Decoding Functional High-Density Lipoprotein Particle Surfaceome Interactions
Abstract
:1. Introduction
2. Results
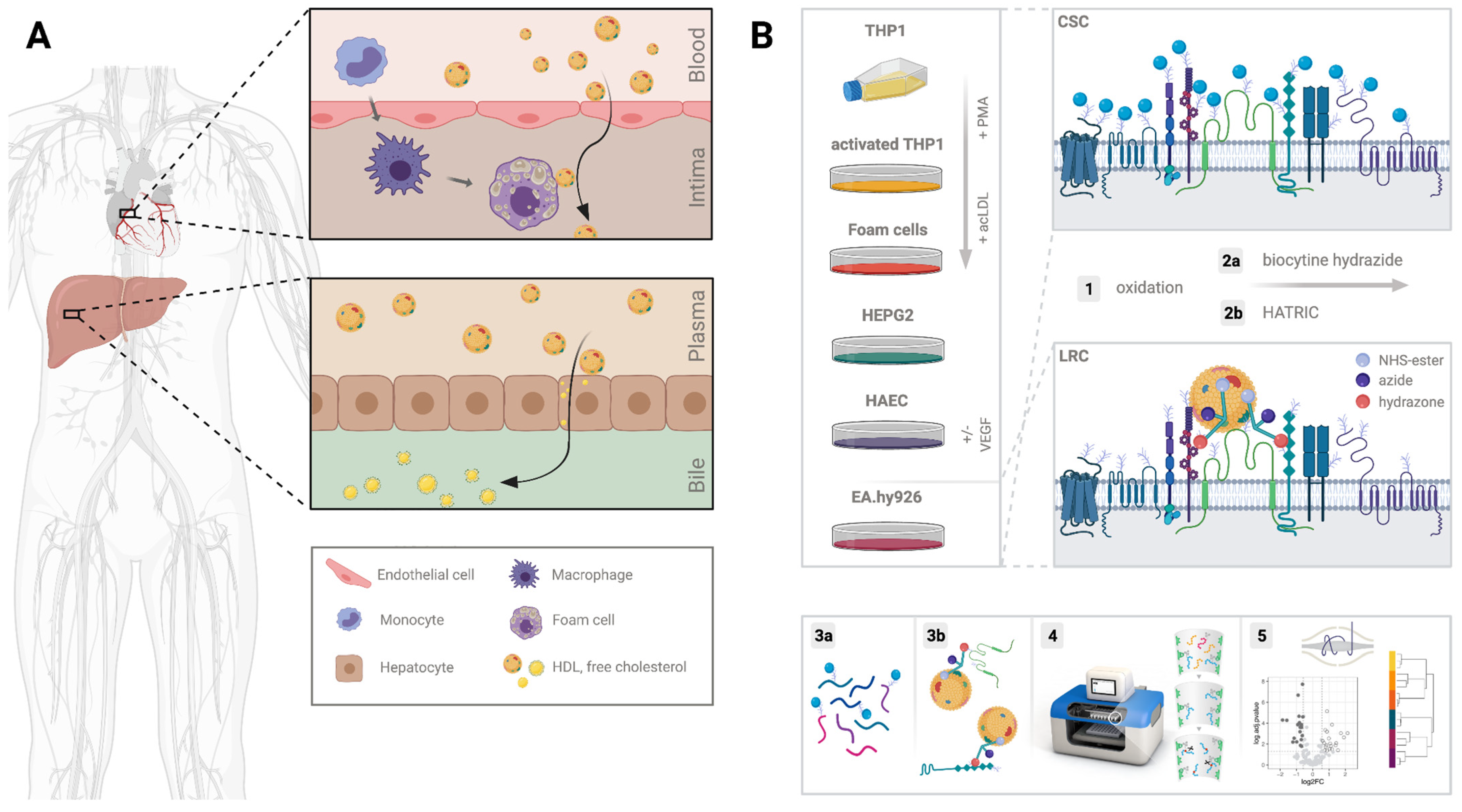
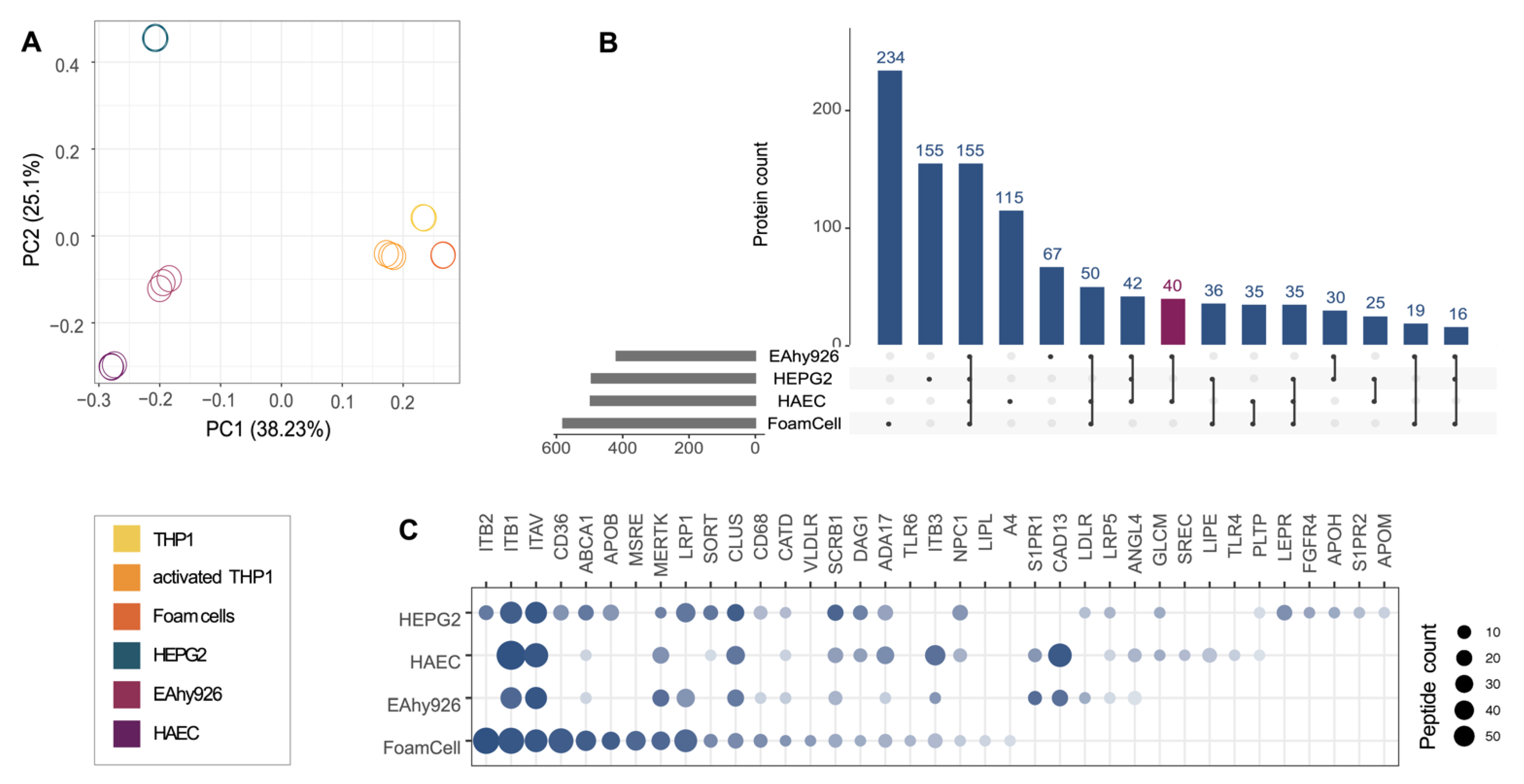
2.1. Characterization of the Potential Receptor Interaction Space of HDL
2.2. VEGF-A Treatment Triggers Reorganization of the Surfaceome of HAECs
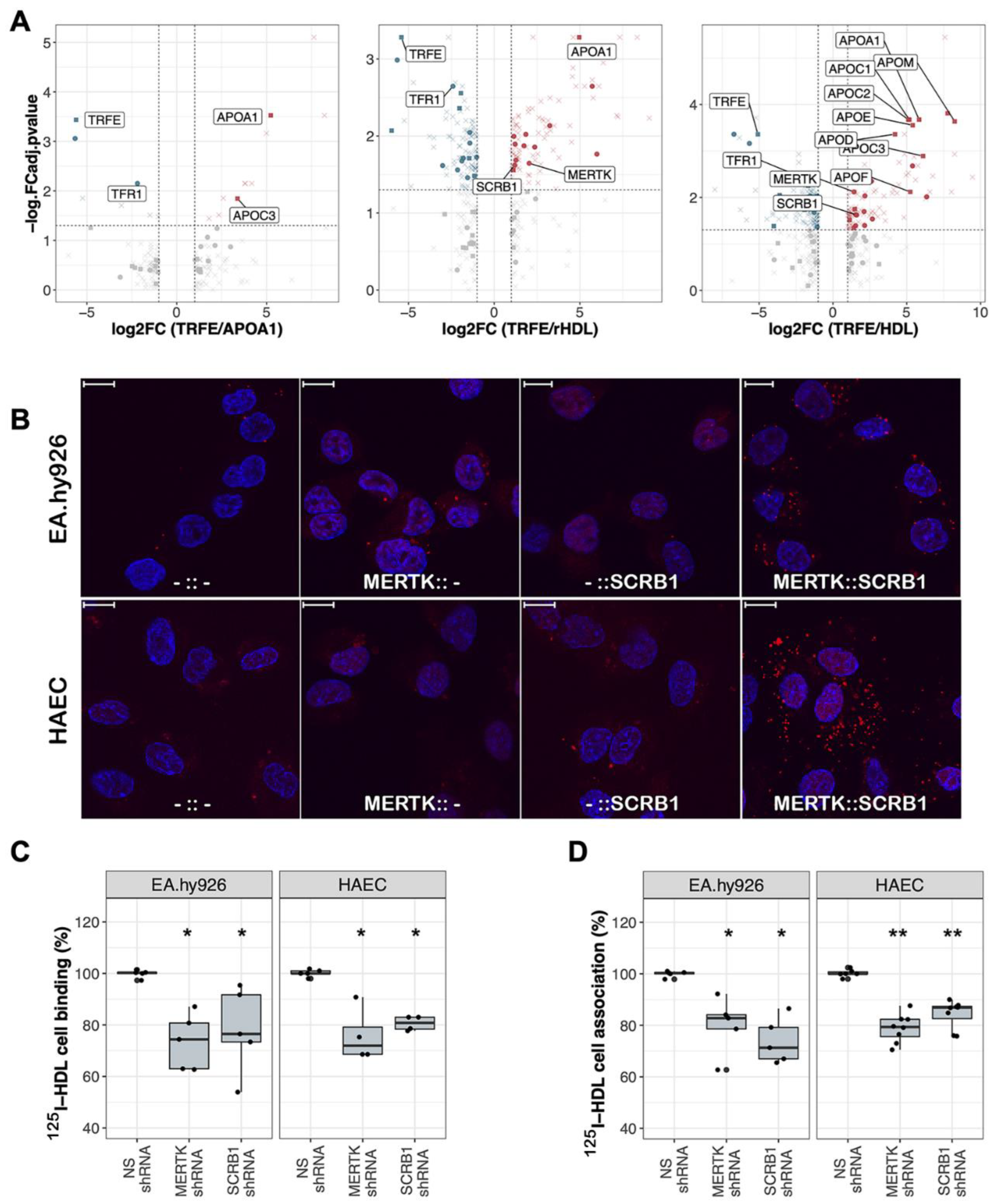
2.3. Endothelial MERTK Is a co-Receptor of HDL, Resides Proximal to SCRB1, and Contributes to HDL Binding and Uptake
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. HDL Isolation, APOA1 Purification, and LDL Isolation and Modification
4.3. Cell Surface Capture of Plasma Membrane Proteins
4.4. HATRIC-Based Identification of HDL Receptors
4.5. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analyses
4.6. Statistical Data Evaluation and Visualization
4.7. MERTK and SCRB1 Silencing
4.8. Quantitative Real-Time PCR and Western Blot
4.9. HDL Binding and Association Experiments
4.10. Proximity Ligation Assay
4.11. Nile Red Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| acLDL | Acetylated LDL |
| auto-CSC | Automated cell surface capture |
| HDL | High-density lipoprotein |
| THP1 | Human acute monocytic leukemia cells |
| HAECs | Human aortic endothelial cells |
| EA.hy926 | Human endothelial somatic hybrid cells |
| HEPG2 | Human hepatocellular carcinoma cells |
| LRC | Ligand–receptor capture |
| oxLDL | Oxidized LDL |
| PMA | Phorbol 12-myristate 13-acetate |
| PLA | Proximity ligation assay |
| rHDL | Reconstituted HDL |
| RCT | Reverse cholesterol transport |
| List of human gene and protein names discussed in this paper: | |
| ABCA1 | Phospholipid-transporting ATPase ABCA1 (ABCA1) |
| ABCA7 | Phospholipid-transporting ATPase ABCA7 (ABCA7) |
| ABCG1 | ATB-binding cassette G1 (ABCG1) |
| ACVR2A | Activin receptor type-2A (AVR2A) |
| APOA1 | Apolipoprotein A1 (APOA1) |
| APOB | Apolipoprotein B (APOB) |
| APOC3 | Apolipoprotein C-III (APOC3) |
| APOH | Beta-2-glycoprotein 1 (APOH) |
| APOM | Apolipoprotein M (APOM) |
| ATP5MF | Ecto-F1-ATPase (ATPK) |
| CD4 | T-cell surface glycoprotein CD4 (CD4) |
| CD14 | Monocyte differentiation antigen CD14 (CD14) |
| CD36 | Platelet glycoprotein 4 (CD36) |
| EGFR | Epidermal growth factor receptor (EGFR) |
| ITGAM | Integrin alpha-M (ITAM) |
| ITGAV | Integrin alpha-V (ITAV) |
