HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis
Abstract
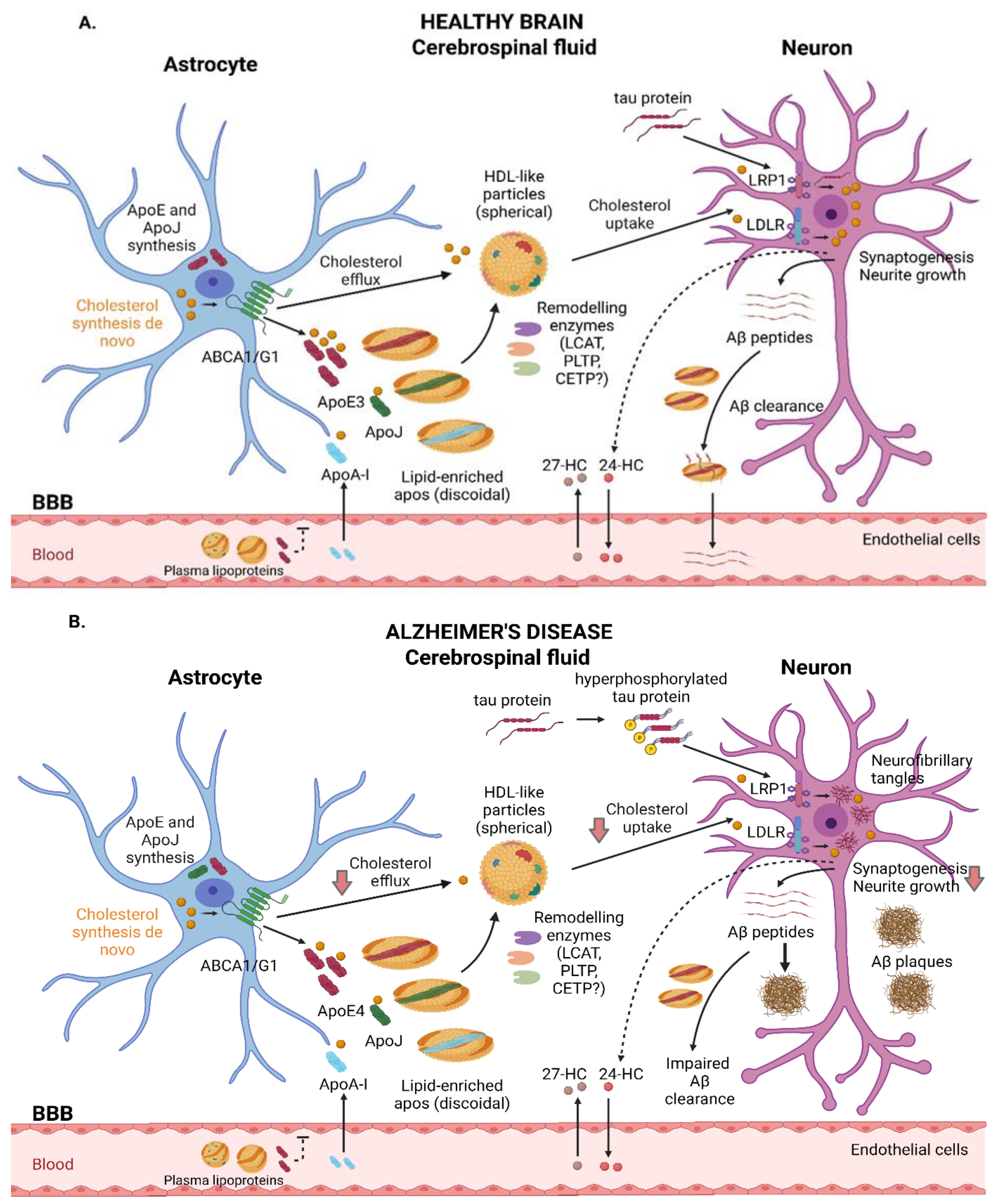
:1. Introduction
1.1. Alzheimer’s Disease
1.2. Involvement of Lipids in AD Pathology
1.3. Literature Search Strategy
2. HDL-Mediated Cholesterol Trafficking in the CNS
2.1. Transporters of Cholesterol in the Brain
2.2. Apolipoprotein E in the CNS in the Context of AD
2.2.1. Aβ Peptides, Cholesterol Transporters, and ApoE Interplay
2.2.2. ApoE4 and AD Pathogenesis
2.3. HDL-like Lipoprotein Metabolism in the CNS
2.4. Cholesterol Efflux to HDL-like Particles in AD
2.5. Cholesterol HDL-like Uptake in AD
3. Lipid-Based Therapies in the CNS with Respect to AD
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- The Global Impact of Dementia 2013–2050|Alzheimer’s Disease International (ADI). Available online: https://www.alzint.org/resource/policy-brief-the-global-impact-of-dementia-2013–2050/ (accessed on 14 June 2022).
- De Souza, L.C.; Sarazin, M.; Goetz, C.; Dubois, B. Clinical Investigations in Primary Care. Front. Neurol. Neurosci. 2009, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s Disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Selkoe, D.J. Cell Biology of Protein Misfolding: The Examples of Alzheimer’s and Parkinson’s Diseases. Nat. Cell Biol. 2004, 6, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Skovronsky, D.M.; Lee, V.M.Y.; Trojanowski, J.Q. Neurodegenerative Diseases: New Concepts of Pathogenesis and Their Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Boström, K.; Jungbjer, B.; Olsson, L. Membrane Lipids of Adult Human Brain: Lipid Composition of Frontal and Temporal Lobe in Subjects of Age 20 to 100 Years. J. Neurochem. 1994, 63, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol Metabolism and Homeostasis in the Brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef]
- Björkhem, I.; Meaney, S.; Fogelman, A.M. Brain Cholesterol: Long Secret Life behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Brain Cholesterol Metabolism and Its Defects: Linkage to Neurodegenerative Diseases and Synaptic Dysfunction. Acta Nat. 2016, 8, 58. [Google Scholar] [CrossRef]
- Orth, M.; Bellosta, S. Cholesterol: Its Regulation and Role in Central Nervous System Disorders. Cholesterol 2012, 2012, 292598. [Google Scholar] [CrossRef]
- Beecham, G.W.; Hamilton, K.; Naj, A.C.; Martin, E.R.; Huentelman, M.; Myers, A.J.; Corneveaux, J.J.; Hardy, J.; Vonsattel, J.P.; Younkin, S.G.; et al. Genome-Wide Association Meta-Analysis of Neuropathologic Features of Alzheimer’s Disease and Related Dementias. PLOS Genet. 2014, 10, e1004606. [Google Scholar] [CrossRef]
- Blain, J.F.; Poirier, J. Could Lipoprotein Lipase Play a Role in Alzheimer’s Disease? Sci. World J. 2004, 4, 531. [Google Scholar] [CrossRef]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-Wide Association Study Identifies Variants at CLU and CR1 Associated with Alzheimer’s Disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nat. Genet. 2013, 45, 1452. [Google Scholar] [CrossRef]
- Parks-Leduc, L.; Feldman, G.; Bardi, A. Personality Traits and Personal Values: A Meta-Analysis. Personal. Soc. Psychol. Rev. 2015, 19, 3–29. [Google Scholar] [CrossRef]
- Papassotiropoulos, A.; Wollmer, M.A.; Aguzzi, A.; Hock, C.; Nitsch, R.M.; de Quervain, D.J.F. The Prion Gene Is Associated with Human Long-Term Memory. Hum. Mol. Genet. 2005, 14, 2241–2246. [Google Scholar] [CrossRef]
- Poirier, J.; Bertrand, P.; Kogan, S.; Gauthier, S.; Gauthier, S.; Davignon, J.; Bouthillier, D.; Davignon, J. Apolipoprotein E Polymorphism and Alzheimer’s Disease. Lancet 1993, 342, 697–699. [Google Scholar] [CrossRef]
- Sun, J.H.; Yu, J.T.; Tan, L. The Role of Cholesterol Metabolism in Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 947–965. [Google Scholar] [CrossRef]
- Wavrant-Devrièze, F.; Pérez-Tur, J.; Lambert, J.C.; Frigard, B.; Pasquier, F.; Delacourte, A.; Amouyel, P.; Hardy, J.; Chartier-Harlin, M.C. Association between the Low Density Lipoprotein Receptor-Related Protein (LRP) and Alzheimer’s Disease. Neurosci. Lett. 1997, 227, 68–70. [Google Scholar] [CrossRef]
- Wollmer, M.A.; Sleegers, K.; Ingelsson, M.; Zekanowski, C.; Brouwers, N.; Maruszak, A.; Brunner, F.; Huynh, K.D.; Kilander, L.; Brundin, R.M.; et al. Association Study of Cholesterol-Related Genes in Alzheimer’s Disease. Neurogenetics 2007, 8, 179–188. [Google Scholar] [CrossRef]
- Picard, C.; Julien, C.; Frappier, J.; Miron, J.; Théroux, L.; Dea, D.; Breitner, J.C.S.; Poirier, J. Alterations in Cholesterol Metabolism-Related Genes in Sporadic Alzheimer’s Disease. Neurobiol. Aging 2018, 66, 180.e1–180.e9. [Google Scholar] [CrossRef]
- Dong, H.K.; Gim, J.A.; Yeo, S.H.; Kim, H.S. Integrated Late Onset Alzheimer’s Disease (LOAD) Susceptibility Genes: Cholesterol Metabolism and Trafficking Perspectives. Gene 2017, 597, 10–16. [Google Scholar] [CrossRef]
- Farfel, J.M.; Yu, L.; de Jager, P.L.; Schneider, J.A.; Bennett, D.A. Association of APOE with Tau-Tangle Pathology with and without β-Amyloid. Neurobiol. Aging 2016, 37, 19. [Google Scholar] [CrossRef]
- Strittmatter, W.J.; Weisgraber, K.H.; Huang, D.Y.; Dong, L.M.; Salvesen, G.S.; Pericak-Vance, M.; Schmechel, D.; Saunders, A.M.; Goldgaber, D.; Roses, A.D. Binding of Human Apolipoprotein E to Synthetic Amyloid Beta Peptide: Isoform-Specific Effects and Implications for Late-Onset Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1993, 90, 8098–8102. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kashif, S.; Kamran, S.; Razzaq, A.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Escola-Gil, J.C.; Kovanen, P.T. HDL Functionality in Reverse Cholesterol Transport--Challenges in Translating Data Emerging from Mouse Models to Human Disease. Biochim. Biophys. Acta 2016, 1861, 566–583. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; Eckardstein, A.V.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Mahley, R.W. Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef]
