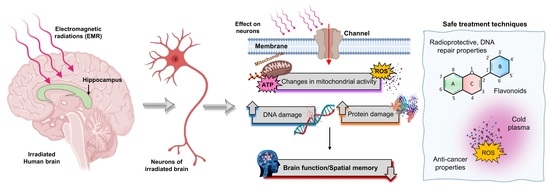
Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects
Abstract
:1. Introduction
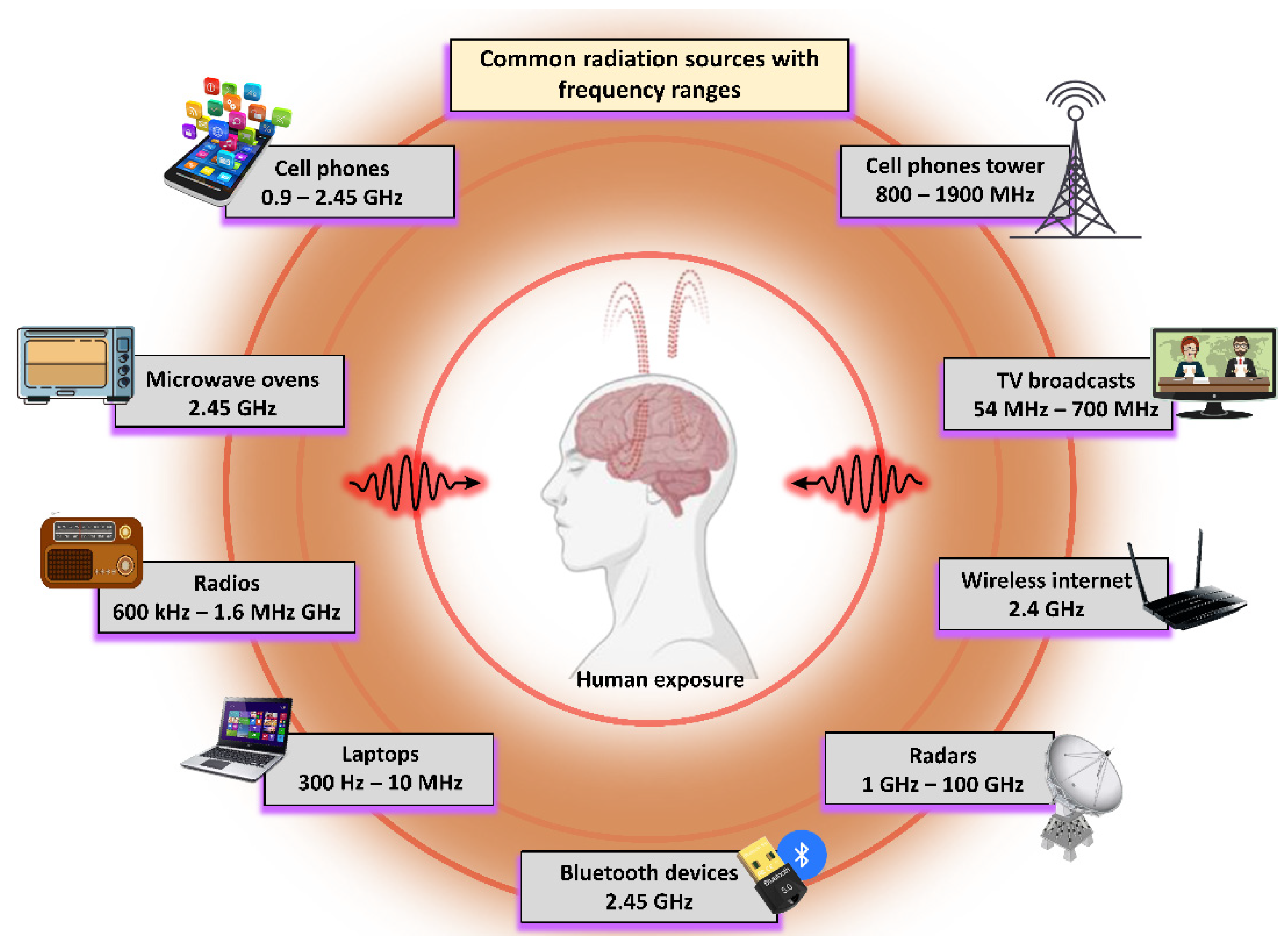
1.1. Interactions of Biological Systems with Electromagnetic Radiation (EMR)
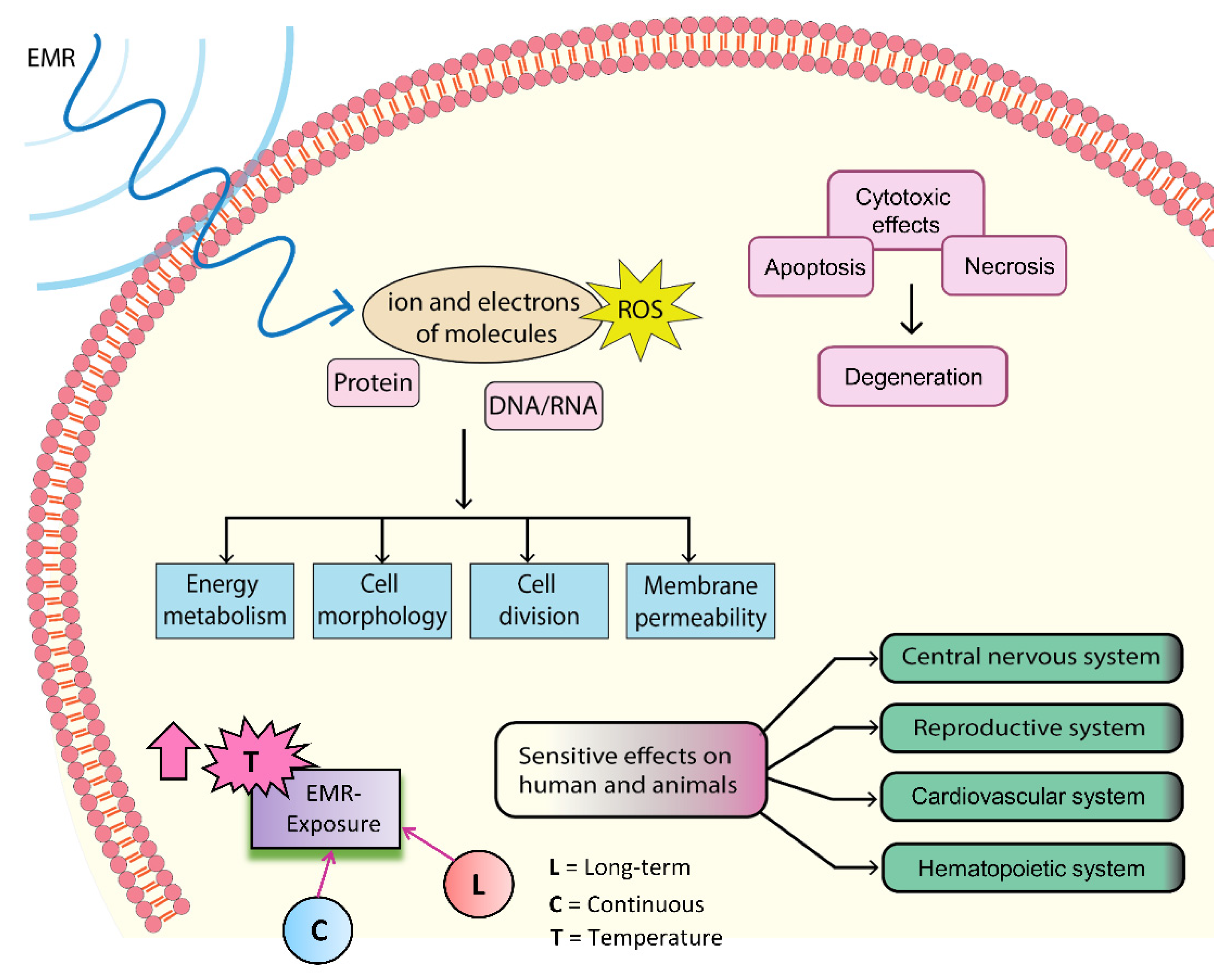
1.2. Possible Biological Effects and Mechanisms of EMR
2. Biological Effects of Microwaves
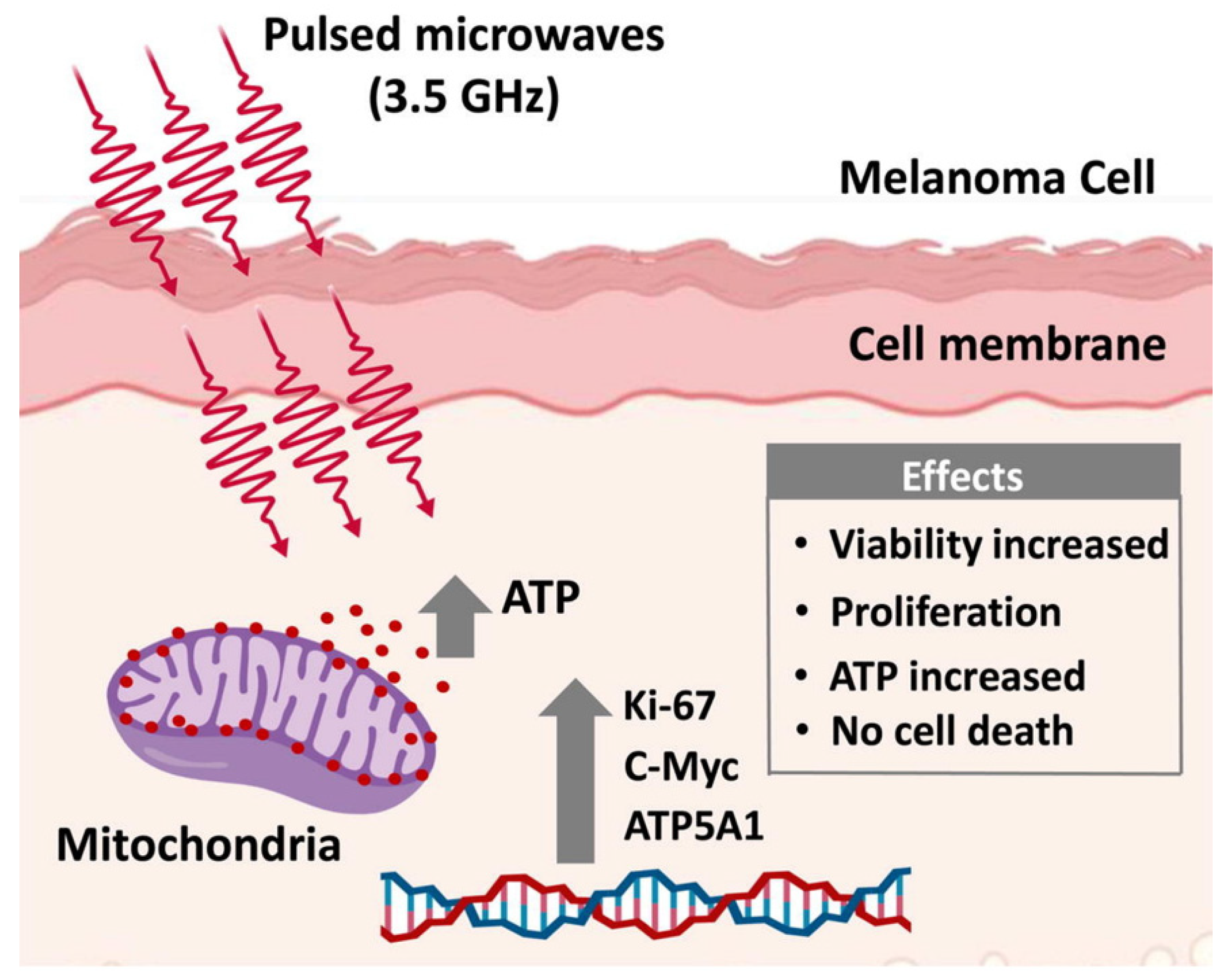
2.1. Effect of Microwave on Skin
2.2. Effect of Microwave on the Reproductive System
2.3. Effect of Millimeter Range Radiations
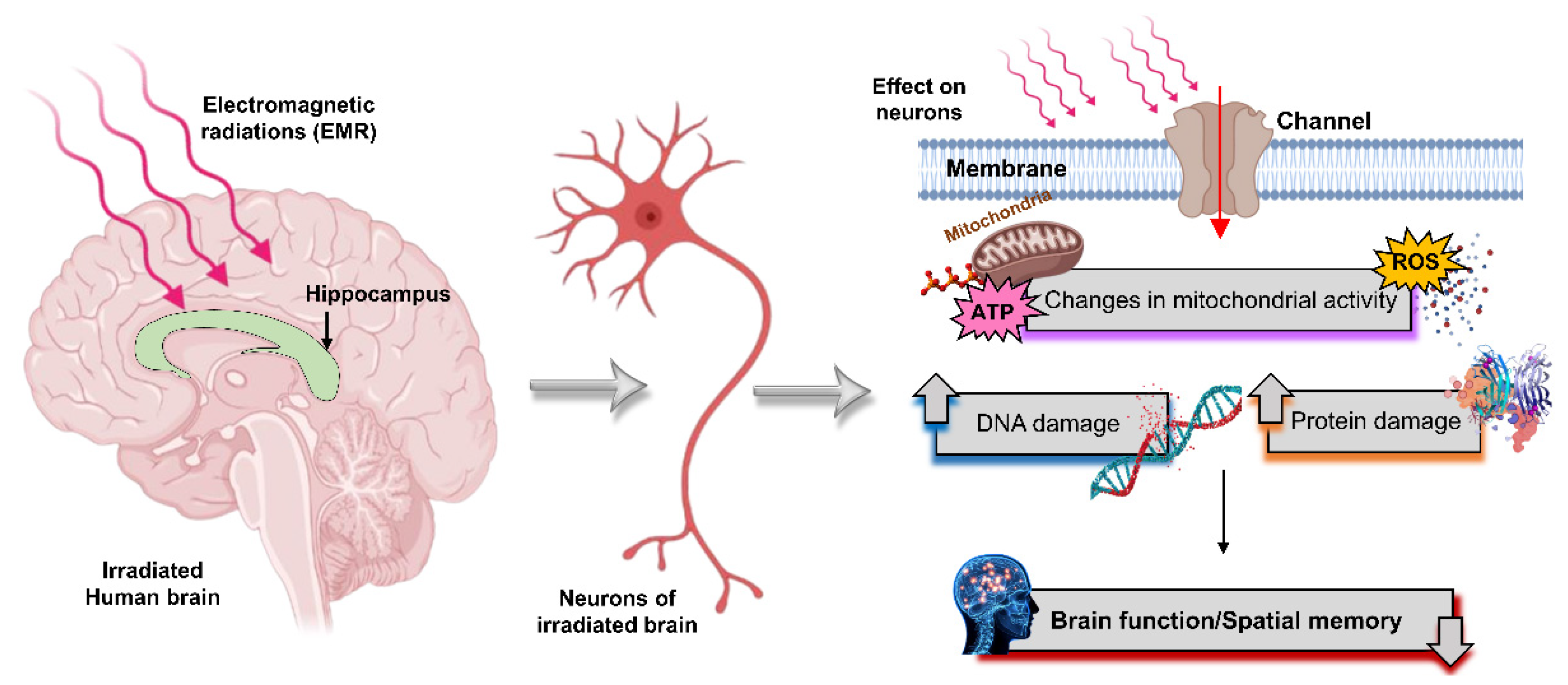
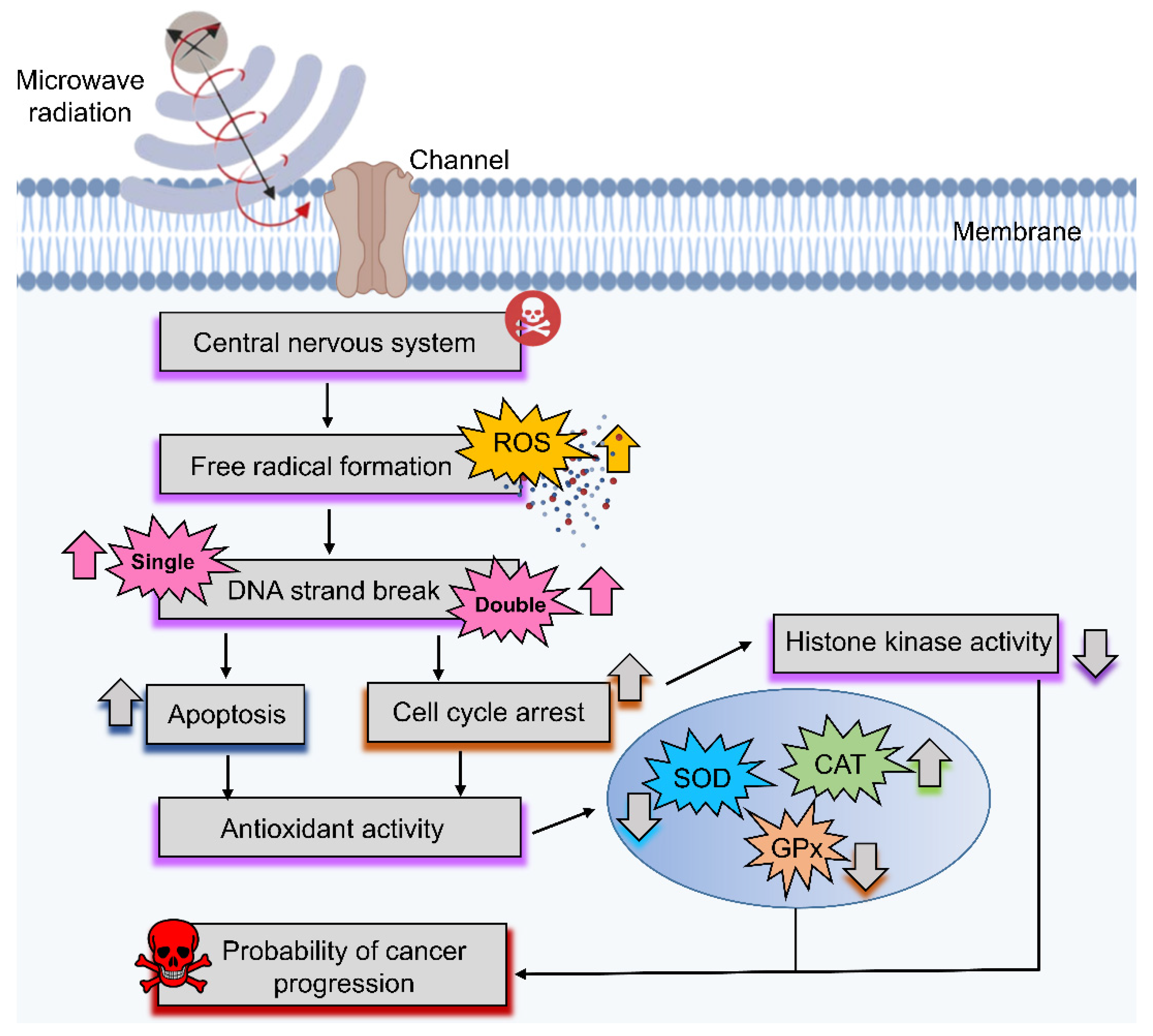
2.4. Effect of Microwave Radiation on the Brain
2.4.1. Positive Effects
2.4.2. Neutral Effects
2.4.3. Negative Effects
| Ref. No. | Frequency | Study Type | Main Findings | Effects |
|---|---|---|---|---|
| [123] | 2.45 GHz | in vivo | Irradiated rats showed a significant decrease in spatial learning and memory performance. | Negative |
| [111] | 2.45 GHz | in vivo | Microwave exposure led to oxidative/nitrosative stress that induced p53 activation of hippocampal neuronal and nonneuronal apoptosis related to memory loss. | Negative |
| [124] | 0.9, 1.8, and 2.45 GHz | in vivo | Microwaves decreased cognitive functions while increasing HSP70 levels and DNA damage in the brain. | Negative |
| [105] | 2.45 and 16.5 GHz | in vivo | Microwave exposure caused DNA single-strand breaks. | Negative |
| [125] | 0.9 GHz | in vitro | No obvious changes were observed in promyelocytic leukemia (HL-60) and neuroblastoma (SK-N-SH) cell lines following microwave exposure. | Neutral |
| [98] | 0.935 GHz | in vitro | No effects in murine microglial (N9) and human neuroblastoma (SH-SY5Y) cells following microwave exposure. | Neutral |
| [126] | 0.9 GHz | in vitro | Increased apoptotic sub-G1 DNA content in human neuroblastoma (SH-SY5Y) cells. Short-term exposures induced a transient rise in Egr-1 mRNA levels, along with activating MAPK subtypes ERK1/2 and SAPK/JNK. | Negative |
| [127] | 0.8–0.9 GHz | in vivo | Microwave exposure led to significant epigenetic modulations in the hippocampus. | Negative |
| [128] | 2.856 GHz | in vivo | Rats exposed to 10 and 50 mW·cm-2 microwaves showed a significant decrease in spatial learning and memory, whereas 5 mW·cm-2 showed no change. | Negative |
| [93] | 2.856 GHz | in vivo | Phospholipid and triglyceride (TG) metabolisms were significantly modified in exposed rats. | Positive |
| [129] | 2.856 GHz | in vivo, in vitro | Microwave exposure at 30 mW·cm-2 altered synaptic structure, amino acid release, and calcium influx. | Negative |
| [130] | 1.7 GHz | in vitro | No effects on human-adipose-tissue-derived stem cells (ASCs) or liver cancer stem cells (Huh7) following microwave exposure. | Neutral |
| [131] | 1.8 GHz | in vitro | Microwave exposure may have decreased the excitatory synaptic activity and the number of excitatory synapses in rat hippocampal neurons. | Negative |
| [132] | 1.8 GHz | in vivo | Hippocampi were injured by long-term microwave exposure, leading to the impairment of cognitive function owing to neurotransmitter disruption. | Negative |
| [133] | 1.8 GHz | in vitro | Microwave exposure at indicated frequencies during the early developmental stage may have influenced dendritic development and excitatory synapse formation in hippocampal neurons. | Negative |
| [94] | 1.8 GHz | in vitro | Microwave exposure significantly increased permeability for 14C-sucrose. | Positive |
| [134] | 1.9 GHz | in vitro | No significant changes were observed across three human-derived immune cell lines (HL-60, Mono-Mac-6, TK6) following microwave exposure. | Neutral |
| [95] | 0.8–1 GHz | in vitro | Microwave radiation exposure across a given frequency range may have induced a considerable survival adaptive response. | Positive |
| [135] | 1 GHz | in vitro | Microwave radiation did not influence efflux in rat brain tissue. | Neutral |
| [136] | 9.3 GHz | in vivo | Irradiation did not affect neuron ability, as no lasting or delayed effects were observed at the analyzed frequency. | Neutral |
| [69] | 50 GHz | in vivo | Microwave exposure caused DNA double-stranded breaks, and changed antioxidant enzymes in the neurological system due to free radical formation. | Negative |
| [96] | 5.8 GHz | in vivo | Microwave exposure did not show any obvious effects on the hippocampal synaptic plasticity of the selected rats at the indicated frequencies. | Neutral |
| [97] | 5.8 GHz | in vitro | Microwave exposure had little to no effect on DNA strand breaks, micronucleus formation, and Hsp expression in eye cells at the assessed frequencies. | Neutral |
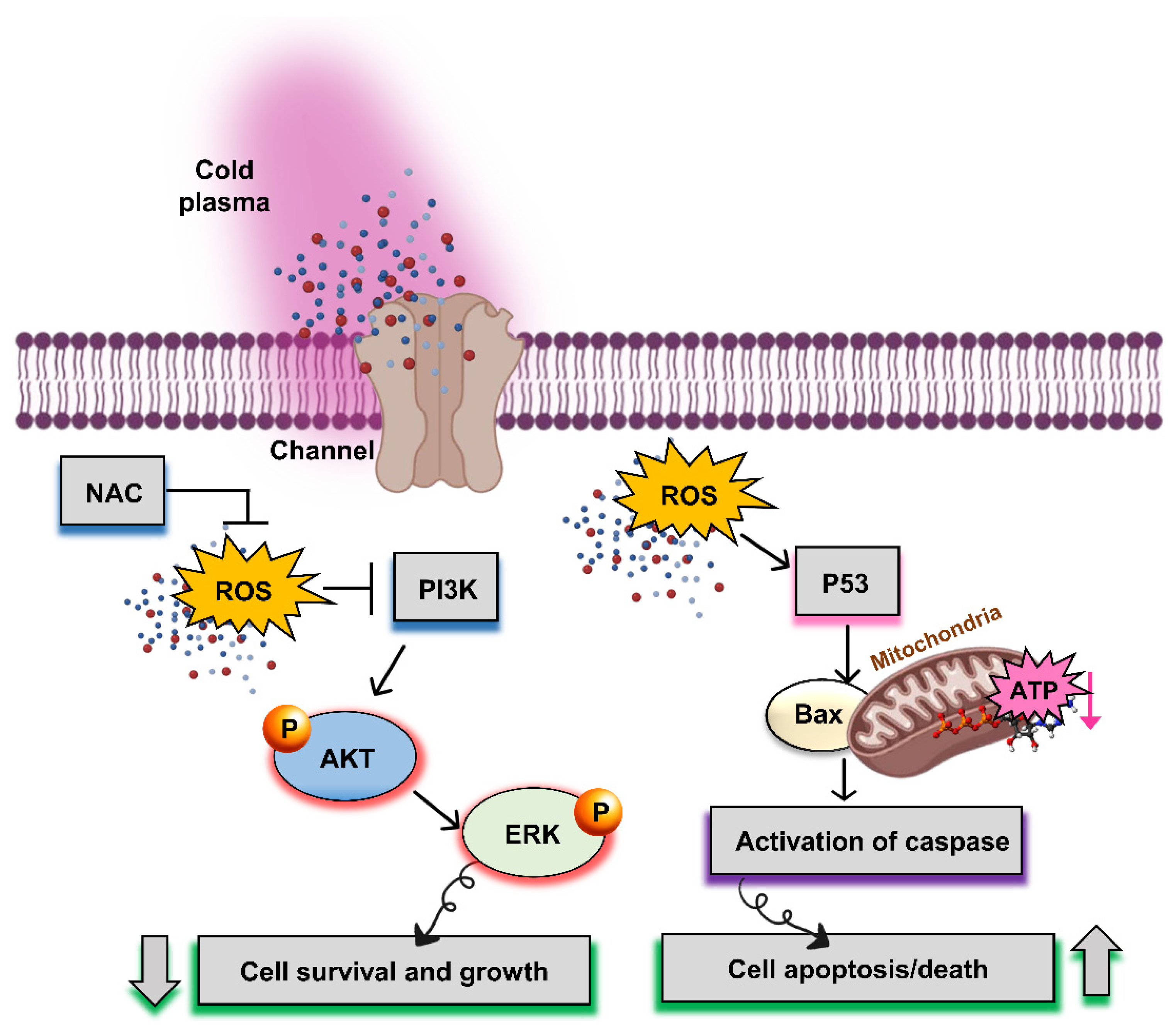
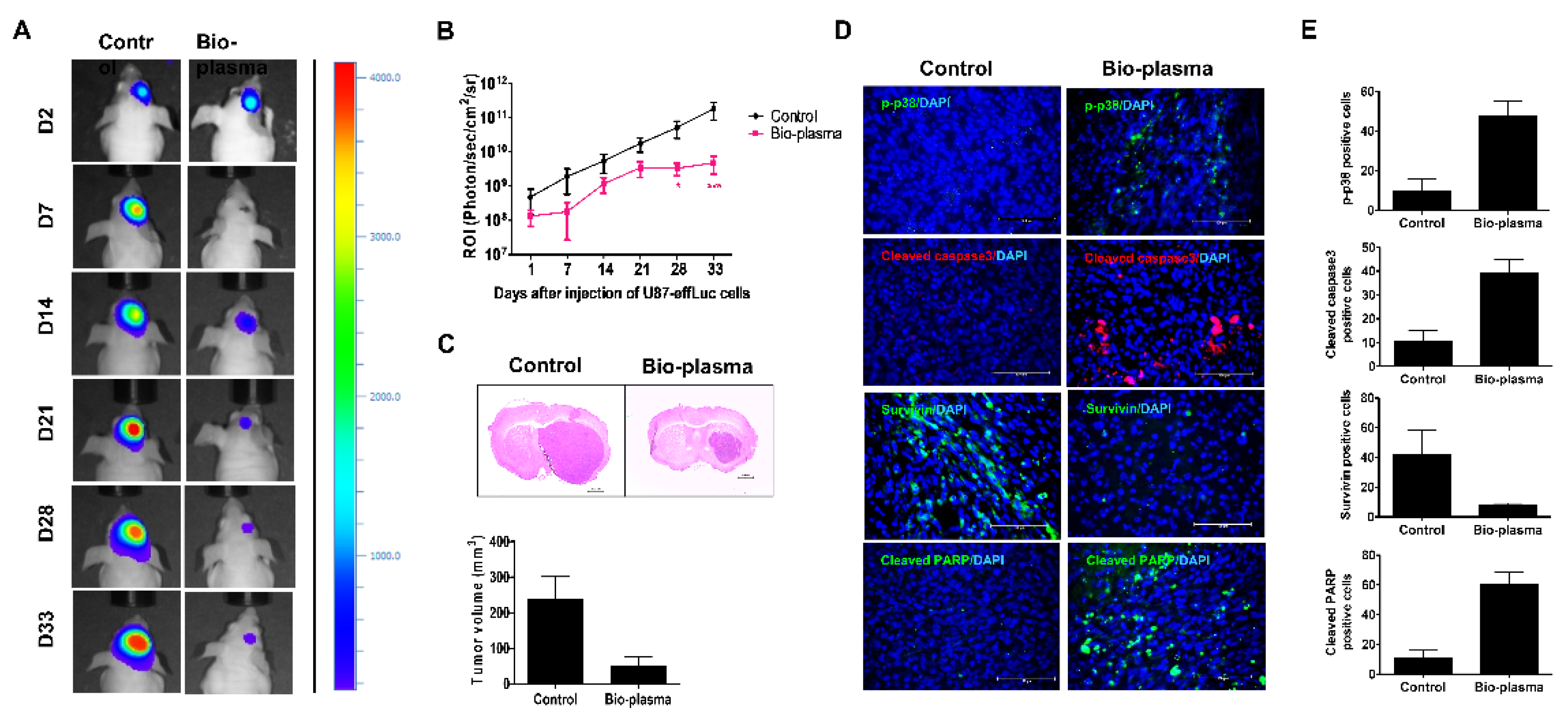
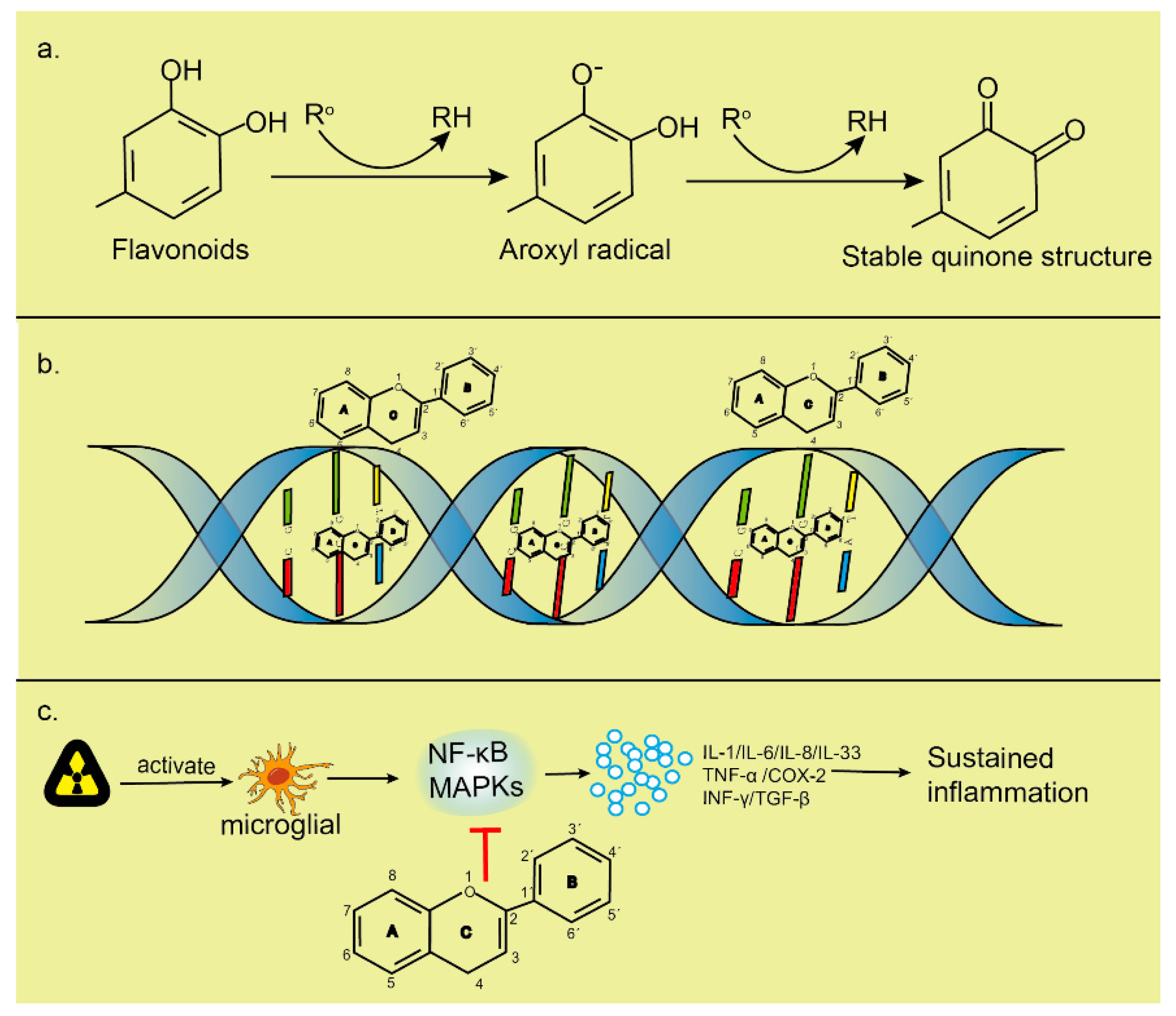
2.5. Protective Techniques to Treat Cancers
2.5.1. Nonthermal Atmospheric Pressure Plasma
2.5.2. Flavonoids
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osepchuk, J.M. A History of Microwave Heating Applications. IEEE Trans. Microw. Theory Tech. 1984, 32, 1200–1224. [Google Scholar] [CrossRef]
