Abstract
This historical review aimed to summarize the main changes that colorectal carcinoma (CRC) staging systems suffered over time, starting from the creation of the classical Duke’s classification, modified Astler–Coller staging, internationally used TNM (T—primary tumor, N—regional lymph nodes’ status, M—distant metastases) staging system, and ending with molecular classifications and epithelial–mesenchymal transition (EMT) concept. Besides currently used staging parameters, this paper briefly presents the author’s contribution in creating an immunohistochemical (IHC)-based molecular classification of CRC. It refers to the identification of three molecular groups of CRCs (epithelial, mesenchymal and hybrid) based on the IHC markers E-cadherin, β-catenin, maspin, and vimentin. Maspin is a novel IHC antibody helpful for tumor budding assessment, which role depends on its subcellular localization (cytoplasm vs. nuclei). The long road of updating the staging criteria for CRC has not come to an end. The newest prognostic biomarkers, aimed to be included in the molecular classifications, exert predictive roles, and become more and more important for targeted therapy decisions.
1. Introduction
Colorectal cancer, being mostly colorectal carcinomas (CRC), represents the third most diagnosed cancer and the second cause of cancer-related death [1]. Over the years, numerous studies focused on various aspects regarding risk factors, carcinogenesis, diagnostic markers, procedures, staging, and therapy. Although some questions have been answered and important mechanisms have been deciphered, further research is constantly needed, aiming to discover new prognostic markers, diagnostic methods, therapeutic agents, and an updated, more optimized staging system [2,3,4].
The current review is focused on the CRC staging systems, from the classical Dukes’ classification to the TNM (T—primary tumor, N—regional lymph nodes, M—distant metastases) stages included in the latest published edition of the American Joint Committee of Cancer (AJCC), with continuous updates over the past decades, together with relevant prognostic markers used by recent studies in the search for molecular classification of CRC, including our team’s contribution to the field.
2. Methodology
For this review of internationally used staging systems, peer-reviewed publications identified on PubMed, Scopus and web of science databases using as keywords colorectal carcinoma AND “staging”, “staging system Dukes”, “staging Astler Coller”, “staging system AJCC”, and “TNM” or “molecular classification” were included. The databases were searched from inception to 4 July 2022.
Only articles written in the English language, based on human tissue studies, with available abstract and full text, were taken into consideration. Staging-related data were also extracted from older versions of the AJCC manuals available online at https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx (accessed on 3 July 2022). After the title’s evaluation, the selection required checking the abstracts and, in the end, the full-text variant of the articles was read.
After deduplication, the initial search resulted in 659 papers. After removing letters to the editor, and articles with unavailable full text in English, a number of 286 papers was selected for full-text screening and 66 of them were included in the present review.
3. Dukes-MAC Era
Staging systems are used to enable the prediction of survival, an internationally appropriate and uniform case evaluation, and treatment decision. Dukes proposed in 1932 the first staging system of CRC, starting with the rectum and then for colorectal segments [5,6]. The first variant of Dukes’ classification included three stages, based on the extent of tumor spread. Stage A represented tumors limited to the rectal wall, stage B dedicated to those that go beyond the wall, but without lymph node (LN) metastases and C for those with positive LN [5,6]. Three years later, stage C was divided into C1 and C2, depending on the location of metastatic LN—regional ones (C1) or LNs located beyond the level of hemorrhoidal/inferior mesenteric vessels ligature (Table 1) [6,7,8].

Table 1.
Localized colorectal carcinomas: Dukes MAC versus AJCC staging system.
Further subclassification and modification of these stages were conducted by Astler and Coller (MAC) in 1954. They proposed splitting former stage A into A and B1. Only superficial tumors limited to the mucosa were included in stage A and those infiltrating the submucosa, but not crossing muscularis propria in stage B1. Ex-stage B becomes B2. No metastases were included in stages A, B1 and B2. Cases with positive LNs were included in C stages, respectively, C1 and C2, corresponding to B1 and B2 with associated LN metastases (Table 1) [7,8,9].
Turnbull proposed in 1967 a new stage—stage D—for tumors with distant systemic spread or direct invasion of the peritoneum—which is partially equivalent to the TNM stage IV introduced in 1977 (Table 1 and Table 2) and kept in the AJCC manual [7,8,10,11]. Crossing of the colorectal wall or direct invasion of the surrounding structures is considered a distinct stage from 1974 when Gunderson and Sosin proposed stage B3 for cases without LN metastases and C3 for those with positive LNs (Table 1 and Table 2) [7,8,9,12].

Table 2.
Metastatic colorectal carcinomas: Dukes MAC versus AJCC staging system.
Although new parameters and sub-divisions were further included in the TNM staging system, the Dukes paradigm of considering lymph nodes as one of the strongest prognostic parameter is kept even in the 8th edition of the AJCC Manual [13].