- Camponova, P.; le Page, A.; Berrougui, H.; Lamoureux, J.; Pawelec, G.; Witkowski, M.J.; Fulop, T.; Khalil, A.; Slimane, M.; Mellal, B. Alteration of High-Density Lipoproteins Functionality in Alzheimer’s Disease Patients. Can. J. Physiol. Pharmacol. 2017, 95, 894–903. [Google Scholar] [CrossRef]
- Sovic, A.; Balazs, Z.; Hrzenjak, A.; Reicher, H.; Panzenboeck, U.; Malle, E.; Sattler, W. Scavenger Receptor Class B, Type I Mediates Uptake of Lipoprotein-Associated Phosphatidylcholine by Primary Porcine Cerebrovascular Endothelial Cells. Neurosci. Lett. 2004, 368, 11–14. [Google Scholar] [CrossRef]
- Husemann, J.; Loike, J.D.; Kodama, T.; Silverstein, S.C. Scavenger Receptor Class B Type I (SR-BI) Mediates Adhesion of Neonatal Murine Microglia to Fibrillar β-Amyloid. J. Neuroimmunol. 2001, 114, 142–150. [Google Scholar] [CrossRef]
- Khalil, A.; Berrougui, H.; Pawelec, G.; Fulop, T. Impairment of the ABCA1 and SR-BI-Mediated Cholesterol Efflux Pathways and HDL Anti-Inflammatory Activity in Alzheimer’s Disease. Mech. Ageing Dev. 2012, 133, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Panzenboeck, U.; Balazs, Z.; Sovic, A.; Hrzenjak, A.; Levak-Frank, S.; Wintersperger, A.; Malle, E.; Sattler, W. ABCA1 and Scavenger Receptor Class B, Type I, Are Modulators of Reverse Sterol Transport at an in Vitro Blood-Brain Barrier Constituted of Porcine Brain Capillary Endothelial Cells. J. Biol. Chem. 2002, 277, 42781–42789. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Guillemin, G.J.; Glaros, E.N.; Lim, C.K.; Garner, B. Quantitation of ATP-Binding Cassette Subfamily-A Transporter Gene Expression in Primary Human Brain Cells. NeuroReport 2006, 17, 891–896. [Google Scholar] [CrossRef]
- Tarr, P.T.; Edwards, P.A. ABCG1 and ABCG4 are Coexpressed in Neurons and Astrocytes of the CNS and Regulate Cholesterol Homeostasis through SREBP-2. J. Lipid Res. 2008, 49, 169–182. [Google Scholar] [CrossRef]
- Ben Aissa, M.; Lewandowski, C.T.; Ratia, K.M.; Lee, S.H.; Layden, B.T.; Ladu, M.J.; Thatcher, G.R.J. Discovery of Nonlipogenic ABCA1 Inducing Compounds with Potential in Alzheimer’s Disease and Type 2 Diabetes. ACS Pharmacol. Transl. Sci. 2021, 4, 143–154. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 Act Sequentially to Remove Cellular Cholesterol and Generate Cholesterol-Rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef]
- Hirsch-Reinshagen, V.; Zhou, S.; Burgess, B.L.; Bernier, L.; McIsaac, S.A.; Chan, J.Y.; Tansley, G.H.; Cohn, J.S.; Hayden, M.R.; Wellington, C.L. Deficiency of ABCA1 Impairs Apolipoprotein E Metabolism in Brain. J. Biol. Chem. 2004, 279, 41197–41207. [Google Scholar] [CrossRef]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Foss, D.; Mahley, R.W. Astrocytes Synthesize Apolipoprotein E and Metabolize Apolipoprotein E-Containing Lipoproteins. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1987, 917, 148–161. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Legleiter, J.; Han, X.; Fryer, J.D.; Kowalewski, T.; Holtzman, D.M. ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-Secreted ApoE. J. Biol. Chem. 2004, 279, 40987–40993. [Google Scholar] [CrossRef]
- Eckert, G.P.; Vardanian, L.; Rebeck, G.W.; Burns, M.P. Regulation of Central Nervous System Cholesterol Homeostasis by the Liver X Receptor Agonist TO-901317. Neurosci. Lett. 2007, 423, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Deng, A.; Irizarry, M.C.; Fitzgerald, M.L.; Rebeck, G.W. Induction of the Cholesterol Transporter ABCA1 in Central Nervous System Cells by Liver X Receptor Agonists Increases Secreted Aβ Levels. J. Biol. Chem. 2002, 277, 48508–48513. [Google Scholar] [CrossRef] [PubMed]
- Abildayeva, K.; Jansen, P.J.; Hirsch-Reinshagen, V.; Bloks, V.W.; Bakker, A.H.F.; Ramaekers, F.C.S.; de Vente, J.; Groen, A.K.; Wellington, C.L.; Kuipers, F.; et al. 24(S)-Hydroxycholesterol Participates in a Liver X Receptor-Controlled Pathway in Astrocytes That Regulates Apolipoprotein E-Mediated Cholesterol Efflux. J. Biol. Chem. 2006, 281, 12799–12808. [Google Scholar] [CrossRef] [PubMed]
- Koldamova, R.P.; Lefterov, I.M.; Ikonomovic, M.D.; Skoko, J.; Lefterov, P.I.; Isanski, B.A.; DeKosky, S.T.; Lazo, J.S. 22R-Hydroxycholesterol and 9-Cis-Retinoic Acid Induce ATP-Binding Cassette Transporter A1 Expression and Cholesterol Efflux in Brain Cells and Decrease Amyloid β Secretion. J. Biol. Chem. 2003, 278, 13244–13256. [Google Scholar] [CrossRef]
- Benarroch, E.E. Brain Cholesterol Metabolism and Neurologic Disease. Neurology 2008, 71, 1368–1373. [Google Scholar] [CrossRef]
- Jeong, W.; Lee, H.; Cho, S.; Seo, J. ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer’s Disease. Mol. Cells 2019, 42, 739. [Google Scholar] [CrossRef]
- Martinez, A.E.; Weissberger, G.; Kuklenyik, Z.; He, X.; Meuret, C.; Parekh, T.; Rees, J.C.; Parks, B.A.; Gardner, M.S.; King, S.M. The Small HDL Particle Hypothesis of Alzheimer’s Disease. Alzheimer’s Dement. 2022, 1–14. [Google Scholar] [CrossRef]
- Lund, E.G.; Xie, C.; Kotti, T.; Turley, S.D.; Dietschy, J.M.; Russell, D.W. Knockout of the Cholesterol 24-Hydroxylase Gene in Mice Reveals a Brain-Specific Mechanism of Cholesterol Turnover. J. Biol. Chem. 2003, 278, 22980–22988. [Google Scholar] [CrossRef]
- Lütjohann, D.; Breuer, O.; Ahlborg, G.; Nennesmo, I.; Sidén, Å.; Diczfalusy, U.; Björkhem, I. Cholesterol Homeostasis in Human Brain: Evidence for an Age-Dependent Flux of 24S-Hydroxycholesterol from the Brain into the Circulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9799. [Google Scholar] [CrossRef]
- Chang, T.Y.; Yamauchi, Y.; Hasan, M.T.; Chang, C. Cellular Cholesterol Homeostasis and Alzheimer’s Disease. J. Lipid Res. 2017, 58, 2239–2254. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Kusumo, H.; Costa, L.G.; Guizzetti, M. Cholesterol Efflux Is Differentially Regulated in Neurons and Astrocytes: Implications for Brain Cholesterol Homeostasis. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2013, 1831, 263–275. [Google Scholar] [CrossRef]