- Al_Dulamey, Q.K. The Development of Microwave Applications in Medical Field. Rafidain J. Sci. 2021, 30, 23–39. [Google Scholar] [CrossRef]
- Liu, S.; Cai, W.; Luo, Y.; Dou, J.; Wu, J.; Wu, H.; Han, Z.; Yu, J.; Liang, P. CEUS Versus MRI in Evaluation of the Effect of Microwave Ablation of Breast Cancer. Ultrasound Med. Biol. 2022, 48, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Jeong, K.; Mumtaz, S.; Choi, E.H. Electromagnetic pulse shielding effectiveness of circular multi-waveguides for fluids. Results Phys. 2020, 16, 102946. [Google Scholar] [CrossRef]
- Mumtaz, S.; Uhm, H.; Lim, J.S.; Choi, E.H. Output-power enhancement of vircator based on second virtual cathode formed by wall charge on a dielectric reflector. IEEE Trans. Electron Devices 2022, 69, 2043–2050. [Google Scholar] [CrossRef]
- Mumtaz, S.; Munnaf, S.A.; Choi, E.H. Numerical study on the formation of second virtual cathode by using different material floating zone plate inside drift tube region. In Proceedings of the 2021 22nd International Vacuum Electronics Conference (IVEC), Rotterdam, The Netherlands, 27–30 April 2021; pp. 1–2. [Google Scholar]
- Afzal, A.M.; Iqbal, M.Z.; Dastgeer, G.; Nazir, G.; Mumtaz, S.; Usman, M.; Eom, J. WS2/GeSe/WS2 Bipolar Transistor-Based Chemical Sensor with Fast Response and Recovery Times. ACS Appl. Mater. Interfaces 2020, 12, 39524–39532. [Google Scholar] [CrossRef]
- Jang, J.H.; Mumtaz, S.; Lee, S.W.; Kim, D.-Y.; Lim, J.S.; Kaushik, N.K.; Choi, E.H. Focus of high-power microwaves with positive and negative zone plate to increase the receiving power in axial virtual cathode oscillator. Curr. Appl. Phys. 2021, 29, 89–96. [Google Scholar] [CrossRef]
- Afzal, A.M.; Mumtaz, S.; Iqbal, M.Z.; Iqbal, M.W.; Manzoor, A.; Dastgeer, G.; Iqbal, M.J.; Javed, Y.; Khan, R.; Shad, N.A.; et al. Fast and high photoresponsivity gallium telluride/hafnium selenide van der Waals heterostructure photodiode. J. Mater. Chem. C 2021, 9, 7110–7118. [Google Scholar] [CrossRef]
- Afzal, A.M.; Iqbal, M.Z.; Mumtaz, S.; Akhtar, I. Multifunctional and high-performance GeSe/PdSe2 heterostructure device with a fast photoresponse. J. Mater. Chem. C 2020, 8, 4743–4753. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Mumtaz, S.; Lim, J.S.; Ghimire, B.; Lee, S.W.; Choi, J.J.; Choi, E.H. Enhancing the power of high power microwaves by using zone plate and investigations for the position of virtual cathode inside the drift tube. Phys. Plasmas 2018, 25, 103113. [Google Scholar] [CrossRef]
- Mumtaz, S.; Lamichhane, P.; Lim, J.S.; Yoon, S.H.; Jang, J.H.; Kim, D.; Lee, S.W.; Choi, J.J.; Choi, E.H. Enhancement in the power of microwaves by the interference with a cone-shaped reflector in an axial vircator. Results Phys. 2019, 15, 102611. [Google Scholar] [CrossRef]
- Mumtaz, S.; Chandra Adhikari, B.; Minin, I.V.; Minin, O.V.; Lamichhane, P.; Paneru, R.; Ha Choi, E. Particle in cell simulation for the power enhancement by forming the second virtual cathode in an axial vircator. Results Phys. 2021, 24, 104126. [Google Scholar] [CrossRef]
- Afzal, A.M.; Javed, Y.; Akhtar Shad, N.; Iqbal, M.Z.; Dastgeer, G.; Munir Sajid, M.; Mumtaz, S. Tunneling-based rectification and photoresponsivity in black phosphorus/hexagonal boron nitride/rhenium diselenide van der Waals heterojunction diode. Nanoscale 2020, 12, 3455–3468. [Google Scholar] [CrossRef]
- Saraskanroud, F.M.; Jeffrey, I. Hybrid Approaches in Microwave Imaging Using Quantitative Time- and Frequency-Domain Algorithms. IEEE Trans. Comput. Imaging 2022, 8, 121–132. [Google Scholar] [CrossRef]
- Ullah, R.; Saied, I.; Arslan, T. Measurement of whole-brain atrophy progression using microwave signal analysis. Biomed. Signal Process. Control 2022, 71, 103083. [Google Scholar] [CrossRef]
- Park, W.-K. Real-time detection of small anomaly from limited-aperture measurements in real-world microwave imaging. Mech. Syst. Signal Process. 2022, 171, 108937. [Google Scholar] [CrossRef]
- Mathur, M.; Mathur, D.; Singh, G.; Bhatnagar, S.K.; Nigam, H.; Arora, M. Microwave Imaging Breast Cancer Detection Techniques: A Brief Review. In Optical and Wireless Technologies; Tiwari, M., Maddila, R.K., Garg, A.K., Kumar, A., Yupapin, P., Eds.; Springer: Singapore, 2022; pp. 203–210. [Google Scholar]
- Alam, M.M.; Talukder, M.S.; Samsuzzaman, M.; Khan, A.I.; Kasim, N.; Mehedi, I.M.; Azim, R. W-shaped slot-loaded U-shaped low SAR patch antenna for microwave-based malignant tissue detection system. Chin. J. Phys. 2022, 77, 233–249. [Google Scholar] [CrossRef]
- Alkhodari, M.; Zakaria, A.; Qaddoumi, N. Using prior information to enhance microwave tomography images in bone health assessment. Biomed. Eng. Online 2022, 21, 8. [Google Scholar] [CrossRef]
- Chitra, R.; Sudharsan, G.S.; Rahul, S.G.; Sudheer, S.S.; Amruthavalli, A. Microwaves in Healthcare Systems for Cancer Detection. In Innovations in Mechanical Engineering; Narasimham, G.S.V.L., Babu, A.V., Reddy, S.S., Dhanasekaran, R., Eds.; Springer: Singapore, 2022; pp. 771–782. [Google Scholar]