| KDR | Vascular endothelial growth factor receptor 2 (VGFR2) |
| KIT | Mast/stem cell growth factor receptor Kit (KIT) |
| MEGF8 | Multiple epidermal growth factor-like domains protein 8 (MEGF8) |
| MEGF10 | Multiple epidermal growth factor-like domains protein 10 (MEGF10) |
| MERTK | Tyrosine-protein kinase Mer (MERTK) |
| MRC2 | C-type mannose receptor 2 (MRC2) |
| MSR1 | Macrophage scavenger receptor types I and II (MSRE) |
| PCDHAC1 | Protocadherin alpha-C1 (PCDC1) |
| PLTP | Phospholipid transfer protein (PLTP) |
| S1PR1 | Sphingosine 1-phosphate receptor 1 (S1PR1) |
| S1PR2 | Sphingosine 1-phosphate receptor 2 (S1PR2) |
| S1PR3 | Sphingosine 1-phosphate receptor 3 (S1PR3) |
| SCARA3 | Scavenger receptor class A member 3 (SCAR3) |
| SCARB1 | Scavenger receptor B1 (SCRB1) |
| SLC8A3 | Sodium/calcium exchanger 2 (NAC2) |
| TF | Transferrin (TRFE) |
| TFRC | Transferrin receptor protein 1 (TFR1) |
| VEGFA | Vascular endothelial growth factor A (VEGF-A) |
References
- Goetze, S.; Frey, K.; Rohrer, L.; Radosavljevic, S.; Krützfeldt, J.; Landmesser, U.; Bueter, M.; Pedrioli, P.G.A.; von Eckardstein, A.; Wollscheid, B. Reproducible Determination of High-Density Lipoprotein Proteotypes. J. Proteome Res. 2021, 20, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; von Eckardstein, A. High-Density Lipoproteins. Multifunctional but Vulnerable Protections from Atherosclerosis. Circ. J. 2013, 77, 2432–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Eckardstein, A. High Density Lipoproteins: Is There a Comeback as a Therapeutic Target? In Prevention and Treatment of Atherosclerosis; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 270, pp. 157–200. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.-A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Osto, E.; von Eckardstein, A. The Endothelium Is Both a Target and a Barrier of HDL’s Protective Functions. Cells 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.-C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol Efflux and Atheroprotection: Advancing the Concept of Reverse Cholesterol Transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [Green Version]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Röhrl, C.; Stangl, H. HDL Endocytosis and Resecretion. BBA-Mol. Cell Biol. Lipids 2013, 1831, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.; Robert, J.; Rohrer, L.; von Eckardstein, A.; Lee, W.L. Transendothelial transport of lipoproteins. Atherosclerosis 2020, 315, 111–125. [Google Scholar] [CrossRef]