- Feringa, F.M.; van der Kant, R. Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front. Aging Neurosci. 2021, 13, 333. [Google Scholar] [CrossRef]
- Iwamoto, N.; Abe-Dohmae, S.; Sato, R.; Yokoyama, S. ABCA7 Expression Is Regulated by Cellular Cholesterol through the SREBP2 Pathway and Associated with Phagocytosis. J. Lipid Res. 2006, 47, 1915–1927. [Google Scholar] [CrossRef]
- Tomioka, M.; Toda, Y.; Mañucat, N.B.; Akatsu, H.; Fukumoto, M.; Kono, N.; Arai, H.; Kioka, N.; Ueda, K. Lysophosphatidylcholine Export by Human ABCA7. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 658–665. [Google Scholar] [CrossRef]
- Mulay, V.; Wood, P.; Rentero, C.; Enrich, C.; Grewal, T. Signal Transduction Pathways Provide Opportunities to Enhance HDL and ApoAI-Dependent Reverse Cholesterol Transport. Curr. Pharm. Biotechnol. 2012, 13, 352–364. [Google Scholar] [CrossRef]
- Herz, J. ApoE Receptors in the Nervous System. Curr. Opin. Lipidol. 2009, 20, 190. [Google Scholar] [CrossRef]
- Bassett, C.N.; Montine, K.S.; Neely, M.D.; Swift, L.L.; Montine, T.J. Cerebrospinal Fluid Lipoproteins in Alzheimer’s Disease. J. Neurosci. Res. 2000, 50, 282–286. [Google Scholar] [CrossRef]
- Turri, M.; Marchi, C.; Adorni, M.P.; Calabresi, L.; Zimetti, F. Emerging Role of HDL in Brain Cholesterol Metabolism and Neurodegenerative Disorders. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2022, 1867, 159123. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and Apolipoprotein E Receptors: Normal Biology and Roles in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef]
- Ishiguro, M.; Imai, Y.; Kohsaka, S. Expression and Distribution of Low Density Lipoprotein Receptor-Related Protein MRNA in the Rat Central Nervous System. Mol. Brain Res. 1995, 33, 37–46. [Google Scholar] [CrossRef]
- Rapp, A.; Gmeiner, B.; Hüttinger, M. Implication of ApoE Isoforms in Cholesterol Metabolism by Primary Rat Hippocampal Neurons and Astrocytes. Biochimie 2006, 88, 473–483. [Google Scholar] [CrossRef]
- Swanson, L.W.; Simmons, D.M.; Hofmannt, S.L.; Goldsteint, J.L.; Brownt, M.S. Localization of MRNA for Low Density Lipoprotein Receptor and a Cholesterol Synthetic Enzyme in Rabbit Nervous System by in Situ Hybridization (3-Hydroxy-3-Methylglutaryl-Coenzyme A Synthase/Apoprotein E/Myelination/Watanabe Heritable-Hyperlipidemic Rabbit). Proc. Natl. Acad. Sci. USA 1988, 85, 9821–9825. [Google Scholar] [CrossRef]
- Tooyama, I.; Kawamata, T.; Akiyama, H.; Moestrup, S.K.; Gliemann, J.; McGeer, P.L. Immunohistochemical Study of A2 Macroglobulin Receptor in Alzheimer and Control Postmortem Human Brain. Mol. Chem. Neuropathol. 1993, 18, 153–160. [Google Scholar] [CrossRef]
- Tooyama, I.; Kawamata, T.; Akiyama, H.; Kimura, H.; Moestrup, S.K.; Gliemann, J.; Matsuo, A.; McGeer, P.L. Subcellular Localization of the Low Density Lipoprotein Receptor-Related Protein (A2-Macroglobulin Receptor) in Human Brain. Brain Res. 1995, 691, 235–238. [Google Scholar] [CrossRef]
- Lewandowski, C.T.; Laham, M.S.; Thatcher, G.R.J. Remembering Your A, B, C’s: Alzheimer’s Disease and ABCA1. Acta Pharm. Sin. B 2022, 12, 995–1018. [Google Scholar] [CrossRef]
- El Asmar, Z.; Terrand, J.; Jenty, M.; Host, L.; Mlih, M.; Zerr, A.; Justiniano, H.; Matz, R.L.; Boudier, C.; Scholler, E.; et al. Convergent Signaling Pathways Controlled by LRP1 (Receptor-Related Protein 1) Cytoplasmic and Extracellular Domains Limit Cellular Cholesterol Accumulation. J. Biol. Chem. 2016, 291, 5116. [Google Scholar] [CrossRef]
- Lee, G.H.; D’Arcangelo, G. New Insights into Reelin-Mediated Signaling Pathways. Front. Cell. Neurosci. 2016, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood–Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Bahrami, A.; Barreto, G.E.; Lombardi, G.; Pirro, M.; Sahebkar, A. Emerging Roles for High-Density Lipoproteins in Neurodegenerative Disorders. Biofactors 2019, 45, 725–739. [Google Scholar] [CrossRef]
- Balazs, Z.; Panzenboeck, U.; Hammer, A.; Sovic, A.; Quehenberger, O.; Malle, E.; Sattler, W. Uptake and Transport of High-Density Lipoprotein (HDL) and HDL-Associated α-Tocopherol by an in Vitro Blood–Brain Barrier Model. J. Neurochem. 2004, 89, 939–950. [Google Scholar] [CrossRef]
- Zhou, A.L.; Swaminathan, S.K.; Curran, G.L.; Poduslo, J.F.; Lowe, V.J.; Li, L.; Kandimalla, K.K. Apolipoprotein A-I Crosses the Blood-Brain Barrier through Clathrin-Independent and Cholesterol-Mediated Endocytosis. J. Pharmacol. Exp. Ther. 2019, 369, 481. [Google Scholar] [CrossRef] [PubMed]
- Kober, A.C.; Manavalan, A.P.C.; Tam-Amersdorfer, C.; Holmér, A.; Saeed, A.; Fanaee-Danesh, E.; Zandl, M.; Albrecher, N.M.; Björkhem, I.; Kostner, G.M.; et al. Implications of Cerebrovascular ATP-Binding Cassette Transporter G1 (ABCG1) and Apolipoprotein M in Cholesterol Transport at the Blood-Brain Barrier. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2017, 1862, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem, I.; Heverin, M.; Leoni, V.; Meaney, S.; Diczfalusy, U. Oxysterols and Alzheimer’s Disease. Acta Neurol. Scand. 2006, 114, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, E.; Giannelli, S.; Testa, G.; Sottero, B.; Leonarduzzi, G.; Gamba, P. Cholesterol Dysmetabolism in Alzheimer’s Disease: A Starring Role for Astrocytes? Antioxidants 2021, 10, 1890. [Google Scholar] [CrossRef]
- Heverin, M.; Meaney, S.; Lütjohann, D.; Diczfalusy, U.; Wahren, J.; Björkhem, I. Crossing the Barrier: Net Flux of 27-Hydroxycholesterol into the Human Brain. J. Lipid Res. 2005, 46, 1047–1052. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, T.; Liu, W.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. 27-Hydroxycholesterol Promotes the Transfer of Astrocyte-Derived Cholesterol to Neurons in Co-Cultured SH-SY5Y Cells and C6 Cells. Front. Cell Dev. Biol. 2020, 8, 580599. [Google Scholar] [CrossRef]