- Ryan, T.P. 10—History and development of microwave thermal therapy. In Principles and Technologies for Electromagnetic Energy Based Therapies; Prakash, P., Srimathveeravalli, G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 313–347. ISBN 978-0-12-820594-5. [Google Scholar]
- Zhang, J.; Li, C.; Jiang, W.; Wang, Z.; Zhang, L.; Wang, X. Deep-learning-enabled Microwave-induced Thermoacoustic Tomography based on Sparse Data for Breast Cancer Detection. IEEE Trans. Antennas Propag. 2022, 1. [Google Scholar] [CrossRef]
- Afzal, A.M.; Javed, Y.; Hussain, S.; Ali, A.; Yaqoob, M.Z.; Mumtaz, S. Enhancement in photovoltaic properties of bismuth ferrite/zinc oxide heterostructure solar cell device with graphene/indium tin oxide hybrid electrodes. Ceram. Int. 2020, 46, 9161–9169. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Sahu, B.D.; Bogaerts, A.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, P.; Mumtaz, S.; Lim, J.S.; Kaushik, N.; Lamichhane, P.; Nguyen, L.N.; Jang, J.H.; Yoon, S.H.; Choi, J.J.; Kaushik, N.K.; et al. Pulsed 3.5 GHz high power microwaves irradiation on physiological solution and their biological evaluation on human cell lines. Sci. Rep. 2021, 11, 8475. [Google Scholar] [CrossRef] [PubMed]
- Okoniewski, M.; Stuchly, M.A. A study of the handset antenna and human body interaction. IEEE Trans. Microw. Theory Tech. 1996, 44, 1855–1864. [Google Scholar] [CrossRef]
- Dubois, L.; Sozanski, J.-P.; Tessier, V.; Camart, J.C.; Fabre, J.-J.; Pribetich, J.; Chive, M. Temperature control and thermal dosimetry by microwave radiometry in hyperthermia. IEEE Trans. Microw. Theory Tech. 1996, 44, 1755–1761. [Google Scholar] [CrossRef]
- Vander Vorst, A.; Rosen, A.; Kotsuka, Y.; Djajaputra, D. RF/Microwave Interaction with Biological Tissues; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gao, F.; Zheng, Q.; Zheng, Y. Electrical circuit modeling and analysis of microwave acoustic interaction with biological tissues. Med. Phys. 2014, 41, 53302. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of microwave—A brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
- Lin, J. Electromagnetic Interaction with Biological Systems; Springer Science & Business Media: Berlin/Heidelberg, Germany; PLenum Press: New York, NY, USA, 2012. [Google Scholar]
- Hu, C.; Zuo, H.; Li, Y. Effects of Radiofrequency Electromagnetic Radiation on Neurotransmitters in the Brain. Front. Public Health 2021, 9, 1139. [Google Scholar] [CrossRef]
- Wdowiak, A.; Mazurek, P.A.; Wdowiak, A.; Bojar, I. Effect of electromagnetic waves on human reproduction. Ann. Agric. Environ. Med. 2017, 24, 13. [Google Scholar] [CrossRef]
- Sheppard, A.R.; Swicord, M.L.; Balzano, Q. Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys. 2008, 95, 365–396. [Google Scholar] [CrossRef]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef]
- Porcelli, M.; Cacciapuoti, G.; Fusco, S.; Massa, R.; d’Ambrosio, G.; Bertoldo, C.; De Rosa, M.; Zappia, V. Non-thermal effects of microwaves on proteins: Thermophilic enzymes as model system. FEBS Lett. 1997, 402, 102–106. [Google Scholar] [CrossRef]
- de Pomerai, D.I.; Smith, B.; Dawe, A.; North, K.; Smith, T.; Archer, D.B.; Duce, I.R.; Jones, D.; Candido, E.P.M. Microwave radiation can alter protein conformation without bulk heating. FEBS Lett. 2003, 543, 93–97. [Google Scholar] [CrossRef]
- Kesari, K.K.; Jamal, Q.M.; Sharma, A.; Chauhan, P.; Dhasmana, A.; Siddiqui, M.; Sisodia, R.; Verma, H.N. LPO and ROS Production in Rat Brain Exposed to Microwaves: Computational Elucidation of Melatonin in Repair System; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Zhao, X.; Dong, G.; Wang, C. The non-thermal biological effects and mechanisms of microwave exposure. Int. J. Radiat. Res. 2021, 19, 483–494. [Google Scholar] [CrossRef]
- Szmigielski, S.; Szudzinski, A.; Pietraszek, A.; Bielec, M.; Janiak, M.; Wrembel, J.K. Accelerated development of spontaneous and benzopyrene-induced skin cancer in mice exposed to 2450-MHz microwave radiation. Bioelectromagnetics 1982, 3, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Keshri, G.K.; Karmakar, S.; Mani, K.V.; Chauhan, S.; Yadav, A.; Sharma, M.; Gupta, A. Effects of Microwave 10 GHz Radiation Exposure in the Skin of Rats: An Insight on Molecular Responses. Radiat. Res. 2021, 196, 404–416. [Google Scholar] [CrossRef]
- Franchini, V.; Regalbuto, E.; De Amicis, A.; De Sanctis, S.; Di Cristofaro, S.; Coluzzi, E.; Marinaccio, J.; Sgura, A.; Ceccuzzi, S.; Doria, A.; et al. Genotoxic Effects in Human Fibroblasts Exposed to Microwave Radiation. Health Phys. 2018, 115, 126–139. [Google Scholar] [CrossRef]
- Mumtaz, S.; Bhartiya, P.; Kaushik, N.; Adhikari, M.; Lamichhane, P.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Pulsed high-power microwaves do not impair the functions of skin normal and cancer cells in vitro: A short-term biological evaluation. J. Adv. Res. 2020, 22, 47–55. [Google Scholar] [CrossRef]
- Leszczynski, D. Physiological effects of millimeter-waves on skin and skin cells: An overview of the to-date published studies. Rev. Environ. Health 2020, 35, 493–515. [Google Scholar] [CrossRef]