- Lee, M.-H.; Appleton, K.M.; El-Shewy, H.M.; Sorci-Thomas, M.G.; Thomas, M.J.; Lopes-Virella, M.F.; Luttrell, L.M.; Hammad, S.M.; Klein, R.L. S1P in HDL Promotes Interaction between SR-BI and S1PR1 and Activates S1PR1-Mediated Biological Func-tions: Calcium Flux and S1PR1 Internalization. J. Lipid Res. 2017, 58, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [Green Version]
- Martinez, L.O.; Jacquet, S.; Esteve, J.-P.; Rolland, C.; Cabezón, E.; Champagne, E.; Pineau, T.; Georgeaud, V.; Walker, J.E.; Tercé, F.; et al. Ectopic Beta-Chain of ATP Synthase Is an Apolipoprotein A-I Receptor in Hepatic HDL Endocytosis. Nature 2003, 421, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Cavelier, C.; Ohnsorg, P.M.; Rohrer, L.; von Eckardstein, A. The β-Chain of Cell Surface F0F1 ATPase Modulates apoA-I and HDL Transcytosis through Aortic Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundert, M.; Heeren, J.; Merkel, M.; Carambia, A.; Herkel, J.; Groitl, P.; Dobner, T.; Ramakrishnan, R.; Moore, K.; Rinninger, F. Scavenger receptor CD36 mediates uptake of high density lipoproteins in mice and by cultured cells. J. Lipid Res. 2011, 52, 745–758. [Google Scholar] [CrossRef] [Green Version]
- van Oostrum, M.; Müller, M.; Klein, F.; Bruderer, R.; Zhang, H.; Pedrioli, P.G.A.; Reiter, L.; Tsapogas, P.; Rolink, A.; Wollscheid, B. Classification of Mouse B Cell Types Using Surfaceome Proteotype Maps. Nat. Commun. 2019, 10, 5734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobotzki, N.; Schafroth, M.A.; Rudnicka, A.; Koetemann, A.; Marty, F.; Goetze, S.; Yamauchi, Y.; Carreira, E.M.; Wollscheid, B. HATRIC-based identification of receptors for orphan ligands. Nat. Commun. 2018, 9, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bausch-Fluck, D.; Milani, E.S.; Wollscheid, B. Surfaceome nanoscale organization and extracellular interaction networks. Curr. Opin. Chem. Biol. 2018, 48, 26–33. [Google Scholar] [CrossRef]
- van Deventer, S.; Arp, A.B.; van Spriel, A.B. Dynamic Plasma Membrane Organization: A Complex Symphony. Trends Cell Biol. 2020, 31, 119–129. [Google Scholar] [CrossRef]