4. TNM-Based Staging System
From 1977 until nowadays, the well-known and internationally utilized pathological TNM (pTNM) staging suffered periodical changes which are included in well-known AJCC manuals (Table 2). Continuous stage refinement represents a necessity due to differences in patient survival correlated with various parameters, proved by multiple studies [7,11,14,15]. An evidence-based medicine group was created in 2013 to establish the potentially new content’s level of evidence so that only level I-III data were included in the last edition of AJCC [11,16].
The most significant changes were added starting with the 6th edition of AJCC in 2002. Although the T, N, and M parameters were not modified, following the Dukes paradigm, cases were sub-classified based on the number of metastatic nodes and the clinical stages I, II and III was suggested to be included in the histopathological reports [13]. For therapeutic purposes, the guidelines of the European Society of Medical Oncology (ESMO) grouped patients from stage III into low (T1-3N1) and high-risk (T4 or N2). This sub-grouping serves for the length of chemotherapy (short or prolonged) and prognostic assessment [17].
Problems regarding the influence of pre-operative therapy were also firstly addressed in the 6th edition of AJCC with the addition of the letter “y” in front of the pTNM stage, with no other evaluation differences compared to untreated tumors [14]. Precise criteria for appreciation of response to chemoradiotherapy were included and perfected in the next two staging manuals, represented by Ryan’s scheme which grades tumor regression as grade 0 (complete response, with no identifiable tumor cells), 1 (a nearly complete response to therapy with evident tumor regression displaying only single tumor cells or rare small groups of tumor cells), 2 (partial response, when more than single tumor cells/small groups of tumor cells are still present, but regression is noticeable) or 3 (poor response or no response, with no tumor regression and presence of tumor cells in over 50% of the examined tissue) [11,15,18,19].
Another useful aspect refers to the presence of multiple synchronous colorectal tumors. It is represented by the symbol “(m)” inserted at the end of the pTNM stage [14].
In the last two editions of the AJCC manuals, it was included in the stage N1c for cases with the presence of tumor deposits in the absence of LN metastases (7th edition), based on a more unfavorable prognosis compared with N0 staged cases. A distinct M1c stage was proposed for the presence of peritoneal carcinomatosis (8th edition). It was based on the worse outcome of these cases compared with those spreading in other organs. The T4 stage was also sub-divided into T4a and T4b (Table 3 and Table 4) [11,16,20,21,22].

Table 3.
Changes of colorectal carcinomas staging system (pT = depth of tumor infiltration) according to AJCC (based on references [7,8,11,13,14,15] and AJCC manuals accessed at https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx (accessed on 3 July 2022)).

Table 4.
Changes of colorectal carcinomas staging system (metastatic stations) according to AJCC (based on references [7,8,11,13,14,15] and AJCC manuals accessed at https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx (accessed on 3 July 2022)).
Currently, it is indicated to include in the histopathological reports, along with the TNM stage criteria (Table 2), those prognostic parameters which can be identified after macro- or microscopic assessment. It is about the presence/absence of lymphovascular and/or perineural invasion of the tumor cells, high-grade tumor, the status of the resection margins (R0—tumor-free margins, R1—microscopically identified tumor invasion of the margins, R2—macroscopic evidence of margin infiltration), perforation, obstruction, number of examined lymph nodes and preoperative serum level of carcinoembryonic antigen (CEA). If few than 12 lymph nodes were harvested the risk of recurrence is higher, especially for poorly (G3) or undifferentiated tumors which are considered high-grade carcinomas [23].
As the systemic inflammatory response (SIR) plays role in carcinogenesis, more and more studies are focused on the prognostic role of the SIR-related parameters such as neutrophil-to-lymphocytes ratio (NLR) or lymphocytes-to-monocytes ratio (LMR). LMR represents the ratio between preoperative lymphocyte and monocyte counts assessed at baseline. LMR and NLR values are correlated with the TNM stage. High preo-operative NLR (over 3.11) and low LMR are indicators of poorer overall survival rates [24,25].
5. Macroscopic Assessment-Mesorectal Fascia
Rectal cancer accounts for one-third of all CRCs. There are some aspects that regard this segment only. Starting with the last (8th) edition of AJCC, it is recommended, for rectal carcinomas, to evaluate the quality of mesorectal fascia [7,11,14]. The peritumoral mesorectum can either be complete, nearly complete, or incomplete, based on the outer surface’s aspect (smooth, irregular, or in small quantity), presence of defects in the mesorectal adipose tissue (less than 5 mm, more than 5 mm but without exposing the outer muscle layer of the rectal wall or with visible muscularis propria), grade of coning (none, moderate, marked) and the aspect of the circumferential mesorectal resection margin (regular or irregular) [11,26]. There is a direct association between the quality of mesorectal excision and the status of circumferential margin (either open surgery or non-invasive procedures), respectively, the risk of tumor recurrence. Complete removal with intact fascia (R0 resection), which is also known as total mesorectal resection (TME) represents an independent favorable prognostic factor directly correlated with a recurrence-free survival rate [11,26,27,28]. As high NLR and low LMR were correlated with a high SRI and incomplete fascia, these pre-operatively serum indicators can guide surgeons to choose the best therapeutic approach [24].