- Avendaño, C.; Mata, A.; Sanchez Sarmiento, C.A.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45.e2. [Google Scholar] [CrossRef]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef]
- McGill, J.J.; Agarwal, A. The Impact of Cell Phone, Laptop Computer, and Microwave Oven Usage on Male Fertility. In Male Infertility: A Complete Guide to Lifestyle and Environmental Factors; du Plessis, S.S., Agarwal, A., Sabanegh Edmund, S.J., Eds.; Springer: New York, NY, USA, 2014; pp. 161–177. ISBN 978-1-4939-1040-3. [Google Scholar]
- Dong, G.; Zhou, H.; Gao, Y.; Zhao, X.; Liu, Q.; Li, Z.; Zhao, X.; Yin, J.; Wang, C. Effects of 1.5-GHz high-power microwave exposure on the reproductive systems of male mice. Electromagn. Biol. Med. 2021, 40, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Taheri, M.; Khalili, M.A.; Hosseininejad Mohebati, A.; Zardast, M.; Hosseini, M.; Palmerini, M.G.; Doostabadi, M.R. The detrimental effect of cell phone radiation on sperm biological characteristics in normozoospermic. Andrologia 2022, 54, e14257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Behari, J.; Sisodia, R. Influence of electromagnetic fields on reproductive system of male rats. Int. J. Radiat. Biol. 2013, 89, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bilgici, B.; Gun, S.; Avci, B.; Akar, A.; Engiz, B.K. What is adverse effect of wireless local area network, using 2.45 GHz, on the reproductive system? Int. J. Radiat. Biol. 2018, 94, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Jonwal, C.; Sisodia, R.; Saxena, V.K.; Kesari, K.K. Effect of 2.45 GHz microwave radiation on the fertility pattern in male mice. Gen. Physiol. Biophys. 2018, 37, 453–460. [Google Scholar] [CrossRef]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Mostafa, T. In vitro effect of cell phone radiation on motility, DNA fragmentation and clusterin gene expression in human sperm. Int. J. Fertil. Steril. 2015, 9, 129–136. [Google Scholar] [CrossRef]
- Gorpinchenko, I.; Nikitin, O.; Banyra, O.; Shulyak, A. The influence of direct mobile phone radiation on sperm quality. Cent. Eur. J. Urol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
- Er, H.; Tas, G.G.; Soygur, B.; Ozen, S.; Sati, L. Acute and Chronic Exposure to 900 MHz Radio Frequency Radiation Activates p38/JNK-mediated MAPK Pathway in Rat Testis. Reprod. Sci. 2022, 29, 1471–1485. [Google Scholar] [CrossRef]
- PLoSkonos, V.M.; Zulbalaeva, F.D.; Kurbangalieva, R.N.; Ripp, V.S.; Neborak, V.E.; Blagonravov, L.M.; Syatkin, P.S.; Sungrapova, K.; Hilal, A. Assessing the biological effects of microwave irradiation on human semen in vitro and determining the role of seminal plasma polyamines in this process. Biomed. Rep. 2022, 16, 38. [Google Scholar] [CrossRef]
- Mirbeik, A.; Ashinoff, R.; Jong, T.; Aued, A.; Tavassolian, N. Real-time high-resolution millimeter-wave imaging for in-vivo skin cancer diagnosis. Sci. Rep. 2022, 12, 4971. [Google Scholar] [CrossRef] [PubMed]
- Lindley-Hatcher, H.; Stantchev, R.I.; Chen, X.; Hernandez-Serrano, A.I.; Hardwicke, J.; Pickwell-MacPherson, E. Real time THz imaging—Opportunities and challenges for skin cancer detection. Appl. Phys. Lett. 2021, 118, 230501. [Google Scholar] [CrossRef]
- Furman, O.; Komoshvili, K.; Levitan, J.; Yahalom, A.; Marks, H.; Borodin, D.; Liberman-Aronov, S. The Lack of Toxic Effect of High-Power Short-Pulse 101 GHz Millimeter Waves on Healthy Mice. Bioelectromagnetics 2020, 41, 188–199. [Google Scholar] [CrossRef]
- Zhadobov, M.; Chahat, N.; Sauleau, R.; Le Quement, C.; Le Drean, Y. Millimeter-wave interactions with the human body: State of knowledge and recent advances. Int. J. Microw. Wirel. Technol. 2011, 3, 237–247. [Google Scholar] [CrossRef]
- Kues, H.A.; D’Anna, S.A.; Osiander, R.; Green, W.R.; Monahan, J.C. Absence of ocular effects after either single or repeated exposure to 10 mW/cm2 from a 60 GHz CW source. Bioelectromagnetics 1999, 20, 463–473. [Google Scholar] [CrossRef]
- Qi, M.; Liu, R.; Li, B.; Wang, S.; Fan, R.; Zhao, X.; Xu, D. Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors 2022, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Kovalevska, L.; Golenkov, O.; Kulahina, Y.; Callender, T.; Sizov, F.; Kashuba, E. A Comparative Study on the Viability of Normal and Cancerous Cells upon Irradiation with a Steady Beam of THz Rays. Life 2022, 12, 376. [Google Scholar] [CrossRef]
- Hu, E.; Wang, L.; Zhang, Q.; Li, P.; Zhang, P.; Wu, D.; Lu, X. Studying the influence of 3.1 THz irradiation on the endocytosis of neuronal cells. J. Opt. Soc. Am. B 2022, 39, 129–136. [Google Scholar] [CrossRef]
- Homenko, A.; Kapilevich, B.; Kornstein, R.; Firer, M.A. Effects of 100 GHz radiation on alkaline phosphatase activity and antigen–antibody interaction. Bioelectromagnetics 2009, 30, 167–175. [Google Scholar] [CrossRef]
- Lin, J.C. The Microwave Auditory Effect. In Auditory Effects of Microwave Radiation; Lin, J.C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 127–173. ISBN 978-3-030-64544-1. [Google Scholar]
