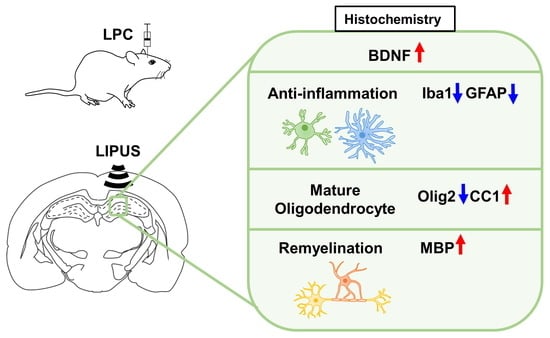
Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis
Abstract
:1. Introduction
2. Results
2.1. Ultrasound Treatment Enhances Remyelination
2.2. Ultrasound Treatment Reduces Astrocytic Activation and the Density of Microglia in the Vicinity of the Demyelination Lesion
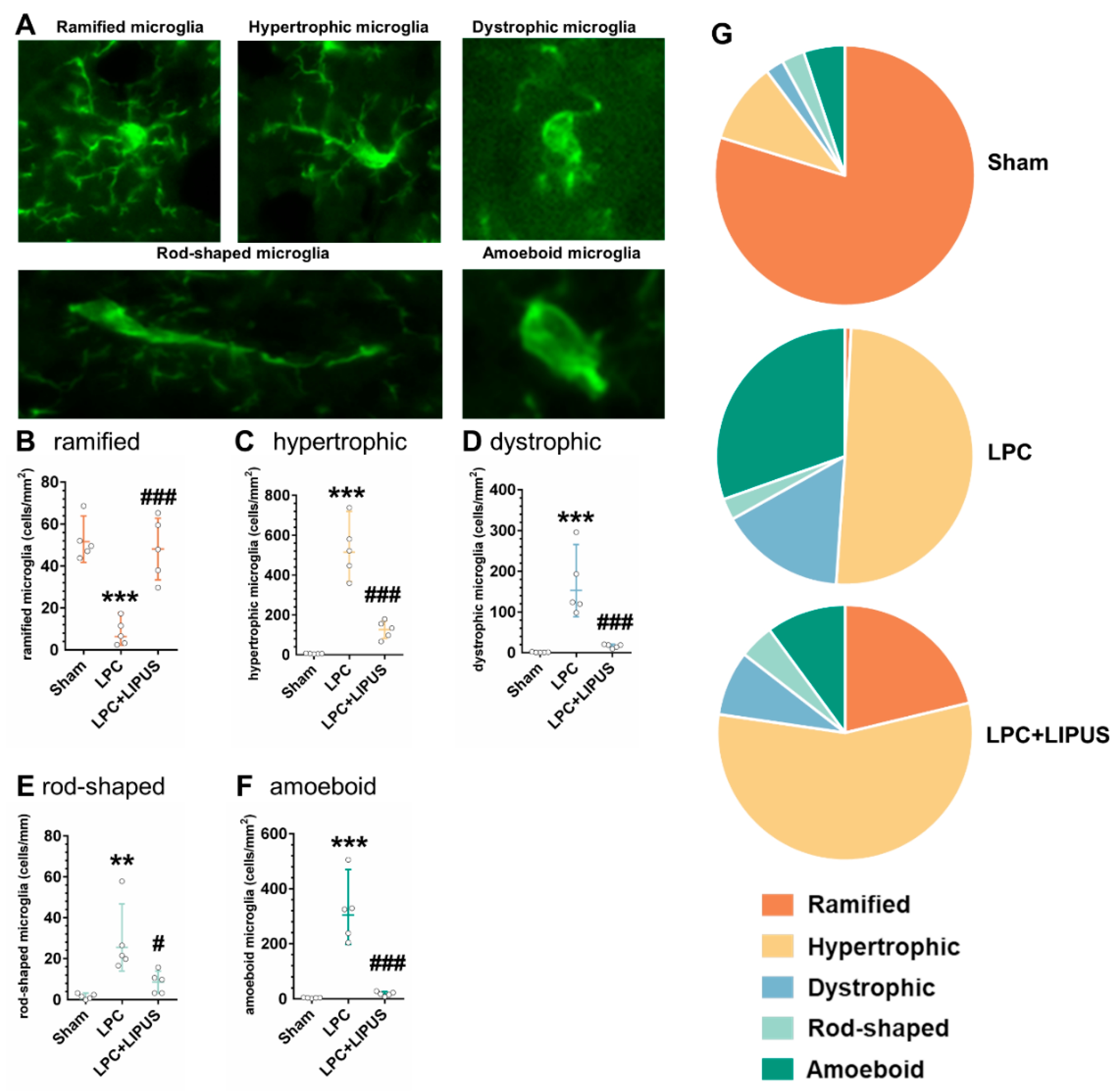
2.3. Microglial Phenotypes in the Demyelination Lesion of the Hippocampus
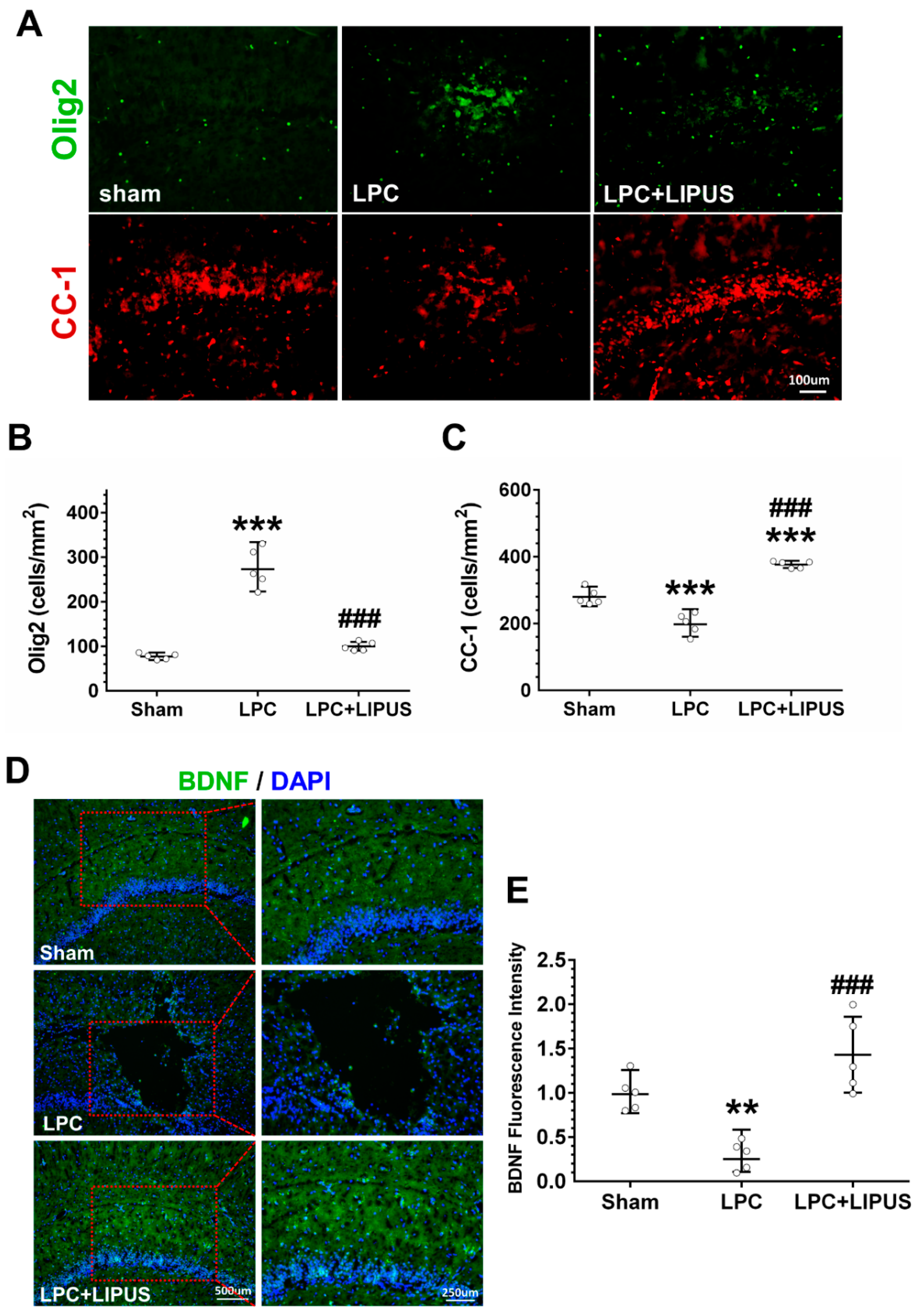
2.4. Ultrasound Treatment Enhances the Maturation of Oligodendrocytes
3. Discussion
4. Materials and Methods
4.1. Toxic Model of Demyelination and Remyelination
4.2. TUS Setup and Treatment Protocols
4.3. Tissue Processing
4.4. Immunofluorescence
4.5. Statistical Analysis
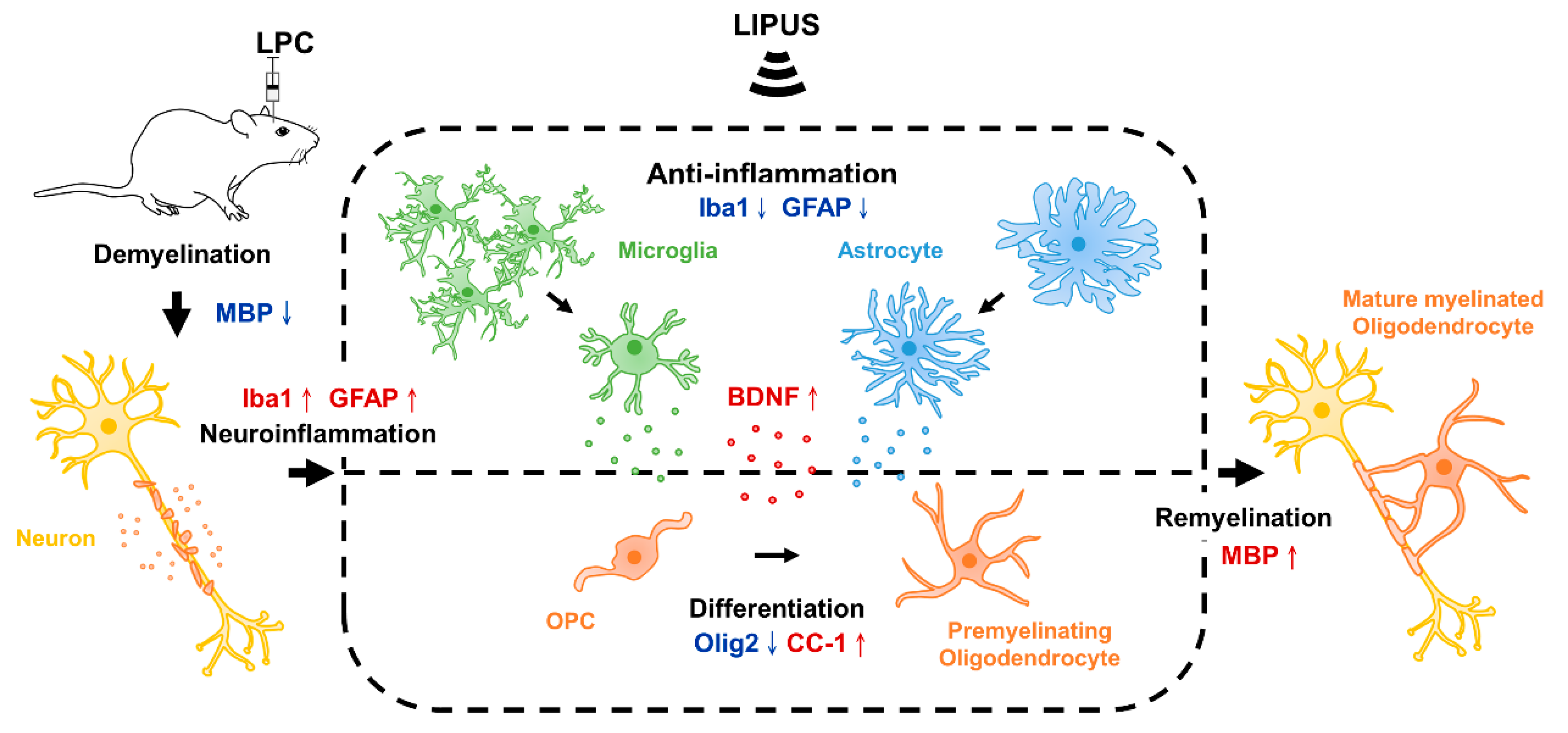
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawcer, S.; Franklin, R.J.; Ban, M. Multiple sclerosis genetics. Lancet Neurol. 2014, 13, 700–709. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Barkhof, F.; Bodini, B.; De Stefano, N.; Golay, X.; Nicolay, K.; Pelletier, D.; Pouwels, P.J.; Smith, S.A.; Wheeler-Kingshott, C.A.; et al. Pathogenesis of multiple sclerosis: Insights from molecular and metabolic imaging. Lancet Neurol. 2014, 13, 807–822. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132 Pt 5, 1175–1189. [Google Scholar] [CrossRef]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Bruck, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128, 2705–2712. [Google Scholar] [CrossRef]
- Mills, J.H.; Kim, D.G.; Krenz, A.; Chen, J.F.; Bynoe, M.S. A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J. Immunol. 2012, 188, 5713–5722. [Google Scholar] [CrossRef]
- Behrangi, N.; Fischbach, F.; Kipp, M. Mechanism of Siponimod: Anti-Inflammatory and Neuroprotective Mode of Action. Cells 2019, 8, 24. [Google Scholar] [CrossRef]