- Velagapudi, S.; Yalcinkaya, M.; Piemontese, A.; Meier, R.; Nørrelykke, S.F.; Perisa, D.; Rzepiela, A.; Stebler, M.; Stoma, S.; Zanoni, P.; et al. VEGF-A Regulates Cellular Localization of SR-BI as Well as Transendothelial Transport of HDL but Not LDL. Arter. Thromb. Vasc. Biol. 2017, 37, 794–803. [Google Scholar] [CrossRef] [Green Version]
- McShane, L.; Tabas, I.; Lemke, G.; Kurowska-Stolarska, M.; Maffia, P. TAM receptors in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1286–1295. [Google Scholar] [CrossRef] [Green Version]
- Kalxdorf, M.; Gade, S.; Christian Eberl, H.; Bantscheff, M. Monitoring Cell-surfaceN-Glycoproteome Dynamics by Quantitative Proteomics Reveals Mechanistic Insights into Macrophage Differentiation. Mol. Cell. Proteom. 2017, 16, 770–785. [Google Scholar] [CrossRef] [Green Version]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef] [PubMed]
- Franko, A.; Hartwig, S.; Kotzka, J.; Ruoß, M.; Nüssler, A.K.; Königsrainer, A.; Häring, H.-U.; Lehr, S.; Peter, A. Identification of the Secreted Proteins Originated from Primary Human Hepatocytes and HepG2 Cells. Nutrients 2019, 11, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huuskonen, J.; Olkkonen, V.; Jauhiainen, M.; Ehnholm, C. The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis 2001, 155, 269–281. [Google Scholar] [CrossRef]
- Rohrer, L.; Ohnsorg, P.M.; Lehner, M.; Landolt, F.; Rinninger, F.; von Eckardstein, A. High-Density Lipoprotein Transport Through Aortic Endothelial Cells Involves Scavenger Receptor BI and ATP-Binding Cassette Transporter G1. Circ. Res. 2009, 104, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.-I.; Ueda, K.; Yokoyama, S. Human ABCA7 Supports Apolipoprotein-mediated Release of Cellular Cholesterol and Phospholipid to Generate High Density Lipoprotein. J. Biol. Chem. 2004, 279, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Prabhudas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017, 198, 3775–3789. [Google Scholar] [CrossRef] [Green Version]