- Kesari, K.K.; Behari, J. Fifty-gigahertz Microwave Exposure Effect of Radiations on Rat Brain. Appl. Biochem. Biotechnol. 2008, 158, 126. [Google Scholar] [CrossRef]
- Sylvestre, D.A.; Otoki, Y.; Metherel, A.H.; Bazinet, R.P.; Slupsky, C.M.; Taha, A.Y. Effects of hypercapnia / ischemia and dissection on the rat brain metabolome. Neurochem. Int. 2022, 156, 105294. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, W.; Wang, H.; Zhang, J.; Wang, H.; Dong, J.; Yao, B.; Xu, X.; Zhao, L.; Peng, R. Microwave radiation induces neuronal autophagy through miR-30a-5p/AMPKα2 signal pathway. Biosci. Rep. 2022, 42, BSR20212584. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Barnes, R.A.; Downey, A.X.; Freeman, D.A.; Johnson, L.R.; Rodriguez, R.A.; Sloan, M.A.; Valdez, C.M.; Voorhees, W.B.; Whitmore, J.N. Temperature Dynamics in Rat Brains Exposed to Near-Field Waveguide Outputs at 2.8 GHz. Bioelectromagnetics 2022, 43, 14–24. [Google Scholar] [CrossRef] [PubMed]
- De Seze, R.; Poutriquet, C.; Gamez, C.; Maillot-Maréchal, E.; Robidel, F.; Lecomte, A.; Fonta, C. Repeated exposure to nanosecond high power pulsed microwaves increases cancer incidence in rat. PLoS ONE 2020, 15, e0226858. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Hahn, G.M. The Use of Microwaves for the Hyperthermic Treatment of Cancer: Advantages and Disadvantages. In Photochemical and Photobiological Reviews; Smith, K.C., Ed.; Springer: Boston, MA, USA, 1978; Volume 3, pp. 277–301. ISBN 978-1-4684-2580-2. [Google Scholar]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Benford, J. Space Applications of High-Power Microwaves. IEEE Trans. Plasma Sci. 2008, 36, 569–581. [Google Scholar] [CrossRef]
- Keglevich, G. The Impact of Microwaves on Organophosphorus Chemistry. Chem. Rec. 2019, 19, 65–76. [Google Scholar] [CrossRef]
- Scapaticci, R.; Bucci, O.M.; Catapano, I.; Crocco, L. Differential Microwave Imaging for Brain Stroke Followup. Int. J. Antennas Propag. 2014, 2014, 312528. [Google Scholar] [CrossRef]
- Gavrilin, E.V.; Dunaevskiy, G.E.; Antipov, V.B. Microwave Treatment of Cold Injuries. J. Emerg. Trauma Shock 2021, 14, 108–110. [Google Scholar] [CrossRef]
- Bayat, M.; Karimi, N.; Karami, M.; Haghighi, A.B.; Bayat, K.; Akbari, S.; Haghani, M. Chronic exposure to 2.45 GHz microwave radiation improves cognition and synaptic plasticity impairment in vascular dementia model. Int. J. Neurosci. 2021, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Bakian-Dogaheh, K.; Stang, J.; Tabatabaeenejad, A.; Moghaddam, M. A Versatile and Shelf-Stable Dielectric Coupling Medium for Microwave Imaging. IEEE Trans. Biomed. Eng. 2022, 69, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Raterink, A.; Farshkaran, A. Microwave-Based Detection of the Bladder State as a Support Tool for Urinary Incontinence [Bioelectromagnetics]. IEEE Antennas Propag. Mag. 2022, 64, 112–122. [Google Scholar] [CrossRef]
- Chitra, R.; Sudharsan, G.S.; Rahul, S.G.; Sudheer, S.S.; Amruthavalli, A. Microwaves in Health Care for Breast Cancer Detection. In Proceedings of the International Conference on Computational Intelligence and Computing; Mandal, J.K., Roy, J.K., Eds.; Springer: Singapore, 2022; pp. 273–284. [Google Scholar]
- Hammouch, N.; Ammor, H.; Himdi, M. A Compact Flexible UWB Antenna for Biomedical Applications: Especially for Breast Cancer Detection. In WITS 2020; Bennani, S., Lakhrissi, Y., Khaissidi, G., Mansouri, A., Khamlichi, Y., Eds.; Springer: Singapore, 2022; pp. 1061–1072. [Google Scholar]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A Feasibility Study on Microwave Imaging for Brain Stroke Monitoring. Prog. Electromagn. Res. B 2012, 40, 305–312. [Google Scholar] [CrossRef]
- Deowan, M.E.; Nuhel, A.K.; Hossain, M.S.; Ullah, A.; Rahman, M.A. Study of UWB Near Field Microwave Imaging System for the Diagnosis of Lungs Damage Due to Fluid Accumulations. In Proceedings of the 2022 International Conference for Advancement in Technology (ICONAT), Goa, India, 21–22 January 2022; pp. 1–6. [Google Scholar]
- Bhargava, D.; Rattanadecho, P. Microwave imaging of breast cancer: Simulation analysis of SAR and temperature in tumors for different age and type. Case Stud. Therm. Eng. 2022, 31, 101843. [Google Scholar] [CrossRef]
- Lalitha, K.; Manjula, J. Non-invasive microwave head imaging to detect tumors and to estimate their size and location. Phys. Med. 2022, 13, 100047. [Google Scholar] [CrossRef]
- Kandasamy, L.; Manjula, J. Ground Penetrating Radar Algorithm to Sense the Depth of Blood Clot in Microwave Head Imaging. Curr. Med. Imaging 2022, 18, 845–854. [Google Scholar] [CrossRef]
- Chiaramello, E.; Parazzini, M.; Fiocchi, S.; Ravazzani, P.; Wiart, J. Stochastic Dosimetry Based on Low Rank Tensor Approximations for the Assessment of Children Exposure to WLAN Source. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 131–137. [Google Scholar] [CrossRef]
- Semenov, S.; Seiser, B.; Stoegmann, E.; Auff, E. Electromagnetic tomography for brain imaging: From virtual to human brain. In Proceedings of the 2014 IEEE Conference on Antenna Measurements & Applications (CAMA), Antibes Juan-les-Pins, France, 16–19 November 2014; pp. 1–4. [Google Scholar]