- Dietschy, J.M. Central Nervous System: Cholesterol Turnover, Brain Development and Neurodegeneration. Biol. Chem. 2009, 390, 287–293. [Google Scholar] [CrossRef]
- Rantham Prabhakara, J.P.; Feist, G.; Thomasson, S.; Thompson, A.; Schommer, E.; Ghribi, O. Differential Effects of 24-Hydroxycholesterol and 27-Hydroxycholesterol on Tyrosine Hydroxylase and α-Synuclein in Human Neuroblastoma SH-SY5Y Cells. J. Neurochem. 2008, 107, 1722–1729. [Google Scholar] [CrossRef]
- Leoni, V. Oxysterols as Markers of Neurological Disease—A Review. Scand. J. Clin. Lab. Investig. 2009, 69, 22–25. [Google Scholar] [CrossRef]
- Hughes, T.M.; Rosano, C.; Evans, R.W.; Kuller, L.H. Brain Cholesterol Metabolism, Oxysterols, and Dementia. J. Alzheimers Dis. 2013, 33, 891. [Google Scholar] [CrossRef]
- Leoni, V.; Solomon, A.; Lövgren-Sandblom, A.; Minthon, L.; Blennow, K.; Hansson, O.; Wahlund, L.O.; Kivipelto, M.; Björkhem, I. Diagnostic Power of 24S-Hydroxycholesterol in Cerebrospinal Fluid: Candidate Marker of Brain Health. J. Alzheimer’s Dis. 2013, 36, 739–747. [Google Scholar] [CrossRef]
- Djelti, F.; Braudeau, J.; Hudry, E.; Dhenain, M.; Varin, J.; Bièche, I.; Marquer, C.; Chali, F.; Ayciriex, S.; Auzeil, N.; et al. CYP46A1 Inhibition, Brain Cholesterol Accumulation and Neurodegeneration Pave the Way for Alzheimer’s Disease. Brain 2015, 138, 2383–2398. [Google Scholar] [CrossRef]
- Fonseca, A.C.R.G.; Resende, R.; Oliveira, C.R.; Pereira, C.M.F. Cholesterol and Statins in Alzheimer’s Disease: Current Controversies. Exp. Neurol. 2010, 223, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.G.; Hamby, M.E.; McReynolds, M.L.; Ray, W.J. The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 14. [Google Scholar] [CrossRef]
- Marsillach, J.; Adorni, M.P.; Zimetti, F.; Papotti, B.; Zuliani, G.; Cervellati, C. HDL Proteome and Alzheimer’s Disease: Evidence of a Link. Antioxidants 2020, 9, 1224. [Google Scholar] [CrossRef]
- Van Valkenburgh, J.; Meuret, C.; Martinez, A.E.; Kodancha, V.; Solomon, V.; Chen, K.; Yassine, H.N. Understanding the Exchange of Systemic HDL Particles Into the Brain and Vascular Cells Has Diagnostic and Therapeutic Implications for Neurodegenerative Diseases. Front. Physiol. 2021, 12, 1335. [Google Scholar] [CrossRef]
- Shobab, L.A.; Hsiung, G.Y.R.; Feldman, H.H. Cholesterol in Alzheimer’s Disease. Lancet Neurol. 2005, 4, 841–852. [Google Scholar] [CrossRef]
- Poirier, J. Apolipoprotein E Represents a Potent Gene-Based Therapeutic Target for the Treatment of Sporadic Alzheimer’s Disease. Alzheimer’s Dement. 2008, 4, S91–S97. [Google Scholar] [CrossRef]
- Hirsch-Reinshagen, V.; Wellington, C.L. Cholesterol Metabolism, Apolipoprotein E, Adenosine Triphosphate-Binding Cassette Transporters, and Alzheimer’s Disease. Curr. Opin. Lipidol. 2007, 18, 325–332. [Google Scholar] [CrossRef]
- Poirier, J. Apolipoprotein E, Cholesterol Transport and Synthesis in Sporadic Alzheimer’s Disease. Neurobiol. Aging 2005, 26, 355–361. [Google Scholar] [CrossRef]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. INAUGURAL ARTICLE by a Recently Elected Academy Member:Apolipoprotein E4: A Causative Factor and Therapeutic Target in Neuropathology, Including Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5644. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.; Huang, Y.; Weisgraber, K. Detrimental Effects of Apolipoprotein E4: Potential Therapeutic Targets in Alzheimers Disease. Curr. Alzheimer Res. 2007, 4, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Fenili, D.; McLaurin, J.A. Cholesterol and Apoe: A Target for Alzheimer’s Disease Therapeutics. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 553–567. [Google Scholar] [CrossRef]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the Brain: Implications for Neurological and Psychiatric Disorders. Clin. Lipidol. 2017, 5, 555–573. [Google Scholar] [CrossRef]
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential Roles of Microglia and Monocytes in the Inflamed Central Nervous System. J. Exp. Med. 2014, 211, 1533–1549. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Geroldi, C.; Blanchettl, A.; Trabucch, M.I.; Govoni, S.; Franceschini, G.; Calabresi, L. Apolipoprotein E Epsilon 4 Allele Frequency in Vascular Dementia and Alzheimer’s Disease. Stroke 1994, 25, 1703. [Google Scholar] [CrossRef]
- MS Hartz, A.; Bauer, B.A.B.C. ABC Transporters in the CNS—An Inventory. Curr. Pharm. Biotechnol. 2011, 12, 656–673. [Google Scholar] [CrossRef]
- Vance, J.E.; Hayashi, H. Formation and Function of Apolipoprotein E-Containing Lipoproteins in the Nervous System. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2010, 1801, 806–818. [Google Scholar] [CrossRef]
- Flowers, S.A.; Rebeck, G.W. APOE in the Normal Brain. Neurobiol. Dis. 2020, 136, 104724. [Google Scholar] [CrossRef]
- Robert, J.; Button, E.B.; Yuen, B.; Gilmour, M.; Kang, K.; Bahrabadi, A.; Stukas, S.; Zhao, W.; Kulic, I.; Wellington, C.L. Clearance of Beta-Amyloid Is Facilitated by Apolipoprotein E and Circulating High-Density Lipoproteins in Bioengineered Human Vessels. eLife 2017, 6, e29595. [Google Scholar] [CrossRef]
- Ma, J.; Yee, A.; Brewer, H.B.; Das, S.; Potter, H. Amyloid-Associated Proteins A1-Antichymotrypsin and Apolipoprotein E Promote Assembly of Alzheimer β-Protein into Filaments. Nature 1994, 372, 92–94. [Google Scholar] [CrossRef]
- Sanan, D.A.; Weisgraber, K.H.; Russell, S.J.; Mahley, R.W.; Huang, D.; Saunders, A.; Schmechel, D.; Wisniewski, T.; Frangione, B.; Roses, A.D.; et al. Apolipoprotein E Associates with Beta Amyloid Peptide of Alzheimer’s Disease to Form Novel Monofibrils. Isoform ApoE4 Associates More Efficiently than ApoE3. J. Clin. Investig. 1994, 94, 860–869. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, Z.; Peng, Y. Potential Utility of Retinal Imaging for Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2018, 10, 188. [Google Scholar] [CrossRef]