- Shinomiya, T.; Li, X.K.; Amemiya, H.; Suzuki, S. An immunosuppressive agent, FTY720, increases intracellular concentration of calcium ion and induces apoptosis in HL-60. Immunology 1997, 91, 594–600. [Google Scholar] [CrossRef]
- Rommer, P.S.; Zettl, U.K. Managing the side effects of multiple sclerosis therapy: Pharmacotherapy options for patients. Expert Opin. Pharm. 2018, 19, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Ocrelizumab: A Review in Multiple Sclerosis. Drugs 2022, 82, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, L.E.; Feng, J.; Clayton, B.L.L.; Oh, S.H.; Sakaie, K.; Tesar, P.J.; Wang, Y.; Cohen, J.A. Developing therapeutic strategies to promote myelin repair in multiple sclerosis. Expert Rev. Neurother. 2019, 19, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Vachapova, V.; Shihman, B.; Miler, A.; Karni, A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: Reversal by glatiramer acetate. J. Neuroimmunol. 2005, 167, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, C.G.; VonDran, M.W.; Stillman, A.A.; Huang, Y.; Hempstead, B.L.; Dreyfus, C.F. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8186–8196. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Curran, G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef]
- Chen, T.T.; Lan, T.H.; Yang, F.Y. Low-Intensity Pulsed Ultrasound Attenuates LPS-Induced Neuroinflammation and Memory Impairment by Modulation of TLR4/NF-kappaB Signaling and CREB/BDNF Expression. Cereb. Cortex 2019, 29, 1430–1438. [Google Scholar] [CrossRef]
- Liu, S.H.; Lai, Y.L.; Chen, B.L.; Yang, F.Y. Ultrasound Enhances the Expression of Brain-Derived Neurotrophic Factor in Astrocyte Through Activation of TrkB-Akt and Calcium-CaMK Signaling Pathways. Cereb. Cortex 2017, 27, 3152–3160. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, L.; Xia, X.; Lin, Z.; Zhou, W.; Pang, N.; Bian, T.; Yuan, T.; Niu, L.; Zheng, H. Transcranial Ultrasound Stimulation Suppresses Neuroinflammation in a Chronic Mouse Model of Parkinson’s Disease. IEEE Trans. Bio-Med. Eng. 2021, 68, 3375–3387. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Wang, Z.; Wang, X.; Yan, J.; Li, X. The Effect of Low-Intensity Transcranial Ultrasound Stimulation on Behavior in a Mouse Model of Parkinson’s Disease Induced by MPTP. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1017–1021. [Google Scholar] [CrossRef]
- Xu, T.; Lu, X.; Peng, D.; Wang, G.; Chen, C.; Liu, W.; Wu, W.; Mason, T.J. Ultrasonic stimulation of the brain to enhance the release of dopamine—A potential novel treatment for Parkinson’s disease. Ultrason. Sonochem. 2020, 63, 104955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Niu, L.; Meng, L.; Lin, Z.; Zou, J.; Xia, X.; Huang, X.; Zhou, W.; Bian, T.; Zheng, H. Noninvasive Ultrasound Deep Brain Stimulation for the Treatment of Parkinson’s Disease Model Mouse. Research 2019, 2019, 1748489. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Chen, R.C.; Lu, W.W.; Liu, S.H.; Yang, F.Y. Protective effects of low-intensity pulsed ultrasound on aluminum-induced cerebral damage in Alzheimer’s disease rat model. Sci. Rep. 2015, 5, 9671. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Chang, C.W.; Lee, Y.H.; Yang, F.Y. Protective Effect of Low-Intensity Pulsed Ultrasound on Memory Impairment and Brain Damage in a Rat Model of Vascular Dementia. Radiology 2017, 282, 113–122. [Google Scholar] [CrossRef]
- Olmstead, T.A.; Chiarelli, P.A.; Griggs, D.J.; McClintic, A.M.; Myroniv, A.N.; Mourad, P.D. Transcranial and pulsed focused ultrasound that activates brain can accelerate remyelination in a mouse model of multiple sclerosis. J. Ther. Ultrasound. 2018, 6, 11. [Google Scholar] [CrossRef]
- Kalakh, S.; Mouihate, A. Demyelination-Induced Inflammation Attracts Newly Born Neurons to the White Matter. Mol. Neurobiol. 2017, 54, 5905–5918. [Google Scholar] [CrossRef]