- Xia, Z.; Li, M.; Tian, Y.; Li, Y.; Li, B.; Zhang, G.; Lv, J.; Fu, Q.; Zhou, H.; Dong, J. Lipidomics of Serum and Hippocampus Reveal the Protective Effects of Fermented Soybean Lipid on Rats of Microwave-Induced Cognitive Damage. ACS Chem. Neurosci. 2021, 12, 2122–2132. [Google Scholar] [CrossRef]
- Schirmacher, A.; Winters, S.; Fischer, S.; Goeke, J.; Galla, H.-J.; Kullnick, U.; Ringelstein, E.B.; Stögbauer, F. Electromagnetic fields (1.8 GHz) increase the permeability to sucrose of the blood–brain barrier in vitro. Bioelectromagnetics 2000, 21, 338–345. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Mosleh-Shirazi, M.A.; Tavassoli, A.R.; Taheri, M.; Mehdizadeh, A.R.; Namazi, S.A.S.; Jamali, A.; Ghalandari, R.; Bonyadi, S.; Haghani, M.; et al. Increased Radioresistance to Lethal Doses of Gamma Rays in Mice and Rats after Exposure to Microwave Radiation Emitted by a GSM Mobile Phone Simulator. Dose-Response 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Rui, G.; Liu, L.-Y.; Guo, L.; Xue, Y.-Z.; Lai, P.-P.; Gao, P.; Xing, J.-L.; Li, J.; Ding, G.-R. Effects of 5.8 GHz microwave on hippocampal synaptic plasticity of rats. Int. J. Environ. Health Res. 2021, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, J.; Tonomura, H.; Koyama, S.; Narita, E.; Shinohara, N. Effects of Exposure to 5.8 GHz Electromagnetic Field on Micronucleus Formation, DNA Strand Breaks, and Heat Shock Protein Expressions in Cells Derived From Human Eye. IEEE Trans. Nanobiosci. 2019, 18, 257–260. [Google Scholar] [CrossRef]
- Zielinski, J.; Ducray, A.D.; Moeller, A.M.; Murbach, M.; Kuster, N.; Mevissen, M. Effects of pulse-modulated radiofrequency magnetic field (RF-EMF) exposure on apoptosis, autophagy, oxidative stress and electron chain transport function in human neuroblastoma and murine microglial cells. Toxicol. Vitr. 2020, 68, 104963. [Google Scholar] [CrossRef]
- LAI, H. Single-and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int. J. Radiat. Biol. 1996, 69, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Alkis, M.E.; Akdag, M.Z.; Dasdag, S. Effects of Low-Intensity Microwave Radiation on Oxidant-Antioxidant Parameters and DNA Damage in the Liver of Rats. Bioelectromagnetics 2021, 42, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yuan, C.; Feng, Y.; Qian, J.; Huang, X.; Chen, Q.; Zhou, S.; Ding, Y.; Zhai, B.; Mei, W.; et al. Microwave-assisted synthesis of ruthenium(ii) complexes containing levofloxacin-induced G2/M phase arrest by triggering DNA damage. RSC Adv. 2021, 11, 4444–4453. [Google Scholar] [CrossRef] [PubMed]
- Alkis, M.E.; Bilgin, H.M.; Akpolat, V.; Dasdag, S.; Yegin, K.; Yavas, M.C.; Akdag, M.Z. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn. Biol. Med. 2019, 38, 32–47. [Google Scholar] [CrossRef]
- Hasan, I.; Rubayet Jahan, M.; Nabiul Islam, M.; Rafiqul Islam, M. Effect of 2400 MHzmobile phone radiation exposure on the behavior and hippocampus morphology in Swiss mouse model. Saudi J. Biol. Sci. 2022, 29, 102–110. [Google Scholar] [CrossRef]
- Jorge-Mora, T.; Folgueiras, M.A.; Leiro-Vidal, J.M.; Jorge-Barreiro, F.J.; Ares-Pena, F.J.; Lopez-Martin, E. Exposure to 2.45 GHz Microwave Radiation Provokes Cerebral Changes in Induction of Hsp-90 α/β Heat Shock Protein in Rat. Prog. Electromagn. Res. 2010, 100, 351–379. [Google Scholar] [CrossRef]
- Paulraj, R.; Behari, J. Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutat. Res. Mol. Mech. Mutagen. 2006, 596, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L. The Role of DNA Single- and Double-Strand Breaks in Cell Killing by Ionizing Radiation. Radiat. Res. 1998, 150, S42–S51. [Google Scholar] [CrossRef]
- Cox, R. Molecular Mechanisms of Radiation Oncogenesis. Int. J. Radiat. Biol. 1994, 65, 57–64. [Google Scholar] [CrossRef]
- D’Andrea, J.A.; Chou, C.K.; Johnston, S.A.; Adair, E.R. Microwave effects on the nervous system. Bioelectromagnetics 2003, 24, S107–S147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, W.-J.; Chen, W.-W. Microwaves and Alzheimer’s disease (Review). Exp. Med. 2016, 12, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Majumdar, A.; Kumar, G.; Shukla, A. Rats exposed to 2.45 GHz of non-ionizing radiation exhibit behavioral changes with increased brain expression of apoptotic caspase 3. Pathophysiology 2018, 25, 19–30. [Google Scholar] [CrossRef]
- Shahin, S.; Banerjee, S.; Singh, S.P.; Chaturvedi, C.M. 2.45 GHz Microwave Radiation Impairs Learning and Spatial Memory via Oxidative/Nitrosative Stress Induced p53-Dependent/Independent Hippocampal Apoptosis: Molecular Basis and Underlying Mechanism. Toxicol. Sci. 2015, 148, 380–399. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Wang, H.; Zhang, J.; Yu, C.; Tan, S.; Wang, H.; Xu, X.; Dong, J.; Yao, B.; et al. Autophagy mediates the degradation of synaptic vesicles: A potential mechanism of synaptic plasticity injury induced by microwave exposure in rats. Physiol. Behav. 2018, 188, 119–127. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, H.; Xu, X.; Zhao, L.; Zhang, J.; Dong, J.; Yao, B.; Wang, H.; Zhou, H.; Gao, Y.; et al. Effects of 1.5 and 4.3 GHz microwave radiation on cognitive function and hippocampal tissue structure in Wistar rats. Sci. Rep. 2021, 11, 10061. [Google Scholar] [CrossRef]
