Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Mechanically Stimulated Osteoclasts Lead to Protein Synthesis in Co-Cultured FDB Muscle
2.2. RAW 264.7 Release ATP by Mechanical Stimulation
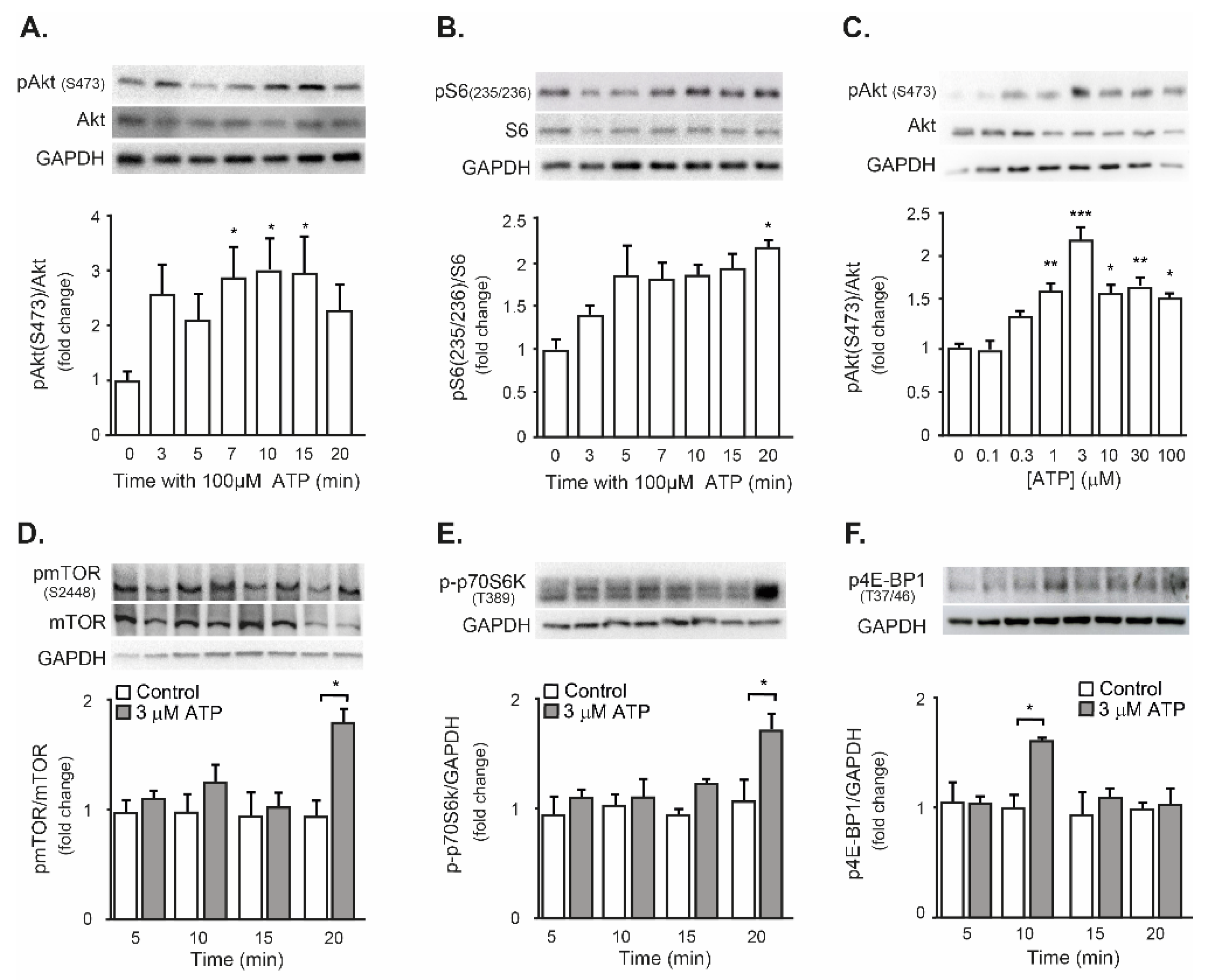
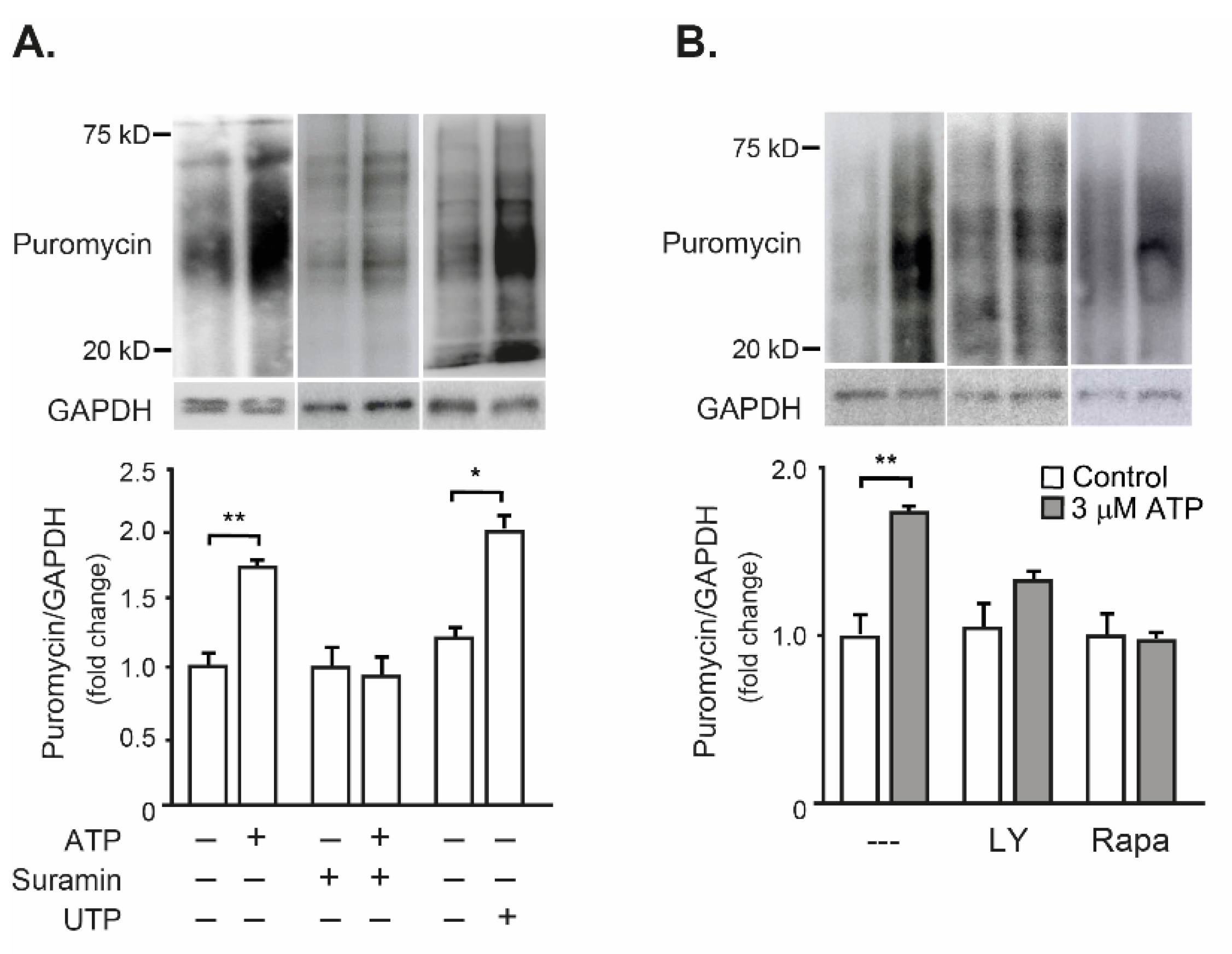
2.3. Exogenous ATP Promotes Protein Synthesis in Mouse Isolated FDB Muscle, through the P2R-Akt-mTOR Pathway
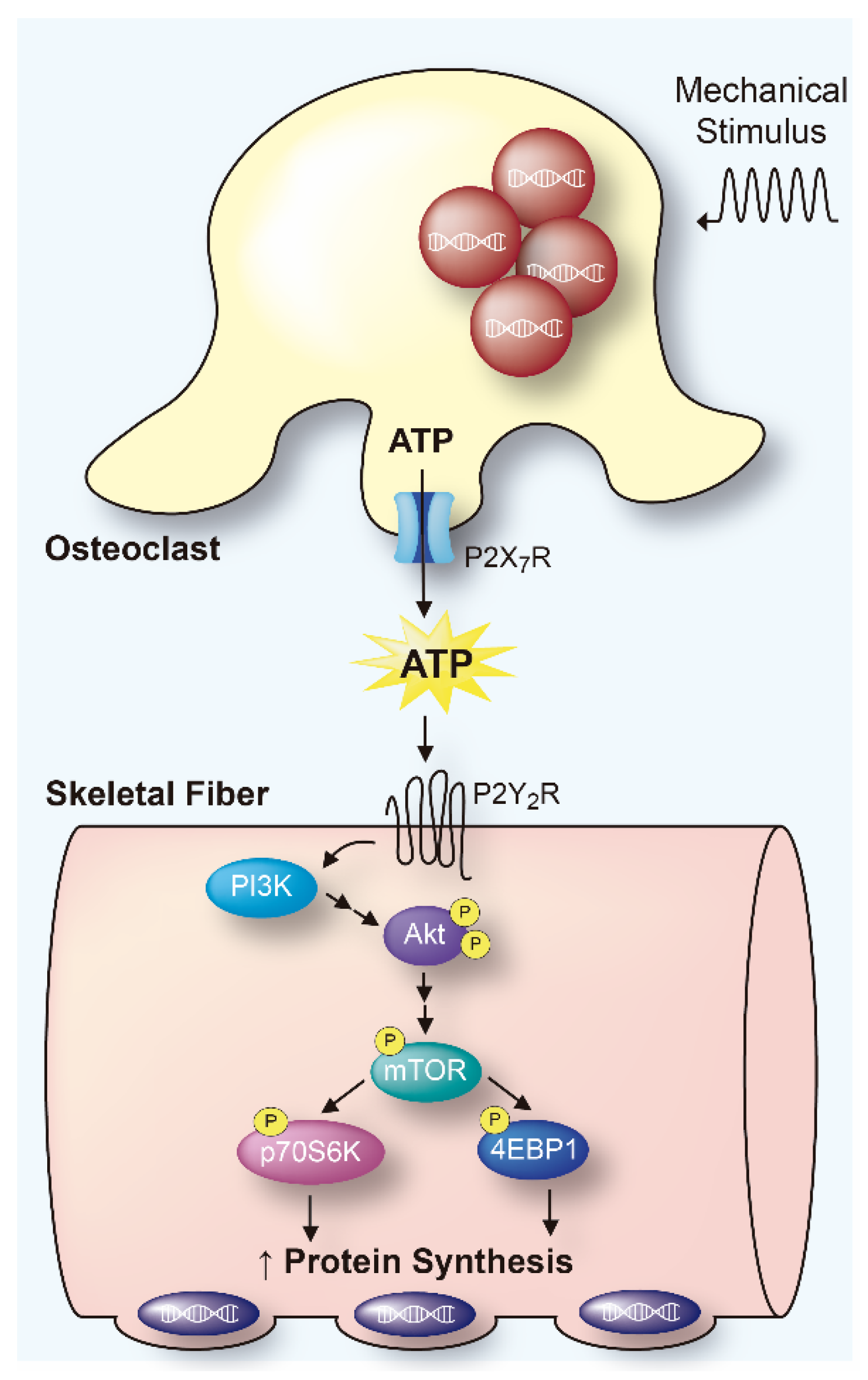
2.4. Mechanically Stimulated Osteoclasts Lead to Protein Synthesis in Co-Cultured FDB Muscle, through ATP Release and Activation of the P2R-Akt-mTOR Pathway
3. Discussion
3.1. Mechanically Evoked ATP Release from Osteoclasts
3.2. eATP as a Protein Synthesis Inductor in Skeletal Muscle
3.3. ATP as a Signaling Molecule for Bone-Muscle Crosstalk
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. Preparation of Osteoclast-like Cell-Enriched Populations
4.3. TRAP Staining
4.4. Total RNA Extraction, Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
4.5. Fluorescence-Activated Cell Sorting
4.6. Mechanical Stimulation and Extracellular ATP Measurement
4.7. Cell Viability
4.8. Immunofluorescence
4.9. Muscle Dissection and Stimulation
4.10. Immunoblot
4.11. Protein Synthesis Assay
4.12. Paracrine Communication Assay (Transwell® Chambers)
4.13. Statistical Analysis
5. Conclusions
- Purified osteoclasts release ATP to the extracellular medium after mechanical stimulation, in a regulated and non-lytic way.