6. Preoperative Imagistic Assessment-Particular Issues
Preoperative imagistic evaluation of CRC can be performed with computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) combined with CT (PET/CT). They have advantages and limitations. CT is useful for lymph node assessment. MRI is mainly used for checking a suspicion of relapses, especially for rectal tumors and suspected hepatic metastases. PET/CT is indicated to evaluate the whole body and check distant metastases [29].
Proper staging of CRC requires a precise evaluation of LN status. It should be performed by a transdisciplinary team and start before surgery with CT or MRI scans. The suspect LNs are evaluated based on imagistic criteria such as size (less than 5 mm, between 5 and 10 mm or greater than 10 mm), shape, contour, and heterogeneity [30]. Afterward, the LN stations map that was published in the 3rd edition of the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma can be used by the radiologist to encircle the imagistically identified LN groups (Figure 1). This adapted map is meant to assure the surgical removal of all suspect LNs, thus avoiding pathological sub-staging [29,30]. Based on such maps, new valid evidence-based results will help future staging updates [31,32,33]. CT provides a lower performance, compared with MRI, for evaluation of the depth of infiltration, especially for low-T stages [29].
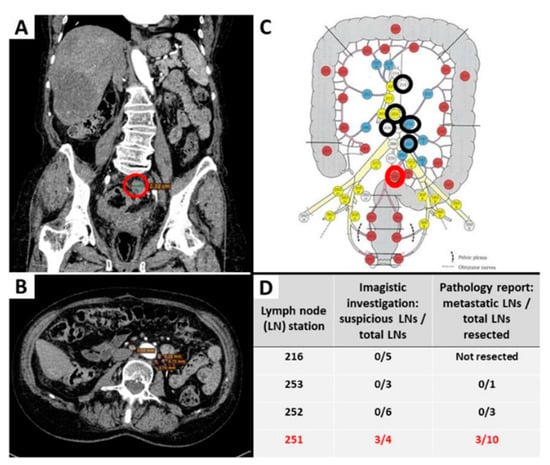
Figure 1.
Evaluation of the lymph node status before and after surgical intervention for removal of a rectal carcinoma. Examples of imagistically identified LNs are shown on abdominopelvic CT scan, coronal (A) and axial view (B). Suspect LNs were present only in the perirectal group 251, the largest one measuring 23 mm ((A,C), highlighted with a red circle), confirmed as metastatic LNs on histopathological examination (table (D)). Other identified LNs were homogenous, measuring less than 10 mm, considered non-suspicious ((B)—example of periaortic LNs), marked with a black circle on the map (C), and correlated with the absence of metastasis after microscopic evaluation (D). The map with lymph node stations was adapted by our team with permission from Yamamoto S et al. [30,31].
For rectal cancers, pelvic MRI is the gold standard imaging way for evaluation of primary tumors and local recurrences, even for cases with a large post-radiotherapy fibrotic scar. The risk of local relapse can be predicted based on the distance between the tumor and circumferential resection margins, combined with the presence or absence of extramural invasion [29].
Further changes and ways of perfecting the evaluation methods as a response to current challenges in the diagnostic and therapeutical case management are continuously being studied and new valid evidence-based results will help future staging updates [32,33,34].
For a proper MRI evaluation of the TME, the newest classification beyond TME (BTME) was recently proposed. After pelvic MRI, cases can be grouped based on their localization in the eight compartments: 1. Anterior above peritoneal reflection (sigmoid colon, small bowel, ureters, iliac vessels above peritoneal reflection, lateral pelvic sidewall fascia); 2. Anterior below Peritoneal Reflection (Genitourinary organs and pubic symphysis); 3. Central (Rectum and perirectal fat); 4. Posterior (Coccyx, pre-/retro-sacral area, sciatic nerve); 5. Lateral (Internal and external iliac vessels, lateral pelvic lymph nodes, piriformis and internal obturator muscles); 6. Infralevator (levator ani muscles, external sphincter, ischio-anal fossa); 7. Anterior urogenital (Perineal, vaginal, distal urethra, crus penis). The worse survival was reported for patients with tumors located in the first compartment (anterior below peritoneal reflection) same as for those with tumors involving multiple compartments [35].
[18F]-FDG PET combined with MR ([18F]-FDG PET/MR) was recently proved to have high specificity and sensitivity for the diagnosis of CRC, evaluation of the free margins (distance from tumors) and identification of distant metastases. Due to limited spatial resolution, the preoperative T stage cannot be properly performed with PET/CT. High specificity, but low sensitivity was also proved for N staging [29].
7. Molecular Classification
7.1. Consensus Molecular Subtype Classification
Differences in tumor behavior and response to therapy in same-stage CRC cases have increased the need for gene-expression studies and the creation of a molecular classification that would facilitate targeted therapy [2,4,36,37,38,39]. In this regard, four consensus molecular subtypes (CMS 1-4) were introduced in 2005, based on multiple molecular characteristics and the presence or absence of epithelial-mesenchymal transition (EMT) [39].