- Kalakh, S.; Mouihate, A. The promyelinating properties of androstenediol in gliotoxin-induced demyelination in rat corpus callosum. Neuropathol. Appl. Neurobiol. 2015, 41, 964–982. [Google Scholar] [CrossRef]
- Azin, M.; Goudarzvand, M.; Mirnajafi-Zadeh, J.; Javan, M. Field potential recording from rat hippocampus provides a functional evaluation method for assessing demyelination and myelin repair. Neurol. Res. 2013, 35, 837–843. [Google Scholar] [CrossRef]
- Hall, S.M. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J. Cell Sci. 1972, 10, 535–546. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Eames, R.A.; Smith, K.J.; McDonald, W.I. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J. Neurol. Sci. 1977, 33, 31–43. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.C.; Carrier, M.; Tremblay, M.E. Morphology of Microglia Across Contexts of Health and Disease. Methods Mol. Biol. 2019, 2034, 13–26. [Google Scholar]
- Mei, F.; Wang, H.; Liu, S.; Niu, J.; Wang, L.; He, Y.; Etxeberria, A.; Chan, J.R.; Xiao, L. Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 8454–8462. [Google Scholar] [CrossRef]
- Dolgova, N.; Wei, Z.; Spink, B.; Gui, L.; Hua, Q.; Truong, D.; Zhang, Z.; Zhang, Y. Low-Field Magnetic Stimulation Accelerates the Differentiation of Oligodendrocyte Precursor Cells via Non-canonical TGF-beta Signaling Pathways. Mol. Neurobiol. 2021, 58, 855–866. [Google Scholar] [CrossRef]
- Braun, R.; Klein, R.; Walter, H.L.; Ohren, M.; Freudenmacher, L.; Getachew, K.; Ladwig, A.; Luelling, J.; Neumaier, B.; Endepols, H.; et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp. Neurol. 2016, 279, 127–136. [Google Scholar] [CrossRef]
- McTigue, D.M.; Horner, P.J.; Stokes, B.T.; Gage, F.H. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 5354–5365. [Google Scholar] [CrossRef]