- eATP is an inductor of protein synthesis in skeletal muscle, through activation of P2Y receptors and the Akt-mTOR signaling pathway.
- Mechanical stimulation of purified osteoclasts increases protein synthesis in a co-cultured FDB muscle, through the release of ATP to the extracellular environment and activation of the P2-PI3K-mTOR signaling pathway.
- Then, ATP is a possible signaling molecule for bone-muscle crosstalk. Considering that ATP is a ubiquitous molecule, released by multiple cellular components of the musculoskeletal system, it could participate in the fine regulation of muscle mass. This evidence opens a new area of clinical interest in muscle pathologies, in conditions such as the loss of muscle mass during aging (sarcopenia), or in adaptive processes related to muscle use/disuse.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burr, D.B. Muscle strength, bone mass, and age-related bone loss. J. Bone Miner. Res. 1997, 12, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Maurel, D.B.; Jahn, K.; Lara-Castillo, N. Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies. Biomedicines 2017, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Novotny, S.A.; Warren, G.L.; Hamrick, M.W. Aging and the muscle-bone relationship. Physiology 2015, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Saxon, L.; Turner, C.H.; Robling, A.G.; Bass, S.L. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone 2004, 34, 281–287. [Google Scholar] [CrossRef]
- Duncan, C.S.; Blimkie, C.J.; Cowell, C.T.; Burke, S.T.; Briody, J.N.; Howman-Giles, R. Bone mineral density in adolescent female athletes: Relationship to exercise type and muscle strength. Med. Sci. Sports Exerc. 2002, 34, 286–294. [Google Scholar] [CrossRef]
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.; Vanderschueren, D.; Gielen, E.; Jardi, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- McKeehen, J.N.; Novotny, S.A.; Baltgalvis, K.A.; Call, J.A.; Nuckley, D.J.; Lowe, D.A. Adaptations of mouse skeletal muscle to low-intensity vibration training. Med. Sci. Sports Exerc. 2013, 45, 1051–1059. [Google Scholar] [CrossRef]
- Oxlund, B.S.; Ortoft, G.; Andreassen, T.T.; Oxlund, H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 2003, 32, 69–77. [Google Scholar] [CrossRef]
- Perrini, S.; Laviola, L.; Carreira, M.C.; Cignarelli, A.; Natalicchio, A.; Giorgino, F. The GH/IGF1 axis and signaling pathways in the muscle and bone: Mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J. Endocrinol. 2010, 205, 201–210. [Google Scholar] [CrossRef]
- Reyes, M.L.; Hernandez, M.; Holmgren, L.J.; Sanhueza, E.; Escobar, R.G. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J. Bone Miner. Res. 2011, 26, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef]
- Kaufman, H.; Reznick, A.; Stein, H.; Barak, M.; Maor, G. The biological basis of the bone-muscle inter-relationship in the algorithm of fracture healing. Orthopedics 2008, 31, 751. [Google Scholar] [PubMed]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef]
- Burnstock, G.; Arnett, T.R.; Orriss, I.R. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 2013, 9, 541–572. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP signaling in skeletal muscle: From fiber plasticity to regulation of metabolism. Exerc. Sport Sci. Rev. 2014, 42, 110–116. [Google Scholar] [CrossRef]
- Kringelbach, T.M.; Aslan, D.; Novak, I.; Ellegaard, M.; Syberg, S.; Andersen, C.K.; Kristiansen, K.A.; Vang, O.; Schwarz, P.; Jorgensen, N.R. Fine-tuned ATP signals are acute mediators in osteocyte mechanotransduction. Cell Signal. 2015, 27, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewicz, N.; Zimmermann, E.A.; Willie, B.M.; Komarova, S.V. Mechanically stimulated ATP release from murine bone cells is regulated by a balance of injury and repair. Elife 2018, 7, e37812. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.R. The purinergic P2X7 ion channel receptor-a ‘repair’ receptor in bone. Curr. Opin. Immunol. 2018, 52, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Cutarelli, A.; Marini, M.; Tancredi, V.; D’Arcangelo, G.; Murdocca, M.; Frank, C.; Tarantino, U. Adenosine Triphosphate stimulates differentiation and mineralization in human osteoblast-like Saos-2 cells. Dev. Growth Differ. 2016, 58, 400–408. [Google Scholar] [CrossRef]
