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) gene family in Betula luminifera. Front. Plant Sci. 2018, 9, 608. [Google Scholar] [CrossRef]
- Shao, F.; Lu, Q.; Wilson, I.W.; Qiu, D. Genome-wide identification and characterization of the SPL gene family in Ziziphus jujuba. Gene 2017, 627, 315–321. [Google Scholar] [CrossRef]
- Shruti, L.; Leo Bryan, P.; Harley, S. Regulation of the SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE genes/microRNA156 module by the homeodomain proteins PENNYWISE and POUND-FOOLISH in Arabidopsis. Mol. Plant 2011, 4, 1123–1132. [Google Scholar] [CrossRef]
- Cardon, G.H.; Höhmann, S.; Nettesheim, K.; Saedler, H.; Huijser, P. Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: A novel gene involved in the floral transition. Plant J. 1997, 12, 367–377. [Google Scholar] [CrossRef]
- Hou, H.; Yan, X.; Sha, T.; Yan, Q.; Wang, X. The sbp-box gene vpsbp11 from Chinese wild vitis is involved in floral transition and affects leaf development. Int. J. Mol. Sci. 2017, 18, 1493. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Yang, Y.; Chen, X.; Chen, G. Function annotation of an sbp-box gene in Arabidopsis based on analysis of co-expression networks and promoters. Int. J. Mol. Sci. 2009, 10, 116–132. [Google Scholar] [CrossRef]
- Jung, J.; Ju, Y.; Seo, P.J.; Lee, J.; Prak, C. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2011, 69, 577–588. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, J.H.; Kim, W.; Jung, H.S.; Huijser, P.; Ahn, J.H. The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module regulates ambient temperature-responsive flowering via LOWERING LOCUS T in Arabidopsis. Plant Physiol. 2012, 159, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of mir156 and mir172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, H.; Wang, J.; Yang, W.; Zhang, Q. Identification and profiling of novel and conserved micrornas during the flower opening process in Prunus mume via deep sequencing. Mol. Genet. Genom. 2014, 289, 169–183. [Google Scholar] [CrossRef]
- Preston, J.C.; Jorgensen, S.A.; Orozco, R.; Hileman, L.C. Paralogous squamosa promoter binding protein-like (SPL) genes differentially regulate leaf initiation and reproductive phase change in petunia. Planta 2016, 243, 429–440. [Google Scholar] [CrossRef]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of squamosa promoter-binding-like transcription factors and microrna156 in rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef]
- Masahito, S.; Tomotsugu, K.; Nobutaka, M.; Masaru, O. Arabidopsis sbp-box genes SPL10, SPL11 and SPL2 control morphological change in association with shoot maturation in the reproductive phase. Plant Cell Physiol. 2009, 50, 2133. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Kohalmi, S.E.; Amyot, L.; Hannoufa, A. Squamosa promoter binding protein-like 2 controls floral organ development and plant fertility by activating asymmetric leaves 2 in Arabidopsis thaliana. Plant Mol. Biol. 2016, 92, 661–674. [Google Scholar] [CrossRef]
- Shirasawa, K.; Esumi, T.; Hirakawa, H.; Tanaka, H.; Itai, A.; Ghelfi, A.; Nagasaki, H.; Isobe, S. Phased genome sequence of an interspecific hybrid flowering cherry, ‘somei-yoshino’ (Cerasus × Yedoensis). DNA Res. 2019, 26, 1–29. [Google Scholar] [CrossRef]
- Song, J.; Ma, D.; Yin, J.; Yang, L.; Gao, C. Genome-wide characterization and expression profiling of squamosa promoter binding protein-like (SBP) transcription factors in wheat (Triticum aestivum L.). Agronomy 2019, 9, 527. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, X.; Gu, S.; Hu, Z.; Hua, X.; Xu, C. Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 2008, 407, 1–11. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Functional evolution in the plant squamosa-promoter binding protein-like (SPL) gene family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef]
- Hideki, I.; Fyodor, K. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010, 11, 97–108. [Google Scholar] [CrossRef]
- Michael, F. Bias in Plant Gene Content Following Different Sorts of Duplication: Tandem, Whole-Genome, Segmental, or by Transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef]
- Conant, G.C.; Wolfe, K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, S. Unraveling the Distribution and Evolution of miR156-targeted SPLs in Plants by Phylogenetic Analysis. Plant Divers. Resour. 2012, 34, 33–46. [Google Scholar] [CrossRef]
- Jores, T.; Tonnies, J.; Wrightsman, T.; Buckler, E.S.; Cuperus, J.T.; Fields, S.; Queitsch, C. Synthetic promoter designs enabled by a comprehensive analysis of plant core promoters. Nat. Plants 2021, 7, 842–855. [Google Scholar] [CrossRef]
- Lee, J.; Oh, M.; Park, H.; Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef]
- Schmid, M. Dissection of floral induction pathways using global expression analysis. Development 2003, 130, 6001–6012. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. SQUAMOSA-PROMOTER BINDING PROTEIN 1 initiates flowering in Antirrhinum majus through the activation of meristem identity genes. Plant J. 2010, 62, 704–712. [Google Scholar] [CrossRef]
- Matsoukas, I.G. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front. Genet. 2014, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Blümel, M.; Dally, N.; Jung, C. Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. Flor-id: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2015, 44, D1167–D1171. [Google Scholar] [CrossRef] [PubMed]
- Beemster, G.T.S.; Fiorani, F.; Inzé, D. Cell cycle: The key to plant growth control? Trends Plant Sci. 2003, 8, 154–158. [Google Scholar] [CrossRef]
- Mizukami, Y. A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant Biol. 2001, 4, 533–539. [Google Scholar] [CrossRef]
- Usami, T.; Horiguchi, G.; Yano, S.; Tsukaya, H. The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development 2009, 136, 955–964. [Google Scholar] [CrossRef]
- Jung, S.; Lee, T.; Cheng, C.-H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 years of GDR: New data and functionality in the Genome Database for Rosaceae. Nucleic Acids Res. 2019, 47, D1137–D1145. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Farideh, C.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Marc, G.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, 265–268. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. Smart: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef]
- Chou, K.; Shen, H. Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Sci. 2010, 2, 1090–1103. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Xia, R. Tbtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (itol) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; De Peer, Y.V.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).