- S1rav, B.; Seyhan, N. Effects of radio-frequency radiation on the permeability of blood-brain barrier. Med. Sci. Discov. 2016, 3, 206. [Google Scholar] [CrossRef]
- Tan, S.; Wang, H.; Xu, X.; Zhao, L.; Zhang, J.; Dong, J.; Yao, B.; Wang, H.; Hao, Y.; Zhou, H.; et al. Acute effects of 2.856 GHz and 1.5 GHz microwaves on spatial memory abilities and CREB-related pathways. Sci. Rep. 2021, 11, 12348. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kesari, K.K.; Saxena, V.K.; Sisodia, R. Ten gigahertz microwave radiation impairs spatial memory, enzymes activity, and histopathology of developing mice brain. Mol. Cell. Biochem. 2017, 435, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Lin, T.; Wang, D.; Peng, R.; Wang, S.; Gao, Y.; Xu, X.; Li, Y.; Wang, S.; Zhao, L.; et al. Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int. J. Med. Sci. 2014, 11, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Park, J.-E.; Seo, Y.-K.; Yoon, H.-H.; Kim, C.-W.; Park, J.-K.; Jeon, S. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem. Int. 2013, 62, 418–424. [Google Scholar] [CrossRef]
- Kıvrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef]
- Aydin, B.; Akar, A. Effects of a 900-MHz Electromagnetic Field on Oxidative Stress Parameters in Rat Lymphoid Organs, Polymorphonuclear Leukocytes and Plasma. Arch. Med. Res. 2011, 42, 261–267. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ. Health Perspect. 2004, 112, 687–694. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Zhang, Y.; Zhou, Z.; Yu, Z. Elevation of Plasma Corticosterone Levels and Hippocampal Glucocorticoid Receptor Translocation in Rats: A Potential Mechanism for Cognition Impairment Following Chronic Low-power-density Microwave Exposure. J. Radiat. Res. 2008, 49, 163. [Google Scholar] [CrossRef]
- Deshmukh, P.S.; Megha, K.; Nasare, N.; Banerjee, B.D.; Ahmed, R.S.; Abegaonkar, M.P.; Tripathi, A.K.; Mediratta, P.K. Effect of Low Level Subchronic Microwave Radiation on Rat Brain. Biomed. Environ. Sci. 2016, 29, 858–867. [Google Scholar] [CrossRef]
- Gurisik, E.; Warton, K.; Martin, D.K.; Valenzuela, S.M. An in vitro study of the effects of exposure to a GSM signal in two human cell lines: Monocytic U937 and neuroblastoma SK-N-SH. Cell Biol. Int. 2006, 30, 793–799. [Google Scholar] [CrossRef]
- Buttiglione, M.; Roca, L.; Montemurno, E.; Vitiello, F.; Capozzi, V.; Cibelli, G. Radiofrequency radiation (900 MHz) induces Egr-1 gene expression and affects cell-cycle control in human neuroblastoma cells. J. Cell. Physiol. 2007, 213, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Deshmukh, P.S.; Sharma, S.; Banerjee, B.D. Effect of mobile phone signal radiation on epigenetic modulation in the hippocampus of Wistar rat. Environ. Res. 2021, 192, 110297. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Zhou, H.; Wang, S.; Gao, Y.; Wang, L.; Yong, Z.; Zuo, H.; Zhao, L.; Dong, J.; et al. Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwave exposure. Int. J. Radiat. Biol. 2013, 89, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Sun, C.F.; Zhang, J.; Gao, Y.B.; Wang, L.F.; Zuo, H.Y.; Wang, S.M.; Zhou, H.M.; Xu, X.P.; Dong, J.; et al. Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed. Environ. Sci. 2015, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Min, K.; Jeon, S.; Kim, N.; Pack, J.-K.; Song, K. Continuous Exposure to 1.7 GHz LTE Electromagnetic Fields Increases Intracellular Reactive Oxygen Species to Decrease Human Cell Proliferation and Induce Senescence. Sci. Rep. 2020, 10, 9238. [Google Scholar] [CrossRef]
- Xu, S.; Ning, W.; Xu, Z.; Zhou, S.; Chiang, H.; Luo, J. Chronic exposure to GSM 1800-MHz microwaves reduces excitatory synaptic activity in cultured hippocampal neurons. Neurosci. Lett. 2006, 398, 253–257. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, R.Y.; Wang, S.M.; Wang, L.F.; Gao, Y.B.; Dong, J.; Li, X.; Su, Z.T. Relationship between Cognition Function and Hippocampus Structure after Long-term Microwave Exposure. Biomed. Environ. Sci. 2012, 25, 182–188. [Google Scholar] [CrossRef]
- Ning, W.; Xu, S.; Chiang, H.; Xu, Z.; Zhou, S.; Yang, W.; Luo, J. Effects of GSM 1800 MHz on dendritic development of cultured hippo-campal neurons1. Acta Pharmacol. Sin. 2007, 28, 1873–1880. [Google Scholar] [CrossRef]
- Chauhan, V.; Mariampillai, A.; Kutzner, B.C.; Wilkins, R.C.; Ferrarotto, C.; Bellier, P.V.; Marro, L.; Gajda, G.B.; Lemay, E.; Thansandote, A.; et al. Evaluating the Biological Effects of Intermittent 1.9 GHz Pulse-Modulated Radiofrequency Fields in a Series of Human-Derived Cell Lines. Radiat. Res. 2007, 167, 87–93. [Google Scholar] [CrossRef]