- Nakano, Y.; Addison, W.N.; Kaartinen, M.T. ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone 2007, 41, 549–561. [Google Scholar] [CrossRef]
- Wang, N.; Robaye, B.; Agrawal, A.; Skerry, T.M.; Boeynaems, J.M.; Gartland, A. Reduced bone turnover in mice lacking the P2Y13 receptor of ADP. Mol. Endocrinol. 2012, 26, 142–152. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Du, X.; Sims, S.M.; Dixon, S.J. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J. Biol. Chem. 2005, 280, 16909–16915. [Google Scholar] [CrossRef]
- Orriss, I.R.; Wang, N.; Burnstock, G.; Arnett, T.R.; Gartland, A.; Robaye, B.; Boeynaems, J.M. The P2Y(6) receptor stimulates bone resorption by osteoclasts. Endocrinology 2011, 152, 3706–3716. [Google Scholar] [CrossRef]
- Miyazaki, T.; Iwasawa, M.; Nakashima, T.; Mori, S.; Shigemoto, K.; Nakamura, H.; Katagiri, H.; Takayanagi, H.; Tanaka, S. Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. J. Biol. Chem. 2012, 287, 37808–37823. [Google Scholar] [CrossRef]
- Kadow-Romacker, A.; Hoffmann, J.E.; Duda, G.; Wildemann, B.; Schmidmaier, G. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs 2009, 190, 61–68. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Veldhuijzen, J.P.; van Strien, M.E.; de Jong, M.; Burger, E.H. Inhibition of osteoclastic bone resorption by mechanical stimulation in vitro. Arthritis Rheum. 1990, 33, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Burch, A.; Key, M.L.; Patel, J.J.; Arnett, T.R.; Orriss, I.R. The P2X7 Receptor is an Important Regulator of Extracellular ATP Levels. Front. Endocrinol. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.C.; Siow, N.L.; Cheng, A.W.; Ling, K.K.; Tung, E.K.; Simon, J.; Barnard, E.A.; Tsim, K.W. ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: Transduction and transcription control. J. Neurosci. 2003, 23, 4445–4456. [Google Scholar] [CrossRef]
- Ling, K.K.; Siow, N.L.; Choi, R.C.; Ting, A.K.; Kong, L.W.; Tsim, K.W. ATP potentiates agrin-induced AChR aggregation in cultured myotubes: Activation of RhoA in P2Y1 nucleotide receptor signaling at vertebrate neuromuscular junctions. J. Biol. Chem. 2004, 279, 31081–31088. [Google Scholar] [CrossRef] [PubMed]
- Araya, R.; Riquelme, M.A.; Brandan, E.; Saez, J.C. The formation of skeletal muscle myotubes requires functional membrane receptors activated by extracellular ATP. Brain Res. Brain Res. Rev. 2004, 47, 174–188. [Google Scholar] [CrossRef]
- Mamedova, L.K.; Wang, R.; Besada, P.; Liang, B.T.; Jacobson, K.A. Attenuation of apoptosis in vitro and ischemia/reperfusion injury in vivo in mouse skeletal muscle by P2Y6 receptor activation. Pharmacol. Res. 2008, 58, 232–239. [Google Scholar] [CrossRef]
- Martinello, T.; Baldoin, M.C.; Morbiato, L.; Paganin, M.; Tarricone, E.; Schiavo, G.; Bianchini, E.; Sandona, D.; Betto, R. Extracellular ATP signaling during differentiation of C2C12 skeletal muscle cells: Role in proliferation. Mol. Cell Biochem. 2011, 351, 183–196. [Google Scholar] [CrossRef]
- Bustamante, M.; Fernandez-Verdejo, R.; Jaimovich, E.; Buvinic, S. Electrical stimulation induces IL-6 in skeletal muscle through extracellular ATP by activating Ca(2+) signals and an IL-6 autocrine loop. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E869–E882. [Google Scholar] [CrossRef]
- Buvinic, S.; Almarza, G.; Bustamante, M.; Casas, M.; Lopez, J.; Riquelme, M.; Saez, J.C.; Huidobro-Toro, J.P.; Jaimovich, E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009, 284, 34490–34505. [Google Scholar] [CrossRef]