- Swertfeger, D.; Li, H.; Rebholz, S.; Zhu, X.; Shah, A.S.; Davidson, W.; Lu, L.J. Mapping Atheroprotective Functions and Related Proteins/Lipoproteins in Size Fractionated Human Plasma. Mol. Cell. Proteom. 2017, 16, 680–693. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Sansbury, B.E.; Daemen, M.J.A.P.; Dorweiler, B.; Spite, M.; Fredman, G.; Tabas, I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Investig. 2017, 127, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Tibrewal, N.; Wu, Y.; D’mello, V.; Akakura, R.; George, T.C.; Varnum, B.; Birge, R.B. Autophosphorylation Docking Site Tyr-867 in Mer Receptor Tyrosine Kinase Allows for Dissociation of Multiple Signaling Pathways for Phagocytosis of Apoptotic Cells and Down-Modulation of Lipopolysaccharide-Inducible NF-κB Transcriptional Activation. J. Biol. Chem. 2008, 283, 3618–3627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bausch-Fluck, D.; Goldmann, U.; Müller, S.; van Oostrum, M.; Müller, M.; Schubert, O.T.; Wollscheid, B. The in Silico Human Surfaceome. Proc. Natl. Acad. Sci. USA 2018, 115, E10988–E10997. [Google Scholar] [CrossRef] [Green Version]
- Zannis, V.I.; Fotakis, P.; Koukos, G.; Kardassis, D.; Ehnholm, C.; Jauhiainen, M.; Chroni, A. HDL Biogenesis, Remodeling, and Catabolism. Handb. Exp. Pharmacol. 2015, 224, 53–111. [Google Scholar]
- O’Brien, K.; Vuletic, S.; McDonald, T.O.; Wolfbauer, G.; Lewis, K.; Tu, A.-Y.; Marcovina, S.; Wight, T.N.; Chait, A.; Albers, J.J. Cell-Associated and Extracellular Phospholipid Transfer Protein in Human Coronary Atherosclerosis. Circulation 2003, 108, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiley, C.T.; Chard, L.S.; Gangeswaran, R.; Tysome, J.R.; Briat, A.; Lemoine, N.R.; Wang, Y. Vascular Endothelial Growth Factor A Promotes Vaccinia Virus Entry into Host Cells via Activation of the Akt Pathway. J. Virol. 2013, 87, 2781–2790. [Google Scholar] [CrossRef] [Green Version]
- Bartosch, B.; Vitelli, A.; Granier, C.; Goujon, C.; Dubuisson, J.; Pascale, S.; Scarselli, E.; Cortese, R.; Nicosia, A.; Cosset, F.-L. Cell Entry of Hepatitis C Virus Requires a Set of Co-Receptors That Include the CD81 Tetraspanin and the SR-B1 Scavenger Re-ceptor. J. Biol. Chem. 2003, 278, 41624–41630. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.V.; Chen, H.; Michel, T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millauer, B.; Wizigmann-Voos, S.; Schnürch, H.; Martinez, R.; Møller, N.P.H.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar] [CrossRef]
- Rousseau, S.; Houle, F.; Landry, J.; Huot, J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 1997, 15, 2169–2177. [Google Scholar] [CrossRef] [Green Version]