- Xin, S.H.; Tan, L.; Cao, X.; Yu, J.T.; Tan, L. Clearance of Amyloid Beta and Tau in Alzheimer’s Disease: From Mechanisms to Therapy. Neurotox. Res. 2018, 34, 733–748. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Kumar, A.; Uddin, M.S.; Bungau, S. The Interplay of ABC Transporters in Aβ Translocation and Cholesterol Metabolism: Implicating Their Roles in Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1564–1582. [Google Scholar] [CrossRef]
- Fu, Y.; Hsiao, J.H.T.; Paxinos, G.; Halliday, G.M.; Kim, W.S. ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J. Alzheimer’s Dis. 2016, 54, 569–584. [Google Scholar] [CrossRef]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal Clearance of Amyloid-β by Endocytic Receptor LRP1. J. Neurosci. 2013, 33, 19276–19283. [Google Scholar] [CrossRef]
- Lamartinière, Y.; Boucau, M.C.; Dehouck, L.; Krohn, M.; Pahnke, J.; Candela, P.; Gosselet, F.; Fenart, L. ABCA7 Downregulation Modifies Cellular Cholesterol Homeostasis and Decreases Amyloid-β Peptide Efflux in an in Vitro Model of the Blood-Brain Barrier. J. Alzheimer’s Dis. 2018, 64, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Rawat, V.; Wang, S.; Sima, J.; Bar, R.; Liraz, O.; Gundimeda, U.; Parekh, T.; Chan, J.; Johansson, J.O.; Tang, C.; et al. ApoE4 Alters ABCA1 Membrane Trafficking in Astrocytes. J. Neurosci. 2019, 39, 9611–9622. [Google Scholar] [CrossRef] [PubMed]
- Sierri, G.; Dal Magro, R.; Vergani, B.; Leone, B.E.; Formicola, B.; Taiarol, L.; Fagioli, S.; Kravicz, M.; Tremolizzo, L.; Calabresi, L.; et al. Reduced Levels of ABCA1 Transporter Are Responsible for the Cholesterol Efflux Impairment in β-Amyloid-Induced Reactive Astrocytes: Potential Rescue from Biomimetic HDLs. Int. J. Mol. Sci. 2021, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, M.F.; Ng, C.A.; Rebeck, G.W. ApoE Lipidation as a Therapeutic Target in Alzheimer’s Disease. J. Mol. Sci. 2020, 21, 6336. [Google Scholar] [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic Meta-Analyses of Alzheimer Disease Genetic Association Studies: The AlzGene Database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
- Strittmatter, W.J.; Saunders, A.M.; Goedert, M.; Weisgraber, K.H.; Dong, L.M.; Jakes, R.; Huang, D.Y.; Pericak-Vance, M.; Schmechel, D.; Roses, A.D. Isoform-Specific Interactions of Apolipoprotein E with Microtubule-Associated Protein Tau: Implications for Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1994, 91, 11183. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Remarkable Protein Sheds Light on Cardiovascular and Neurological Diseases. Clin. Chem. 2017, 63, 14–20. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Bradley, W.A.; Gianturco, S.H. ApoE Is Necessary and Sufficient for the Binding of Large Triglyceride-Rich Lipoproteins to the LDL Receptor; ApoB Is Unnecessary. J. Lipid Res. 1986, 27, 40–48. [Google Scholar] [CrossRef]
- Gamba, P.; Testa, G.; Sottero, B.; Gargiulo, S.; Poli, G.; Leonarduzzi, G. The Link between Altered Cholesterol Metabolism and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2012, 1259, 54–64. [Google Scholar] [CrossRef]
- Dafnis, I.; Raftopoulou, C.; Mountaki, C.; Megalou, E.; Zannis, V.I.; Chroni, A. ApoE Isoforms and Carboxyl-Terminal-Truncated ApoE4 Forms Affect Neuronal BACE1 Levels and Aβ Production Independently of Their Cholesterol Efflux Capacity. Biochem. J. 2018, 475, 1839–1859. [Google Scholar] [CrossRef]
- Ye, S.; Huang, Y.; Mü llendorff, K.; Dong, L.; Giedt, G.; Meng, E.C.; Cohen, F.E.; Kuntz, I.D.; Weisgraber, K.H.; Mahley, R.W. Apolipoprotein (Apo) E4 Enhances Amyloid Peptide Production in Cultured Neuronal Cells: ApoE Structure as a Potential Therapeutic Target. Proc. Natl. Acad. Sci. USA 2005, 102, 18700–18705. [Google Scholar] [CrossRef]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E Promotes Astrocyte Colocalization and Degradation of Deposited Amyloid-β Peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. ApoE Isoform–Specific Disruption of Amyloid β Peptide Clearance from Mouse Brain. J. Clin. Investig. 2008, 118, 4002. [Google Scholar] [CrossRef] [PubMed]
- Simonovitch, S.; Schmukler, E.; Bespalko, A.; Iram, T.; Frenkel, D.; Holtzman, D.M.; Masliah, E.; Michaelson, D.M.; Pinkas-Kramarski, R. Impaired Autophagy in APOE4 Astrocytes. J. Alzheimer’s Dis. 2016, 51, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Garai, K.; Wang, Y.; Jiang, H.; Shah, A.; Bu, G.; Frieden, C.; Holtzman, D.M. ApoE Influences Amyloid-β (Aβ) Clearance despite Minimal ApoE/Aβ Association in Physiological Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, E1807. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Bales, K.R.; Tenkova, T.; Fagan, A.M.; Parsadanian, M.; Sartorius, L.J.; Mackey, B.; Olney, J.; McKeel, D.; Wozniak, D.; et al. Apolipoprotein E Isoform-Dependent Amyloid Deposition and Neuritic Degeneration in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2892–2897. [Google Scholar] [CrossRef]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoE4 Markedly Exacerbates Tau-Mediated Neurodegeneration in a Mouse of Tauopathy. Nature 2017, 549, 523. [Google Scholar] [CrossRef]
- Wadhwani, A.R.; Affaneh, A.; van Gulden, S.; Kessler, J.A. Neuronal Apolipoprotein E4 Increases Cell Death and Phosphorylated Tau Release in Alzheimer Disease. Ann. Neurol. 2019, 85, 726–739. [Google Scholar] [CrossRef]
- Rauch, J.N.; Luna, G.; Guzman, E.; Audouard, M.; Challis, C.; Sibih, Y.E.; Leshuk, C.; Hernandez, I.; Wegmann, S.; Hyman, B.T.; et al. LRP1 Is a Master Regulator of Tau Uptake and Spread. Nature 2020, 580, 381. [Google Scholar] [CrossRef]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human IPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154. [Google Scholar] [CrossRef]
- TCW, J.; Qian, L.; Pipalia, N.H.; Chao, M.J.; Liang, S.A.; Shi, Y.; Jain, B.R.; Bertelsen, S.E.; Kapoor, M.; Marcora, E.; et al. Cholesterol and Matrisome Pathways Dysregulated in Astrocytes and Microglia. Cell 2022, 185, 2213–2233. [Google Scholar] [CrossRef]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and Cholesterol Handling in the Brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef]