- Jorquera, G.; Altamirano, F.; Contreras-Ferrat, A.; Almarza, G.; Buvinic, S.; Jacquemond, V.; Jaimovich, E.; Casas, M. Cav1.1 controls frequency-dependent events regulating adult skeletal muscle plasticity. J. Cell Sci. 2013, 126, 1189–1198. [Google Scholar] [CrossRef][Green Version]
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kgamma-Akt-AS160 in skeletal muscle cells. Diabetes 2013, 62, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef] [PubMed]
- Gerasimovskaya, E.V.; Tucker, D.A.; Weiser-Evans, M.; Wenzlau, J.M.; Klemm, D.J.; Banks, M.; Stenmark, K.R. Extracellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin, and p70 S6 kinase signaling pathways. J. Biol. Chem. 2005, 280, 1838–1848. [Google Scholar] [CrossRef]
- Hu, L.Y.; Sun, Z.G.; Wen, Y.M.; Cheng, G.Z.; Wang, S.L.; Zhao, H.B.; Zhang, X.R. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience 2010, 169, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Hoebertz, A.; Arnett, T.R.; Burnstock, G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol. Sci. 2003, 24, 290–297. [Google Scholar] [CrossRef]
- Orriss, I.R.; Burnstock, G.; Arnett, T.R. Purinergic signalling and bone remodelling. Curr. Opin. Pharmacol. 2010, 10, 322–330. [Google Scholar] [CrossRef]
- Li, P.; Bian, X.; Liu, C.; Wang, S.; Guo, M.; Tao, Y.; Huo, B. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium. 2018, 71, 45–52. [Google Scholar] [CrossRef]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef]
- Qiu, F.; Dahl, G. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell Physiol. 2009, 296, C250–C255. [Google Scholar] [CrossRef]
- Wang, J.; Jackson, D.G.; Dahl, G. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J. Gen. Physiol. 2013, 141, 649–656. [Google Scholar] [PubMed]
- Willebrords, J.; Maes, M.; Crespo Yanguas, S.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F. The P2Z purinoceptor: An intriguing role in immunity, inflammation and cell death. Immunol. Today 1995, 16, 524–528. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Brosnan, C.F.; Scemes, E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 2006, 26, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Tournier, N.; Saba, W.; Cisternino, S.; Peyronneau, M.A.; Damont, A.; Goutal, S.; Dubois, A.; Dolle, F.; Scherrmann, J.M.; Valette, H.; et al. Effects of selected OATP and/or ABC transporter inhibitors on the brain and whole-body distribution of glyburide. AAPS J. 2013, 15, 1082–1090. [Google Scholar] [CrossRef]
- Kato, Y.; Omote, H.; Miyaji, T. Inhibitors of ATP release inhibit vesicular nucleotide transporter. Biol. Pharm. Bull. 2013, 36, 1688–1691. [Google Scholar] [CrossRef][Green Version]
- Moriyama, Y.; Hiasa, M.; Sakamoto, S.; Omote, H.; Nomura, M. Vesicular nucleotide transporter (VNUT): Appearance of an actress on the stage of purinergic signaling. Purinergic. Signal. 2017, 13, 387–404. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu. Rev. Fluid. Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J. Bone Miner. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef]
- Buckley, K.A.; Hipskind, R.A.; Gartland, A.; Bowler, W.B.; Gallagher, J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone 2002, 31, 582–590. [Google Scholar] [CrossRef]
- Orriss, I.R. The role of purinergic signalling in the musculoskeletal system. Auton. Neurosci. 2015, 191, 124–134. [Google Scholar] [CrossRef]
- Valladares, D.; Almarza, G.; Contreras, A.; Pavez, M.; Buvinic, S.; Jaimovich, E.; Casas, M. Electrical stimuli are anti-apoptotic in skeletal muscle via extracellular ATP. Alteration of this signal in Mdx mice is a likely cause of dystrophy. PLoS ONE 2013, 8, e75340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS ONE 2015, 10, e0129882. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Verdejo, R.; Casas, M.; Galgani, J.E.; Jaimovich, E.; Buvinic, S. Exercise sensitizes skeletal muscle to extracellular ATP for IL-6 expression in mice. Int. J. Sports Med. 2014, 35, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.C.; Gygi, S.P.; Raught, B.; Polakiewicz, R.D.; Abraham, R.T.; Hoekstra, M.F.; Aebersold, R.; Sonenberg, N. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999, 13, 1422–1437. [Google Scholar] [CrossRef]