- Ait-Oufella, H.; Pouresmail, V.; Simon, T.; Blanc-Brude, O.; Kinugawa, K.; Merval, R.; Offenstadt, G.; Lesèche, G.; Cohen, P.L.; Tedgui, A.; et al. Defective Mer Receptor Tyrosine Kinase Signaling in Bone Marrow Cells Promotes Apoptotic Cell Accumu-lation and Accelerates Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1429–1431. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.; Wang, X.; Li, M.; Lin, P.H.; Yao, Q.; Chen, C. Human protein S inhibits the uptake of AcLDL and expression of SR-A through Mer receptor tyrosine kinase in human macrophages. Blood 2009, 113, 165–174. [Google Scholar] [CrossRef]
- Li, Y.; Gerbod-Giannone, M.-C.; Seitz, H.; Cui, D.; Thorp, E.; Tall, A.R.; Matsushima, G.K.; Tabas, I. Cholesterol-induced Apoptotic Macrophages Elicit an Inflammatory Response in Phagocytes, Which Is Partially Attenuated by the Mer Receptor. J. Biol. Chem. 2006, 281, 6707–6717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wittchen, E.S.; Monaghan-Benson, E.; Hahn, C.; Earp, H.S.; Doerschuk, C.M.; Burridge, K. The role of endothelial MERTK during the inflammatory response in lungs. PLoS ONE 2019, 14, e0225051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimojima, M.; Takada, A.; Ebihara, H.; Neumann, G.; Fujioka, K.; Irimura, T.; Jones, S.; Feldmann, H.; Kawaoka, Y. Tyro3 Family-Mediated Cell Entry of Ebola and Marburg Viruses. J. Virol. 2006, 80, 10109–10116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Gräbnitz, F.; Barandun, N.; Shen, Y.; Wendt, F.; Steiner, S.N.; Severin, Y.; Vetterli, S.U.; Mondal, M.; Prudent, J.R.; et al. Light-mediated discovery of surfaceome nanoscale organization and intercellular receptor interaction networks. Nat. Commun. 2021, 12, 7036. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifu-gally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Von Eckardstein, A.; Funke, H.; Walter, M.; Altland, K. Structural Analysis of Human Apolipoprotein AI Variants. Amino Acid Substitutions Are Nonrandomly Distributed throughout the Apolipoprotein AI Primary Structure. J. Biol. Chem. 1990, 265, 8610–8617. [Google Scholar] [CrossRef]
- E Matz, C.; Jonas, A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 1982, 257, 4535–4540. [Google Scholar] [CrossRef]