Tumors belonging to the CMS1 subtype are hypermutated, with BRAF mutant status, microsatellite instability (MSI-H), and an important immune reaction. CMS1 group is also known as MSI immune. Carcinogenesis seems to be driven via JAK-STAT and PD-1 signaling pathways [39,40]. Although the pathways are similar for MSI and MSS cases belonging to this group, MSS carcinomas’ behavior and answer to therapy are also influenced by CD8+ cytotoxic T cell infiltration amount [40].
CMS2 (canonical) and CMS3 (metabolic) represent epithelial subtypes. CMS2 is chromosomally unstable, with activation of WNT and MYC signaling pathways. CMS3 shows metabolic deregulations and KRAS mutations and comprises MSI-H and one-third of cases that are microsatellite stable (MSS). The CMS3 MSS-carcinomas are architecturally such as MSI tumors [36,37,38,39,40,41].
CRCs with stromal invasion, angiogenesis, and transforming growth factor β (TGF-ß) activation are included in the CMS4 subtype, which is also known as the mesenchymal subtype [36,37,38,39,40,41]. Hypermethylation of the miR-200 family’s promoter was associated with stimulation of the EMT process in this mesenchymal subtype, frequently diagnosed in advanced stages and associated with worse survival parameters and activation of vascular endothelial growth factor (VEGF) and TGF-ß [38,42]. A risk stratification formula based on the expression of six immune genes, recently described by Zhang et al. might become useful in the clinical management of CMS4-type CRC [43].
In one of the recent studies, a refined classification of the CMS2 and CMS3 (epithelial cases) was proposed based on intrinsic epithelial subtype (I), microsatellite instability status (M) and fibrosis (F). It was called “iCMS” or “IMF” classification but implementation in daily practice is not easy to be performed [41].
Studies confirm response and outcome differences between tumors included in the four CMSs, larger cohorts being required for any valid official changes [44,45,46,47]. CMS1, MSI immune subtype, mostly identified in CRC of the right colon, seems to respond well to immunotherapy and to show better prognosis when bevacizumab, a VEGF inhibitor, is associated with the classical treatment scheme [37,44]. Although immunotherapy shows promising results for MSI-H cases from the CMS1 group, the CMS1-MSS carcinomas do not respond to immune checkpoint inhibitors [40].
Heterogenous research results indicate better overall survival (OS) for CRCs CMS2 and CMS3 when bevacizumab or cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, is associated with classical therapy [37,44,45]. The latter also showed significant benefits when used for BRAF/RAS wild type, left-sided metastatic CRCs [42]. Adding cetuximab or irinotecan to CMS4-CRC chemotherapy appears to be more beneficial than adding bevacizumab or oxaliplatin-based therapy [47,48]. As KRAS mutations can be identified in CMS4 carcinomas, resistance to cetuximab should be considered [40].
Besides aiding the molecular classification process, microsatellite status by itself shows important diagnostic and therapeutic implications. It represents the presence of repeated sequences encompassing 1-6 nucleotides, causing mutations of the DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2), mutations that can be inherited (Lynch syndrome) or developed sporadically [36,37]. Screening for mutations of these genes or loss of IHC expression of their corresponding proteins enables the selection of MMR-deficient/MSI-high tumors, which are known to respond to fluoropyrimidine-based therapy and immunotherapy (pembrolizumab and nivolumab being recently approved by the Food and Drug Administration) [36,37,38,41].
7.2. Immunohistochemical-Based Molecular Classification
Multiple studies attempted to molecularly classify CRC using the expression of IHC antibodies for legit reasons such as cost-efficiency and availability in most pathology departments [48,49,50,51,52]. Most research studies used the following panel of antibodies: cytokeratin, CDX2 for epithelial-like tumors, FRMD6, ZEB1, HTR2B for mesenchymal-like tumors, and determination of microsatellite status [50,52,53].
These stains were not enough for the distinction between CMS2 and CMS3, which are mainly driven via the Wnt pathway [40]. Li X. et al. recently added β-catenin to the above-mentioned panel, considering positive nuclear expression an indicator of CMS2, because CTNNB1, the gene encoding β-catenin, appeared to be upregulated in this subtype [49].
Our team also focused on classifying CRC based on IHC reactions and used markers of EMT such as E-cadherin, β- catenin, vimentin, and maspin, evaluated in both tumor center and invasion front/tumor buds (Figure 2) [51,54,55]. We contoured three subtypes: epithelial (diffuse membrane expression of E-cadherin and β-catenin associated with negative vimentin), mesenchymal (loss of E-cadherin expression, positive vimentin and nuclear staining of β-catenin and maspin) and one with mixed epithelial-mesenchymal features called hybrid (epithelial-like pattern in the tumor center and mesenchymal characteristics in the invasion front), all of them exemplified in Figure 2 [51,56].