- Tee, A.R.; Fingar, D.C.; Manning, B.D.; Kwiatkowski, D.J.; Cantley, L.C.; Blenis, J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 13571–13576. [Google Scholar] [CrossRef]
- Arias-Calderon, M.; Almarza, G.; Diaz-Vegas, A.; Contreras-Ferrat, A.; Valladares, D.; Casas, M.; Toledo, H.; Jaimovich, E.; Buvinic, S. Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle. Skelet. Muscle 2016, 6, 15. [Google Scholar] [CrossRef]
- Cianferotti, L.; Brandi, M.L. Muscle-bone interactions: Basic and clinical aspects. Endocrine 2014, 45, 165–177. [Google Scholar] [CrossRef]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Price, C.; Lu, X.L.; Wang, L. Imaging and quantifying solute transport across periosteum: Implications for muscle-bone crosstalk. Bone 2014, 66, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Knothe Tate, M.L.; Yu, N.Y.; Jalilian, I.; Pereira, A.F.; Knothe, U.R. Periosteum mechanobiology and mechanistic insights for regenerative medicine. Bonekey Rep. 2016, 5, 857. [Google Scholar] [CrossRef]
- Silinsky, E.M.; Redman, R.S. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J. Physiol. 1996, 492 Pt 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Saltin, B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985, 366, 233–249. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012, 816, 187–202. [Google Scholar]
- Song, C.; Yang, X.; Lei, Y.; Zhang, Z.; Smith, W.; Yan, J.; Kong, L. Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. J. Cell Physiol. 2019, 234, 11969–11975. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rumney, R.M.; Sunters, A.; Reilly, G.C.; Gartland, A. Application of multiple forms of mechanical loading to human osteoblasts reveals increased ATP release in response to fluid flow in 3D cultures and differential regulation of immediate early genes. J. Biomech. 2012, 45, 549–554. [Google Scholar] [CrossRef]
- Casas, M.; Figueroa, R.; Jorquera, G.; Escobar, M.; Molgo, J.; Jaimovich, E. IP(3)-dependent, post-tetanic calcium transients induced by electrostimulation of adult skeletal muscle fibers. J. Gen. Physiol. 2010, 136, 455–467. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.; Lorentzon, M.; Conaway, H.H.; Lerner, U.H. Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology 2006, 147, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Arriero Mdel, M.; Ramis, J.M.; Perello, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Jiménez, C.; Balanta-Melo, J.; Arias-Calderón, M.; Hernández, N.; Gómez-Valenzuela, F.; Escobar, A.; Jaimovich, E.; Buvinic, S. Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 9444. https://doi.org/10.3390/ijms23169444
Morales-Jiménez C, Balanta-Melo J, Arias-Calderón M, Hernández N, Gómez-Valenzuela F, Escobar A, Jaimovich E, Buvinic S. Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(16):9444. https://doi.org/10.3390/ijms23169444
Chicago/Turabian StyleMorales-Jiménez, Camilo, Julián Balanta-Melo, Manuel Arias-Calderón, Nadia Hernández, Fernán Gómez-Valenzuela, Alejandro Escobar, Enrique Jaimovich, and Sonja Buvinic. 2022. "Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway" International Journal of Molecular Sciences 23, no. 16: 9444. https://doi.org/10.3390/ijms23169444
APA StyleMorales-Jiménez, C., Balanta-Melo, J., Arias-Calderón, M., Hernández, N., Gómez-Valenzuela, F., Escobar, A., Jaimovich, E., & Buvinic, S. (2022). Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway. International Journal of Molecular Sciences, 23(16), 9444. https://doi.org/10.3390/ijms23169444