- Koch, S.; Donarski, N.; Goetze, K.; Kreckel, M.; Stuerenburg, H.-J.; Buhmann, C.; Beisiegel, U. Characterization of Four Lipoprotein Classes in Human Cerebrospinal Fluid. J. Lipid Res. 2001, 42, 1143–1151. [Google Scholar] [CrossRef]
- Ladu, M.J.; Reardon, C.; van Eldik, L.; Fagan, A.M.; Bu, G.; Holtzman, D.; Getz, G.S. Lipoproteins in the Central Nervous System. Ann. N.Y. Acad. Sci. 2000, 903, 167–175. [Google Scholar] [CrossRef]
- Yu, C.; Youmans, K.L.; LaDu, M.J. Lipoprotein Remodelling in the Periphery: A Model for the Brain? Biochim. Biophys. Acta 2010, 1801, 819. [Google Scholar] [CrossRef]
- Dobiášová, M.; Frohlich, J.J. Advances in Understanding of the Role of Lecithin Cholesterol Acyltransferase (LCAT) in Cholesterol Transport. Clin. Chim. Acta 1999, 286, 257–271. [Google Scholar] [CrossRef]
- Hirsch-Reinshagen, V.; Donkin, J.; Stukas, S.; Chan, J.; Wilkinson, A.; Fan, J.; Parks, J.S.; Kuivenhoven, J.A.; Lütjohann, D.; Pritchard, H.; et al. LCAT Synthesized by Primary Astrocytes Esterifies Cholesterol on Glia-Derived Lipoproteins. J. Lipid Res. 2009, 50, 885–893. [Google Scholar] [CrossRef]
- La Marca, V.; Maresca, B.; Spagnuolo, M.S.; Cigliano, L.; Dal Piaz, F.; di Iorio, G.; Abrescia, P. Lecithin-Cholesterol Acyltransferase in Brain: Does Oxidative Stress Influence the 24-Hydroxycholesterol Esterification? Neurosci. Res. 2016, 105, 19–27. [Google Scholar] [CrossRef]
- Manavalan, A.P.C.; Kober, A.; Metso, J.; Lang, I.; Becker, T.; Hasslitzer, K.; Zandl, M.; Fanaee-Danesh, E.; Brijesh Pippal, J.; Sachdev, V.; et al. Phospholipid Transfer Protein Is Expressed in Cerebrovascular Endothelial Cells and Involved in High Density Lipoprotein Biogenesis and Remodeling at the Blood-Brain Barrier. J. Biol. Chem. 2014, 289, 4683–4698. [Google Scholar] [CrossRef]
- Albers, J.J.; Tollefson, J.H.; Wolfbauer, G.; Albright, R.E. Cholesteryl Ester Transfer Protein in Human Brain. Int. J. Clin. Lab. Res. 1992, 21, 264–266. [Google Scholar] [CrossRef]
- Demeester, N.; Castro, G.; Desrumaux, C.; de Geitere, C.; Fruchart, J.; Santens, P.; Mulleners, E.; Engelborghs, S.; de Deyn, P.; Vandekerckhove, J.; et al. Characterization and Functional Studies of Lipoproteins, Lipid Transfer Proteins, and Lecithin:Cholesterol Acyltransferase in CSF of Normal Individuals and Patients with Alzheimer’s Disease. J. Lipid Res. 2000, 84, 276–294. [Google Scholar] [CrossRef]
- Rebeck, G.W.; Alonzo, N.C.; Berezovska, O.; Harr, S.D.; Knowles, R.B.; Growdon, J.H.; Hyman, B.T.; Mendez, A.J. Structure and Functions of Human Cerebrospinal Fluid Lipoproteins from Individuals of Different APOE Genotypes. Exp. Neurol. 1998, 149, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Guizzetti, M.; Chen, J.; Oram, J.F.; Tsuji, R.; Dao, K.; Möller, T.; Costa, L.G. Ethanol Induces Cholesterol Efflux and Up-Regulates ATP-Binding Cassette Cholesterol Transporters in Fetal Astrocytes. J. Biol. Chem. 2007, 282, 18740–18749. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yvan-Charvet, L.; Lütjohann, D.; Mulder, M.; Vanmierlo, T.; Kim, T.-W.; Tall, A.R. ATP-Binding Cassette Transporters G1 and G4 Mediate Cholesterol and Desmosterol Efflux to HDL and Regulate Sterol Accumulation in the Brain. FASEB J. 2008, 22, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Rellin, L.; Heeren, J.; Beisiegel, U. Recycling of Apolipoprotein E Is Not Associated with Cholesterol Efflux in Neuronal Cells. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2008, 1781, 232–238. [Google Scholar] [CrossRef]
- Lindner, K.; Beckenbauer, K.; van Ek, L.C.; Titeca, K.; de Leeuw, S.M.; Awwad, K.; Hanke, F.; Korepanova, A.V.; Rybin, V.; van der Kam, E.L.; et al. Isoform- and Cell-State-Specific Lipidation of ApoE in Astrocytes. Cell Rep. 2022, 38, 110435. [Google Scholar] [CrossRef]
- Minagawa, H.; Gong, J.S.; Jung, C.G.; Watanabe, A.; Lund-Katz, S.; Phillips, M.C.; Saito, H.; Michikawa, M. Mechanism Underlying Apolipoprotein E (ApoE) Isoform-Dependent Lipid Efflux From Neural Cells in Culture. J. Neurosci. Res. 2009, 87, 2498. [Google Scholar] [CrossRef]
- Chan, H.C.; Ke, L.Y.; Lu, H.T.; Weng, S.F.; Chan, H.C.; Law, S.H.; Lin, I.L.; Chang, C.F.; Lu, Y.H.; Chen, C.H.; et al. An Increased Plasma Level of ApoCIII-Rich Electronegative High-Density Lipoprotein May Contribute to Cognitive Impairment in Alzheimer’s Disease. Biomedicines 2020, 8, 542. [Google Scholar] [CrossRef]
- Yassine, H.N.; Feng, Q.; Chiang, J.; Petrosspour, L.M.; Fonteh, A.N.; Chui, H.C.; Harrington, M.G. ABCA1-Mediated Cholesterol Efflux Capacity to Cerebrospinal Fluid Is Reduced in Patients With Mild Cognitive Impairment and Alzheimer’s Disease. J. Am. Heart Assoc. 2016, 5, e002886. [Google Scholar] [CrossRef]
- Marchi, C.; Adorni, M.P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1-And ABCG1-Mediated Cholesterol Efflux Capacity of Cerebrospinal Fluid Is Impaired in Alzheimer’s Disease. J. Lipid Res. 2019, 60, 1449–1456. [Google Scholar] [CrossRef]
- Cipollari, E.; Szapary, H.J.; Picataggi, A.; Billheimer, J.T.; Lyssenko, C.A.; Ying, G.S.; Shaw, L.M.; Kling, M.A.; Kaddurah-Daouk, R.; Rader, D.J.; et al. Correlates and Predictors of Cerebrospinal Fluid Cholesterol Efflux Capacity from Neural Cells, a Family of Biomarkers for Cholesterol Epidemiology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 74, 563–578. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Li, D.; He, C.; He, K.; Xue, T.; Wan, L.; Zhang, C.; Liu, Q. Astrocytic ApoE Reprograms Neuronal Cholesterol Metabolism and Histone-Acetylation-Mediated Memory. Neuron 2021, 109, 957–970. [Google Scholar] [CrossRef]
- Zimetti, F.; Caffarra, P.; Ronda, N.; Favari, E.; Adorni, M.P.; Zanotti, I.; Bernini, F.; Barocco, F.; Spallazzi, M.; Galimberti, D.; et al. Increased PCSK9 Cerebrospinal Fluid Concentrations in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 55, 315–320. [Google Scholar] [CrossRef]