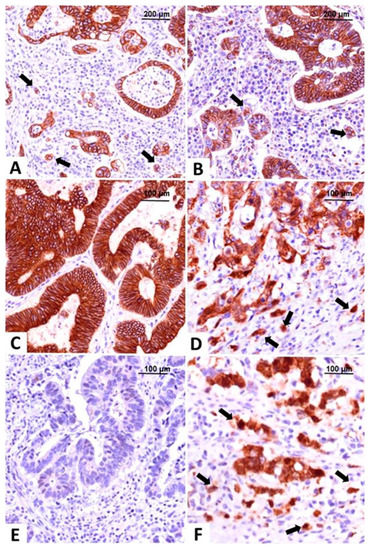
Figure 2.
Molecular classification of colorectal carcinomas based on the immunohistochemical expression of E-cadherin and β-catenin. The epithelial subtype is easily recognized by diffuse membrane staining for E-cadherin (A) and β-catenin (B), in the core and tumor buds (indicated with arrows). The intermediate, hybrid subtype, presents epithelial-type expression in the tumor center, with membrane expression of E-cadherin (C) and β-catenin (D), and buds with mesenchymal immunophenotype showing nuclear β-catenin, indicated with arrows (D). The mesenchymal subtype does not stain for E-cadherin (E) and β-catenin (F) is predominantly nuclear, in both tumor center and buds, indicated with arrows (F). Pictures from the personal collection of authors—referenced data published in 2020–2021 [51,56].
Tumor budding, defined as the single tumor cells or groups of no more than four tumor cells identified in the invasion front, represents an extensively studied parameter with a Hematoxylin-Eosin +/− cytokeratin slide-based evaluation protocol published in 2017, represents an independent prognostic marker not yet included in AJCC staging manual, but its importance and suggestion for addition in the pathological report are mentioned in oncological practice guidelines for both colon and rectal carcinomas [55,56,57,58,59,60]. For a better assessment of budding degree, our team used maspin’s expression which is in the nucleus at the level of the tumor buds and helps with their identification even on the background of an abundant inflammatory stroma [56,61,62,63].
Evaluation of subcellular maspin’s expression, combined with microsatellite status, could also be of therapeutic relevance [56,64]. Cytoplasmic staining identified in serrated MSI carcinomas might indicate favorable prognosis, while nuclear expression evaluated in microsatellite stable carcinomas is associated with high-grade tumor budding, EMT, mesenchymal subtype, worse prognosis, and could indicate response to therapy with fluorouracil [54,63,64].
Proved to be related to EMT and tumor-associated angiogenesis, maspin is opening a window for potential targeted therapy [56,62,65,66,67].
7.3. Precision Medicine
Like other tumors, it is thought that, in the near future, the therapy of CRC will be completely based on molecular diagnostic tests. Deep learning machines can already be used for the evaluation of whole slide images and establishing histological grade, budding degree or other prognostic parameters [18].
The role of pathologist needs to be revisited and next-generation sequencing platforms will replace large parts of ancillary tests. However, as most of the molecular tests are performed from paraffin-embedded tissues, the tissue quality still depends on the pre-analytical processing. Identification of the tumor-rich areas also depends on the pathologist and its role remains crucial for proper staging and lymph node harvesting [67].
8. Summary and Future Perspectives
This review shorty presented the historical evolution of CRC staging systems, using detailed tables to highlight the main modifications and the current interest in molecular classification. It aims for better stratification of cases, above classical staging limitations, in constant search for prognostic biomarkers with beneficial therapeutic impact. Promising discoveries have been made, but further studies are necessary to validate these achievements and include them in future staging manuals.
Author Contributions
Conceptualization, L.B. and S.G.; methodology, I.J.; validation, S.G; investigation, L.B., S.G., R.C. and I.J.; resources, L.B. and S.G.; data curation, L.B. and R.C.; writing—original draft preparation, L.B.; writing—review and editing, S.G. and R.C.; supervision, I.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “George Emil Palade” University of Medicine, Pharmacy, Science and Technologies of Targu Mures, Romania (grant no. 615/5/2019).
Institutional Review Board Statement
Not applicable. This is a review of data from literature.
Informed Consent Statement
Not applicable. This is a review of data from literature.