- Ikeda, O.; Murakami, M.; Ino, H.; Yamazaki, M.; Koda, M.; Nakayama, C.; Moriya, H. Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J. Neuropathol. Exp. Neurol. 2002, 61, 142–153. [Google Scholar] [CrossRef]
- Linker, R.; Gold, R.; Luhder, F. Function of neurotrophic factors beyond the nervous system: Inflammation and autoimmune demyelination. Crit. Rev. Immunol. 2009, 29, 43–68. [Google Scholar] [CrossRef]
- Fletcher, J.L.; Wood, R.J.; Nguyen, J.; Norman, E.M.L.; Jun, C.M.K.; Prawdiuk, A.R.; Biemond, M.; Nguyen, H.T.H.; Northfield, S.E.; Hughes, R.A.; et al. Targeting TrkB with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 7088–7099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azin, M.; Mirnajafi-Zadeh, J.; Javan, M. Fibroblast Growth Factor-2 Enhanced The Recruitment of Progenitor Cells and Myelin Repair in Experimental Demyelination of Rat Hippocampal Formations. Cell J. 2015, 17, 456–540. [Google Scholar] [PubMed]
- Darmani, G.; Bergmann, T.O.; Butts Pauly, K.; Caskey, C.F.; de Lecea, L.; Fomenko, A.; Fouragnan, E.; Legon, W.; Murphy, K.R.; Nandi, T.; et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 2022, 135, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Sarica, C.; Nankoo, J.F.; Fomenko, A.; Grippe, T.C.; Yamamoto, K.; Samuel, N.; Milano, V.; Vetkas, A.; Darmani, G.; Cizmeci, M.N.; et al. Human Studies of Transcranial Ultrasound neuromodulation: A systematic review of effectiveness and safety. Brain Stimul. 2022, 15, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Darmani, G.; Fomenko, A.; Xia, X.; Tran, S.; Nankoo, J.F.; Shamli Oghli, Y.; Wang, Y.; Lozano, A.M.; Chen, R. Induction of Human Motor Cortex Plasticity by Theta Burst Transcranial Ultrasound Stimulation. Ann. Neurol. 2022, 91, 238–252. [Google Scholar] [CrossRef]