- Ahrné, E.; Glatter, T.; Viganò, C.; Von Schubert, C.; Nigg, E.; Schmidt, A. Evaluation and Improvement of Quantification Accuracy in Isobaric Mass Tag-Based Protein Quantification Experiments. J. Proteome Res. 2016, 15, 2537–2547. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
- Prüfer, K.; Muetzel, B.; Do, H.-H.; Weiss, G.; Khaitovich, P.; Rahm, E.; Pääbo, S.; Lachmann, M.; Enard, W. FUNC: A package for detecting significant associations between gene sets and ontological annotations. BMC Bioinform. 2007, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Sarbassov, D.D. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrer, L.; Cavelier, C.; Fuchs, S.; Schlüter, M.A.; Völker, W.; Von Eckardstein, A. Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2006, 1761, 186–194. [Google Scholar] [CrossRef]
- Das, R.; Ganapathy, S.; Mahabeleshwar, G.H.; Drumm, C.; Febbraio, M.; Jain, M.K.; Plow, E.F. Macrophage Gene Expression and Foam Cell Formation Are Regulated by Plasminogen. Circulation 2013, 127, 1209–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, A.; Keating, A.K.; Joung, D.; Sather, S.; Kim, G.K.; Sawczyn, K.K.; Brandão, L.; Henson, P.M.; Graham, D.K. Mer Tyrosine Kinase (MerTK) Promotes Macrophage Survival Following Exposure to Oxidative Stress. J. Leukoc. Biol. 2009, 86, 73–79. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, K.; Goetze, S.; Rohrer, L.; von Eckardstein, A.; Wollscheid, B. Decoding Functional High-Density Lipoprotein Particle Surfaceome Interactions. Int. J. Mol. Sci. 2022, 23, 9506. https://doi.org/10.3390/ijms23169506
Frey K, Goetze S, Rohrer L, von Eckardstein A, Wollscheid B. Decoding Functional High-Density Lipoprotein Particle Surfaceome Interactions. International Journal of Molecular Sciences. 2022; 23(16):9506. https://doi.org/10.3390/ijms23169506
Chicago/Turabian StyleFrey, Kathrin, Sandra Goetze, Lucia Rohrer, Arnold von Eckardstein, and Bernd Wollscheid. 2022. "Decoding Functional High-Density Lipoprotein Particle Surfaceome Interactions" International Journal of Molecular Sciences 23, no. 16: 9506. https://doi.org/10.3390/ijms23169506
APA StyleFrey, K., Goetze, S., Rohrer, L., von Eckardstein, A., & Wollscheid, B. (2022). Decoding Functional High-Density Lipoprotein Particle Surfaceome Interactions. International Journal of Molecular Sciences, 23(16), 9506. https://doi.org/10.3390/ijms23169506