Acknowledgments
The authors would like to express their sincere gratitude for Genoveva Rigmanyi for her technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394(10207), 1467–1480. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30. [Google Scholar] [CrossRef]
- Dukes, C.E. The classification of cancer of the rectum. J. Pathol. Bacteriol. 1932, 35, 323–332. [Google Scholar] [CrossRef]
- I Haq, A.; Schneeweiss, J.; Kalsi, V.; Arya, M. The Dukes staging system: A cornerstone in the clinical management of colorectal cancer. Lancet Oncol. 2009, 10, 1128. [Google Scholar] [CrossRef]
- Horton, J.K.; Tepper, J.E. Staging of Colorectal Cancer: Past, Present, and Future. Clin. Color. Cancer 2005, 4, 302–312. [Google Scholar] [CrossRef]
- Williams, S.T.; Beart, R.W., Jr. Staging of colorectal cancer. Semin. Surg. Oncol. 1992, 8(2), 89–93. [Google Scholar] [CrossRef]
- Astler, V.B.; Coller, F.A. The Prognostic Significance Of Direct Extension Of Carcinoma Of The Colon And Rectum. Ann. Surg. 1954, 139, 846–852. [Google Scholar] [CrossRef]
- Turnbull, R.B.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the colon: The influence of the no-touch isolation technic on survival rates. Ann. Surg. 1967, 166, 420–427. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017; pp. 251–274. [Google Scholar]
- Gunderson, L.L.; Sosin, H. Areas of failure found at reoperation (second or symptomatic look) following “curative resection” for adenocarcinoma of the rectum. Cancer 1974, 34, 1278–1292. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Revisiting Dukes’ paradigm; some node positive colon cancer patients have better prognosis than some node negative patients. Clin. Transl. Oncol. 2017, 20, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Greene, F.L. The Staging of Colorectal Cancer: 2004 and Beyond. CA A Cancer J. Clin. 2004, 54, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Obrocea, F.L.; Sajin, M.; Marinescu, E.C.; Stoica, D. Colorectal cancer and the 7th revision of the TNM staging system: Review of changes and suggestions for uniform pathologic reporting. Romanian J. Morphol. Embryol. 2011, 52. [Google Scholar]
- Tong, G.J.; Zhang, G.Y.; Liu, J.; Zheng, Z.Z.; Chen, Y.; Niu, P.P.; Xu, X.T. Comparison of the eighth version of the American Joint Com-mittee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9(7), 148–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, B.; Wei, G.; Qiu, W.; Li, D.; Li, X.; Liu, X.; Wei, W.; Wang, S.; Liu, Z.; et al. Deep learning with whole slide images can improve the prognostic risk stratification with stage III colorectal cancer. Comput. Methods Programs Biomed. 2022, 221. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.P.; Treanor, D.; White, A.; E Mulcahy, H.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- Washington, M.K.; Berlin, J.; Branton, P.; Burgart, L.J.; Carter, D.K.; Fitzgibbons, P.L.; Halling, K.; Frankel, W.; Jessup, J.; Kakar, S.; et al. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. Arch. Pathol. Lab. Med. 2009, 133, 1539–1551. [Google Scholar] [CrossRef]
- Tong, L.L.; Gao, P.; Wang, Z.N.; Song, Y.X.; Xu, Y.Y.; Sun, Z.; Xing, C.Z.; Xu, H.M. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann. Surg. 2012, 255(2), 208–213. [Google Scholar] [CrossRef]
- Jin, M.; Roth, R.; Rock, J.B.; Washington, M.K.; Lehman, A.; Frankel, W.L. The Impact of Tumor Deposits on Colonic Adenocarcinoma AJCC TNM Staging and Outcome. Am. J. Surg. Pathol. 2015, 39, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D. Treatment of Colorectal Peritoneal Carcinomatosis With Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Son, I.T.; Kim, B.C.; Park, J.H.; Kim, J.Y.; Kim, J.W. Recurrence-Free Survival Outcomes Based on Novel Classification Combining Lymphovascular Invasion, Perineural Invasion, and T4 Status in Stage II–III Colon Cancer. Cancer Manag. Res. 2022, ume 14, 2031–2040. [Google Scholar] [CrossRef]
- Fülöp, Z.Z.; Gurzu, S.; Fülöp, R.L.; Bara, J.T.; Tímár, J.; Drágus, E.; Jung, I. Prognostic Impact of the Neutrophil-to-Lymphocyte and Lymphocyte-to-Monocyte Ratio, in Patients with Rectal Cancer: A Retrospective Study of 1052 Patients. J. Pers. Med. 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Serviddio, G.; Vendemiale, G.; Muscatiello, N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J. Gastroenterol. 2016, 22, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, J.R.; Driman, D.K. The total mesorectal excision specimen for rectal cancer: A review of its pathological assessment. J. Clin. Pathol. 2007, 60, 849–855. [Google Scholar] [CrossRef]