- Nicodemus, N.E.; Becerra, S.; Kuhn, T.P.; Packham, H.R.; Duncan, J.; Mahdavi, K.; Iovine, J.; Kesari, S.; Pereles, S.; Whitney, M.; et al. Focused transcranial ultrasound for treatment of neurodegenerative dementia. Alzheimers Dement. 2019, 5, 374–381. [Google Scholar] [CrossRef]
- Badran, B.W.; Caulfield, K.A.; Stomberg-Firestein, S.; Summers, P.M.; Dowdle, L.T.; Savoca, M.; Li, X.; Austelle, C.W.; Short, E.B.; Borckardt, J.J.; et al. Sonication of the anterior thalamus with MRI-Guided transcranial focused ultrasound (tFUS) alters pain thresholds in healthy adults: A double-blind, sham-controlled study. Brain Stimul. 2020, 13, 1805–1812. [Google Scholar] [CrossRef]
- Fomenko, A.; Chen, K.S.; Nankoo, J.F.; Saravanamuttu, J.; Wang, Y.; El-Baba, M.; Xia, X.; Seerala, S.S.; Hynynen, K.; Lozano, A.M.; et al. Systematic examination of low-intensity ultrasound parameters on human motor cortex excitability and behavior. eLife 2020, 9, e54497. [Google Scholar] [CrossRef]
- Sanguinetti, J.L.; Hameroff, S.; Smith, E.E.; Sato, T.; Daft, C.M.W.; Tyler, W.J.; Allen, J.J.B. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef]
- Beisteiner, R.; Matt, E.; Fan, C.; Baldysiak, H.; Schonfeld, M.; Philippi Novak, T.; Amini, A.; Aslan, T.; Reinecke, R.; Lehrner, J.; et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease-A New Navigated Focal Brain Therapy. Adv. Sci. 2020, 7, 1902583. [Google Scholar] [CrossRef]
- Monti, M.M.; Schnakers, C.; Korb, A.S.; Bystritsky, A.; Vespa, P.M. Non-Invasive Ultrasonic Thalamic Stimulation in Disorders of Consciousness after Severe Brain Injury: A First-in-Man Report. Brain Stimul. 2016, 9, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Pourabdolhossein, F.; Mozafari, S.; Morvan-Dubois, G.; Mirnajafi-Zadeh, J.; Lopez-Juarez, A.; Pierre-Simons, J.; Demeneix, B.A.; Javan, M. Nogo receptor inhibition enhances functional recovery following lysolecithin-induced demyelination in mouse optic chiasm. PLoS ONE 2014, 9, e106378. [Google Scholar] [CrossRef]