- Picard, C.; Poirier, A.; Bélanger, S.; Labonté, A.; Auld, D.; Poirier, J. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Alzheimer’s Disease: A Genetic and Proteomic Multi-Cohort Study. PLoS ONE 2019, 14, e0220254. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and Cardiovascular Diseases: From Cholesterol Lowering to Pleiotropy. Curr. Pharm. Des. 2009, 15, 467. [Google Scholar] [CrossRef]
- Miida, T.; Takahashi, A.; Ikeuchi, T. Prevention of Stroke and Dementia by Statin Therapy: Experimental and Clinical Evidence of Their Pleiotropic Effects. Pharmacol. Ther. 2007, 113, 378–393. [Google Scholar] [CrossRef]
- Pollen, D.A.; Baker, S.; Hinerfeld, D.; Swearer, J.; Evans, B.A.; Evans, J.E.; Caselli, R.; Rogaeva, E.; St George-Hyslop, P.; Moonis, M. Prevention of Alzheimer’s Disease in High Risk Groups: Statin Therapy in Subjects with PSEN1 Mutations or Heterozygosity for Apolipoprotein E Epsilon 4. Alzheimer’s Res. Ther. 2010, 2, 31. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, J.; Zhang, Z. Statins Use and Risk of Dementia: A Dose-Response Meta Analysis. Medicine 2018, 97, e11304. [Google Scholar] [CrossRef]
- Poirier, J.; Miron, J.; Picard, C.; Gormley, P.; Théroux, L.; Breitner, J.; Dea, D. Apolipoprotein E and Lipid Homeostasis in the Etiology and Treatment of Sporadic Alzheimer’s Disease. Neurobiol. Aging 2014, 35 (Suppl. 2), S3–S10. [Google Scholar] [CrossRef]
- Lam, V.; Clarnette, R.; Francis, R.; Bynevelt, M.; Watts, G.; Flicker, L.; Orr, C.F.; Loh, P.; Lautenschlager, N.; Reid, C.M.; et al. Efficacy of Probucol on Cognitive Function in Alzheimer’s Disease: Study Protocol for a Double-Blind, Placebo-Controlled, Randomised Phase II Trial (PIA Study). BMJ Open 2022, 12, e058826. [Google Scholar] [CrossRef]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.D.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef]
- Laclair, K.D.; Manaye, K.F.; Lee, D.L.; Allard, J.S.; Savonenko, A.V.; Troncoso, J.C.; Wong, P.C. Treatment with Bexarotene, a Compound That Increases Apolipoprotein-E, Provides No Cognitive Benefit in Mutant APP/PS1 Mice. Mol. Neurodegener. 2013, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Skerrett, R.; Pellegrino, M.P.; Casali, B.T.; Taraboanta, L.; Landreth, G.E. Combined Liver X Receptor/Peroxisome Proliferator-Activated Receptor γ Agonist Treatment Reduces Amyloid β Levels and Improves Behavior in Amyloid Precursor Protein/Presenilin 1 Mice. J. Biol. Chem. 2015, 290, 21591–21602. [Google Scholar] [CrossRef] [PubMed]
- Riddell, D.R.; Zhou, H.; Comery, T.A.; Kouranova, E.; Lo, C.F.; Warwick, H.K.; Ring, R.H.; Kirksey, Y.; Aschmies, S.; Xu, J.; et al. The LXR Agonist TO901317 Selectively Lowers Hippocampal Abeta42 and Improves Memory in the Tg2576 Mouse Model of Alzheimer’s Disease. Mol. Cell Neurosci. 2007, 34, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Rutten, K.; Dederen, J.; Bloks, V.W.; van Vark-van der Zee, L.C.; Kuipers, F.; Kiliaan, A.; Blokland, A.; Sijbrands, E.J.G.; Steinbusch, H.; et al. Liver X Receptor Activation Restores Memory in Aged AD Mice without Reducing Amyloid. Neurobiol. Aging 2011, 32, 1262–1272. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Cantillana, V.; Wang, H.; Sullivan, P.M.; Kolls, B.J.; Ge, X.; Lin, Y.; Mace, B.; Laskowitz, D.T. ApoE Mimetic Improves Pathology and Memory in a Model of Alzheimer’s Disease. Brain Res. 2020, 1733, 146685. [Google Scholar] [CrossRef]
- Ghosal, K.; Stathopoulos, A.; Thomas, D.; Phenis, D.; Vitek, M.P.; Pimplikar, S.W. The Apolipoprotein-E-Mimetic COG112 Protects Amyloid Precursor Protein Intracellular Domain-Overexpressing Animals from Alzheimer’s Disease-Like Pathological Features. Neurodegener. Dis. 2013, 12, 51–58. [Google Scholar] [CrossRef]
- Fernández-de Retana, S.; Montañola, A.; Marazuela, P.; de La Cuesta, M.; Batlle, A.; Fatar, M.; Grudzenski, S.; Montaner, J.; Hernández-Guillamon, M. Intravenous Treatment with Human Recombinant ApoA-I Milano Reduces Beta Amyloid Cerebral Deposition in the APP23-Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2017, 60, 116–128. [Google Scholar] [CrossRef]
- Handattu, S.P.; Garber, D.W.; Monroe, C.E.; van Groen, T.; Kadish, I.; Nayyar, G.; Cao, D.; Palgunachari, M.N.; Li, L.; Anantharamaiah, G.M. Oral Apolipoprotein A-I Mimetic Peptide Improves Cognitive Function and Reduces Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2009, 34, 525–534. [Google Scholar] [CrossRef]
- Delk, S.C.; Chattopadhyay, A.; Escola-Gil, J.C.; Fogelman, A.M.; Reddy, S.T. Apolipoprotein Mimetics in Cancer. Semin. Cancer Biol. 2021, 73, 158–168. [Google Scholar] [CrossRef]
- Korthauer, L.E.; Giugliano, R.P.; Guo, J.; Sabatine, M.S.; Sever, P.; Keech, A.; Atar, D.; Kurtz, C.; Ruff, C.T.; Mach, F.; et al. No Association between APOE Genotype and Lipid Lowering with Cognitive Function in a Randomized Controlled Trial of Evolocumab. PLoS ONE 2022, 17, e0266615. [Google Scholar] [CrossRef]
| Cholesterol Transporters and Receptors | Cellular Expression | Regulation | Main Functions |
|---|---|---|---|
| SR-BI | Astrocytes, neurons, and capillary endothelial cells | SREBP-2 pathway | Cholesterol diffusion to lipidated ApoE forms |
| ABCA1 | Astrocytes, microglia, neurons, and capillary endothelial cells | LXR/RXR heterodimer/PKA-pathway | Cholesterol efflux to poorly lipidated ApoE |
| ABCG1 | Astrocytes, neurons, and capillary endothelial cells | LXR/RXR heterodimer | Cholesterol efflux to lipidated ApoE forms |
| ABCG4 | Astrocytes, microglia, neurons, and capillary endothelial cells | LXR/RXR heterodimer | Cholesterol efflux to lipidated ApoE forms |
| ABCA7 | Astrocytes, neurons, and microglia | SREBP-2 pathway | Less known roles |
| LDLR | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Cholesterol uptake regulator |