- Ferko, A.; Orhalmi, J.; Dusek, T.; Chobola, M.; Hovorková, E.; Nikolov, D.H. Higher risk of incomplete mesorectal excision and positive circumferential margin in low rectal cancer regardless of surgical technique. Videosurg. Other Miniinvasive Tech. 2014, 9, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Garlipp, B.; Ptok, H.; Schmidt, U.; Stübs, P.; Scheidbach, H.; Meyer, F.; Gastinger, I.; Lippert, H. Factors influencing the quality of total mesorectal excision. Br. J. Surg. 2012, 99, 714–720. [Google Scholar] [CrossRef]
- Mirshahvalad, S.A.; Hinzpeter, R.; Kohan, A.; Anconina, R.; Kulanthaivelu, R.; Ortega, C.; Metser, U.; Veit-Haibach, P. Diagnostic performance of [18F]-FDG PET/MR in evaluating colorectal cancer: A systematic review and meta-analysis. Eur. J. Pediatr. 2022, 1–13. [Google Scholar] [CrossRef]
- Simu, P.; Jung, I.; Banias, L.; Kovacs, Z.; Fulop, Z.; Bara, T.; Simu, I.; Gurzu, S. Synchronous Colorectal Cancer: Improving Accuracy of Detection and Analyzing Molecular Heterogeneity—The Main Keys for Optimal Approach. Diagnostics 2021, 11, 314. [Google Scholar] [CrossRef]
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: The 3d English Edition [Secondary Publication]. J. Anus. Rectum. Colon. 2019, 3, 175–195. [Google Scholar] [CrossRef] [Green Version]
- Dawson, H.; Kirsch, R.; Messenger, D.; Driman, D. A Review of Current Challenges in Colorectal Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Guan, X.; Ma, M.; Zhuang, M.; Ma, T.; Liu, Z.; Chen, H.; Jiang, Z.; Chen, Y.; Wang, G.; et al. Reconsidering the prognostic significance of tumour deposit count in the TNM staging system for colorectal cancer. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, I.; Atares, B.; Tarragona, J.; Bernet, L.; Sardon, J.D.; Pereda, T.; Villar, C.; Mendez, M.C.; Gonzalez-Obeso, E.; Elorriaga, K.; et al. Molecularly determined total tumour load in lymph nodes of stage I-II colon cancer patients correlates with high-risk factors. A multicentre prospective study. Virchows Arch. 2016, 469, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokan, Z.; Simillis, C.; Kontovounisios, C.; Moran, B.; Tekkis, P.; Brown, G. Locally Recurrent Rectal Cancer According to a Standardized MRI Classification System: A Systematic Review of the Literature. J. Clin. Med. 2022, 11, 3511. [Google Scholar] [CrossRef] [PubMed]
- García-Alfonso, P.; García-Carbonero, R.; Garcia-Foncillas, J.; Pérez-Segura, P.; Salazar, R.; Vera, R.; Ramón, Y.; Cajal, S.; Hernán-dez-Losa, J.; Landolfi, S.; et al. Update of the recommendations for the determination of bi-omarkers in colorectal carcinoma: National Consensus of the Spanish Society of Medical Oncology and the Spanish Society of Pathology. Clin. Transl. Oncol. 2020, 22, 1976–1991. [Google Scholar] [CrossRef]
- Martini, G.; Dienstmann, R.; Ros, J.; Baraibar, I.; Cuadra-Urteaga, J.L.; Salva, F.; Ciardiello, D.; Mulet, N.; Argiles, G.; Tabernero, J.; et al. Molecular subtypes and the evolution of treatment management in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kandimalla, R.; Huang, H.; Zhu, L.; Li, Y.; Gao, F.; Goel, A.; Wang, X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2018, 55, 37–52. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Khaliq, A.M.; Erdogan, C.; Kurt, Z.; Turgut, S.S.; Grunvald, M.W.; Rand, T.; Khare, S.; Borgia, J.A.; Hayden, D.M.; Pappas, S.G.; et al. Refining colorectal cancer classification and clinical stratification through a single-cell atlas. Genome Biol. 2022, 23, 1–30. [Google Scholar] [CrossRef]
- Joanito, I.; Wirapati, P.; Zhao, N.; Nawaz, Z.; Yeo, G.; Lee, F.; Eng, C.L.P.; Macalinao, D.C.; Kahraman, M.; Srinivasan, H.; et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 2022, 54, 963–975. [Google Scholar] [CrossRef]
- Fessler, E.; Jansen, M.; Melo, F.D.S.E.; Zhao, L.; Prasetyanti, P.R.; Rodermond, H.; Kandimalla, R.; Linnekamp, J.F.; Franitza, M.; Van Hooff, S.R.; et al. A multidimensional network approach reveals microRNAs as determinants of the mesenchymal colorectal cancer subtype. Oncogene 2016, 35, 6026–6037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wang, L.; Liu, Z.; Shao, B.; Jiang, W.; Shu, P. Integrated analysis identifies an immune-based prognostic signature for the mesenchymal identity in colorectal cancer. Medicine 2020, 99, e20617. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Ou, F.S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of Consensus Molecular Subtype on Survival in Patients With Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2019, 37, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Mooi, J.; Wirapati, P.; Asher, R.; Lee, C.; Savas, P.S.; Price, T.; Townsend, A.; Hardingham, J.; Buchanan, D.; Williams, D.; et al. The prognostic impact of consensus molecular subtypes (CMS) and its predictive effects for bevacizumab benefit in metastatic colorectal cancer: Molecular analysis of the AGITG MAX clinical trial. Ann. Oncol. 2018, 29, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.; Díaz-Rubio, E.; Carrato, A.; Abad, A.; Guillén, C.; Garcia-Alfonso, P.; Gil, S.; Cano, M.T.; Safont, M.J.; Gravalos, C.; et al. Tumour location and efficacy of first-line EGFR inhibitors in KRAS/RAS wild-type metastatic colorectal cancer: Retrospective analyses of two phase II randomised Spanish TTD trials. ESMO Open 2019, 4, e000599. [Google Scholar] [CrossRef]