- Mousavi Majd, A.; Ebrahim Tabar, F.; Afghani, A.; Ashrafpour, S.; Dehghan, S.; Gol, M.; Ashrafpour, M.; Pourabdolhossein, F. Inhibition of GABA A receptor improved spatial memory impairment in the local model of demyelination in rat hippocampus. Behav. Brain Res. 2018, 336, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kalakh, S.; Mouihate, A. Enhanced remyelination during late pregnancy: Involvement of the GABAergic system. Sci. Rep. 2019, 9, 7728. [Google Scholar] [CrossRef]
- Xavier, L.L.; Viola, G.G.; Ferraz, A.C.; Da Cunha, C.; Deonizio, J.M.; Netto, C.A.; Achaval, M. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res. Brain Res. Protoc. 2005, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Leon Chavez, B.A.; Guevara, J.; Galindo, S.; Luna, J.; Ugarte, A.; Villegas, O.; Mena, R.; Eguibar, J.R.; Martinez-Fong, D. Regional and temporal progression of reactive astrocytosis in the brain of the myelin mutant taiep rat. Brain Res. 2001, 900, 152–155. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.-Y.; Huang, L.-H.; Wu, M.-T.; Pan, Z.-Y. Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 10034. https://doi.org/10.3390/ijms231710034
Yang F-Y, Huang L-H, Wu M-T, Pan Z-Y. Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis. International Journal of Molecular Sciences. 2022; 23(17):10034. https://doi.org/10.3390/ijms231710034
Chicago/Turabian StyleYang, Feng-Yi, Li-Hsin Huang, Meng-Ting Wu, and Zih-Yun Pan. 2022. "Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis" International Journal of Molecular Sciences 23, no. 17: 10034. https://doi.org/10.3390/ijms231710034
APA StyleYang, F.-Y., Huang, L.-H., Wu, M.-T., & Pan, Z.-Y. (2022). Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis. International Journal of Molecular Sciences, 23(17), 10034. https://doi.org/10.3390/ijms231710034