| LRP1 | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Cholesterol uptake regulator |
| VLDLR | Astrocytes, microglia, neurons, and capillary endothelial cells | PCSK9 | Bind ligands for neurodevelopment and synaptic functions |
| ApoER2 | Neurons | PCSK9 | Bind other ligands involved in neurodevelopment and synaptic functions |
| Effects of ApoE4 Genotype | |||||
|---|---|---|---|---|---|
| Aβ Metabolism | Tau Pathology | Lipid Metabolism | |||
| ↑ Aβ production | [120,121] | ↑ Neurodegeneration | [127] | ↑ Cholesterol synthesis and accumulation | [130,131] |
| ↓ Aβ clearance | [122,123,124,125] | ↑ Tau phosphorylation and secretion | [128] | ↓ Lipid binding to ApoE | [118] |
| ↑ Aβ binding to ApoE | [25,103] | ↓ Tau binding to LRP1 | [129] | ↑ Oxidative stress and lipid peroxidation | [112] |
| ↑ Aβ aggregation and deposition | [126] | ||||
| Cell Culture | Sample (Acceptor/Carrier) | Mechanism Tested | Activation | Main Findings | Reference |
|---|---|---|---|---|---|
| Fibroblasts | CSF lipoproteins | Baseline efflux | None | CSF lipoproteins induce cholesterol efflux | [142] |
| Neuroglioma cells and primary neurons and astrocytes | CSF lipoproteins | Uptake | None | CSF lipoproteins are internalized by neurons | |
| Rat astrocytes | CSF from AD (n = 3) and controls (n = 3) | Baseline efflux | None | No differences | [141] |
| Primary neurons | ApoA-I from human plasma and recombinant ApoE3 | ABCA1-mediated efflux | 25-HC, 9-cis-RA | 25-HC, 9-cis-RA: ↑ efflux levels | [45] |
| Primary murine wild-type and Abca1−/− astrocytes and microglia | Lipid-free ApoA-I, recombinant ApoE2, ApoE3, ApoE4 | Baseline efflux | None | ABCA1 is involved in mediating cholesterol efflux to ApoA-I and ApoE | [39] |
| Abca1-deficient mouse primary cultured astrocytes | ApoE | Baseline efflux | None | ↓ ApoE and cholesterol in CSF lipoproteins | [41] |
| Rat astrocytes and human astrocytes | ApoA-I, ApoE, and HDL | ABCA1- and ABCG1-mediated efflux | Ethanol, cAMP, or 22(R)-HC plus 9-cis-RA | ↑ ABCA1- and ABCG1-mediated efflux | [143] |
| Murine neuronal cell line HT-22 | HDL alone or HDL associated with ApoE3 or ApoE4 | Baseline efflux | None | No differences in cholesterol efflux depending on ApoE isoform | [145] |
| Abcg1−/− and Abcg4−/− primary astrocytes | HDL | Baseline efflux | None | ↓ Efflux levels | [144] |
| HEK293 | HDL | Baseline efflux | Overexpression of Abcg1 and Abcg4 (transfection) | ↑ Efflux levels | |
| Primary neurons and ApoE-deficient astrocytes | Recombinant ApoE3 and ApoE4 | Baseline efflux | None | Recombinant ApoE3: ↑ efflux compared to recombinant ApoE4 | [147] |
| Human THP-1 monocytes, J774 macrophages, and SR-BI-enriched Fu5AH cells | Plasma, HDL, and ApoA-I from AD patients (n = 39) and controls (n = 20) | Baseline efflux, SR-BI- or ABCA1-mediated efflux | J774 + cAMP | ↓ ABCA1-mediated efflux | [33] |
| Primary cortical astrocytes and neurons | ApoA-I, HDL, ApoE3 | ABCA1-, ABCG1-, and ABCG4-mediated efflux | Ethanol, 22-HC plus 9cis-RA, ABCA1, ABCG1, and ABCG4 siRNAs, probucol (ABCA1 inhibitor) | ABCA1 and ABCG1 are mainly involved in cholesterol efflux in astrocytes, whereas ABCG4 regulates it in neurons | [52] |
| BHK cells | CSF from AD (n = 26), MCI (n = 35), and control (n = 47) individuals | ABCA1-mediated efflux | Mifepristone (induces ABCA1) | ↓ CSF ABCA1-mediated efflux in AD and MCI patients | [149] |
| J774 macrophages | Plasma-isolated HDL from AD (n = 33), MCI (n = 27), and control (n = 27) individuals | Baseline efflux and ABCA1-mediated efflux | cAMP | ↓ HDL baseline efflux in AD patients. No differences in ABCA1-mediated efflux | [30] |
| ApoE3 and ApoE4 primary astrocytes | BSA | ABCA1-mediated efflux | GW3965 (LXR agonist), CS-6253 (ABCA1 agonist peptide) | ↓ ABCA1-mediated efflux from ApoE4 astrocytes | [110] |
| BHK cells | CSF from non-demented ApoE4/4 (n = 3), ApoE3/4 (n = 9), and non ApoE4 (n = 9) carriers | ABCA1-mediated efflux | Mifepristone | ↓ CSF ABCA1-mediated efflux in ApoE4/4 carriers | |
| J774 macrophages | CSF from AD (n = 37), non-AD dementia patients (n = 16), and controls (n = 39) | Baseline and ABCA1-mediated efflux | cAMP | ↓ CSF ABCA1-mediated efflux in AD patients | [150] |
| CHO-K1 cells | ABCG1-mediated efflux | Expression of hABCG1 | ↓ CSF ABCG1-mediated efflux in AD patients | ||
| RAW264.7 murine macrophages | HDL isolated from control (n = 24) and AD patient (n = 44) serum | Baseline and ABCA1-mediated efflux | cAMP | ↓ HDL ABCA1-mediated efflux in AD patients | [148] |
| J774 macrophages and microglia cells | Human ApoA-I and HDL. CSF samples (with no demographic or clinical data) | Baseline and ABCA1/G1-mediated efflux | cAMP | Efflux correlates with CSF concentrations of cholesterol, ApoA-I, ApoE, and ApoJ | [151] |
| A172 astrocytes and SH-SY5Y neurons | T0901317 (LXR agonist) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borràs, C.; Mercer, A.; Sirisi, S.; Alcolea, D.; Escolà-Gil, J.C.; Blanco-Vaca, F.; Tondo, M. HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2022, 23, 9356. https://doi.org/10.3390/ijms23169356
Borràs C, Mercer A, Sirisi S, Alcolea D, Escolà-Gil JC, Blanco-Vaca F, Tondo M. HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. International Journal of Molecular Sciences. 2022; 23(16):9356. https://doi.org/10.3390/ijms23169356
Chicago/Turabian StyleBorràs, Carla, Aina Mercer, Sònia Sirisi, Daniel Alcolea, Joan Carles Escolà-Gil, Francisco Blanco-Vaca, and Mireia Tondo. 2022. "HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis" International Journal of Molecular Sciences 23, no. 16: 9356. https://doi.org/10.3390/ijms23169356
APA StyleBorràs, C., Mercer, A., Sirisi, S., Alcolea, D., Escolà-Gil, J. C., Blanco-Vaca, F., & Tondo, M. (2022). HDL-like-Mediated Cell Cholesterol Trafficking in the Central Nervous System and Alzheimer’s Disease Pathogenesis. International Journal of Molecular Sciences, 23(16), 9356. https://doi.org/10.3390/ijms23169356