- Stintzing, S.; Wirapati, P.; Lenz, H.J.; Neureiter, D.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef] [Green Version]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef]
- Li, X.; Larsson, P.; Ljuslinder, I.; Ling, A.; Löfgren-Burström, A.; Zingmark, C.; Edin, S.; Palmqvist, R. A modified protein marker panel to identify four consensus molecular subtypes in colorectal cancer using immunohistochemistry. Pathol. Res. Pract. 2021, 220, 153379. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Q.; Zhang, L.; Mo, S.; Cai, S.; Huang, D.; Peng, J. Immunohistochemistry-Based Consensus Molecular Subtypes as a Prognostic and Predictive Biomarker for Adjuvant Chemotherapy in Patients with Stage II Colorectal Cancer. Oncologist 2020, 25, e1968–e1979. [Google Scholar] [CrossRef]
- Banias, L.; Jung, I.; Bara, T.; Fulop, Z.; Simu, P.; Simu, I.; Satala, C.; Gurzu, S. Immunohistochemical-based molecular subtyping of colorectal carcinoma using maspin and markers of epithelial-mesenchymal transition. Oncol. Lett. 2019, 19, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Ten Hoorn, S.; Trinh, A.; de Jong, J.; Koens, L.; Vermeulen, L. Classification of Colorectal Cancer in Molecular Subtypes by Im-munohistochemistry. Methods Mol. Biol. 2018, 1765, 179–191. [Google Scholar] [PubMed]
- Trinh, A.; Trumpi, K.; De Sousa, E.; Melo, F.; Wang, X.; de Jong, J.H.; Fessler, E.; Kuppen, P.J.; Reimers, M.S.; Swets, M.; et al. Practical and Robust Identification of Molecular Subtypes in Colorectal Cancer by Immunohistochemistry. Clin. Cancer Res. 2017, 23, 387–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseweir, A.; Kong, C.Y.; Park, J.; Bennett, L.; Powell, A.; Quinn, J.; Van Wyk, H.; Horgan, P.; McMillan, D.; Edwards, J.; et al. A novel tumor-based epithelial-to-mesenchymal transition score that associates with prognosis and metastasis in patients with Stage II/III colorectal cancer. Int. J. Cancer 2018, 144, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Silveanu, C.; Fetyko, A.; Butiurca, V.; Kovacs, Z.; Jung, I. Systematic review of the old and new concepts in the epitheli-al-mesenchymal transition of colorectal cancer. World J. Gastroenterol. 2016, 22, 6764–6775. [Google Scholar] [CrossRef]
- Gurzu, S.; Jung, I. Subcellular Expression of Maspin in Colorectal Cancer: Friend or Foe. Cancers 2021, 13, 366. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Mesina, C.; Stoean, C.L.; Stoean, R.; Sandita, A.V.; Dumitrescu, T.V.; Mogoantă, S.S.; Cristian, D.A.; Mesina-Botoran, M.I.; Mitroi, G.; Gruia, C.L.; et al. Immunohistochemical evaluation of tumor budding in colorectal cancer: An important parameter with prognostic value. Rom. J. Morphol. Embryol. 2019, 60, 841–846. [Google Scholar]
- Zlobec, I.; Berger, M.D.; Lugli, A. Tumour budding and its clinical implications in gastrointestinal cancers. Br. J. Cancer 2020, 123, 700–708. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Banias, L.; Jung, I.; Gurzu, S. Subcellular expression of maspin – from normal tissue to tumor cells. World J. Meta-Analysis 2019, 7, 142–155. [Google Scholar] [CrossRef]
- Banias, L.; Gurzu, S.; Kovacs, Z.; Bara, T.; Jung, I. Nuclear maspin expression: A biomarker for budding assessment in colorectal cancer specimens. Pathol.-Res. Pr. 2017, 213, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Wang, J.Y.; Shia, J.; Zhou, Y.; Ogawa, M.; Hendrickson, R.C.; Klimstra, D.S.; Roehrl, M.H.A. Maspin as a Prognostic Marker for Early Stage Colorectal Cancer With Microsatellite Instability. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Jung, I.; Szalman, K.; Banias, L.; Bara, T.J.; Gurzu, S. Interaction of arylsulfatases A and B with maspin: A possible ex-planation for dysregulation of tumor cell metabolism and invasive potential of colorectal cancer. World J. Clin. Cases. 2019, 7(23), 3990–4003. [Google Scholar] [CrossRef]
- Gurzu, S.; Jung, J.; Azamfirei, L.; Mezei, T.; Cimpean, A.M.; Szentirmay, Z. The angiogenesis in colorectal carcinomas with and without lymph node metastases. Romanian J. Morphol. Embryol. 2008, 49. [Google Scholar]
- Chen, G.; Yang, Z.; Eshleman, J.R.; Netto, G.J.; Lin, M.-T. Molecular Diagnostics for Precision Medicine in Colorectal Cancer: Current Status and Future Perspective. BioMed Res. Int. 2016, 2016, 9850690. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).