- Shelton, W.W., Jr.; Merritt, J.H. In vitro study of microwave effects on calcium efflux in rat brain tissue. Bioelectromagnetics 1981, 2, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Doyle, J.; Stuck, B.E.; Murphy, M.R. Effects of high power microwave pulses on synaptic transmission and long term potentiation in hippocampus. Bioelectromagnetics 2003, 24, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Rana, J.N.; Kim, J.-H.; Choi, E.H.; Kim, Y. A Non-thermal Biocompatible Plasma-Modified Chitosan Scaffold Enhances Osteogenic Differentiation in Bone Marrow Stem Cells. Pharmaceutics 2022, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. (Invited) Plasma Sources for Plasma Medicine. ECS Meet. Abstr. 2021, MA2021-02, 684. [Google Scholar] [CrossRef]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma Cancer Treatment, a Critical Review. Appl. Sci. 2021, 11, 7757. [Google Scholar] [CrossRef]
- Akter, M.; Lim, J.S.; Choi, E.H.; Han, I. Non-Thermal Biocompatible Plasma Jet Induction of Apoptosis in Brain Cancer Cells. Cells 2021, 10, 236. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 33001. [Google Scholar] [CrossRef]
- Attri, P.; Kaushik, N.K.; Kaushik, N.; Hammerschmid, D.; Privat-Maldonado, A.; De Backer, J.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Plasma treatment causes structural modifications in lysozyme, and increases cytotoxicity towards cancer cells. Int. J. Biol. Macromol. 2021, 182, 1724–1736. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.W.; Jung, M.H.; Kim, Y.S.; Kim, K.S.; Suh, D.S.; Kim, K.H.; Choi, E.H.; Kim, J.; Kwon, B.S. Plasma-activated medium inhibits cancer stem cell-like properties and exhibits a synergistic effect in combination with cisplatin in ovarian cancer. Free Radic. Biol. Med. 2022, 182, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma Apoptotic and Oxidative Effects on MCF7 and HCC1806 Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef] [PubMed]
- Gerling, T.; Bansemer, R.; Timmermann, E.; Weltmann, K.-D. Basic Principles and Future Developments in Cold Plasma Therapy. In Textbook of Good Clinical Practice in Cold Plasma Therapy; Metelmann, H.-R., von Woedtke, T., Weltmann, K.-D., Emmert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 257–280. ISBN 978-3-030-87857-3. [Google Scholar]
- Babington, P.; Rajjoub, K.; Canady, J.; Siu, A.; Keidar, M.; Sherman, J.H. Use of cold atmospheric plasma in the treatment of cancer. Biointerphases 2015, 10, 29403. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 45026. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Paneru, R.; Ki, S.H.; Lamichhane, P.; Nguyen, L.N.; Adhikari, B.C.; Jeong, I.J.; Mumtaz, S.; Choi, J.; Kwon, J.S.; Choi, E.H. Enhancement of antibacterial and wettability performances of polyvinyl alcohol/chitosan film using non-thermal atmospheric pressure plasma. Appl. Surf. Sci. 2020, 532, 147339. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 075021. [Google Scholar] [CrossRef]
- Lim, J.S.; Hong, Y.J.; Ghimire, B.; Choi, J.; Mumtaz, S.; Choi, E.H. Measurement of electron density in transient spark discharge by simple interferometry. Results Phys. 2021, 20, 103693. [Google Scholar] [CrossRef]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef]
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 265206. [Google Scholar] [CrossRef]
- Akter, M.; Jangra, A.; Choi, S.A.; Choi, E.H.; Han, I. Non-Thermal Atmospheric Pressure Bio-Compatible Plasma Stimulates Apoptosis via p38/MAPK Mechanism in U87 Malignant Glioblastoma. Cancers 2020, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Sengul, E.; Sharbati, P.; Elitas, M.; Islam, M.; Korvink, J.G. Analysis of U87 glioma cells by dielectrophoresis. Electrophoresis 2022, 43, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Rajjoub, K.; Sherman, J.; Canady, J.; Recek, N.; Yan, D.; Bian, K.; Murad, F.; Keidar, M. Cold Plasma Accelerates the Uptake of Gold Nanoparticles Into Glioblastoma Cells. Plasma Process. Polym. 2015, 12, 1364–1369. [Google Scholar] [CrossRef]
- Sklias, K.; Santos Sousa, J.; Girard, P.-M. Role of Short- and Long-Lived Reactive Species on the Selectivity and Anti-Cancer Action of Plasma Treatment In Vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Horn, S.; Niessner, F.; Sagwal, S.K.; von Woedtke, T.; Emmert, S.; Weltmann, K.-D.; Clemen, R.; Schmidt, A.; et al. Tumor cytotoxicity and immunogenicity of a novel V-jet neon plasma source compared to the kINPen. Sci. Rep. 2021, 11, 136. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.-D.; Metelmann, H.-R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, N.; Li, J.; Liu, Y.; Li, Y.; Wang, X.; Wang, J.; Wang, Y.; Wang, A. Protective effects of low-temperature plasma on cisplatin-induced nephrotoxicity. Life Sci. 2022, 289, 120230. [Google Scholar] [CrossRef]
- Yang, B.; Ren, B.X.; Tang, F.R. Prenatal irradiation–induced brain neuropathology and cognitive impairment. Brain Dev. 2017, 39, 10–22. [Google Scholar] [CrossRef]
- Peng, X.C.; Huang, J.R.; Wang, S.W.; Liu, L.; Liu, Z.Z.; Sethi, G.; Ren, B.X.; Tang, F.R. Traditional Chinese Medicine in Neuroprotection after Brain Insults with Special Reference to Radioprotection. Evid. Based Complement. Altern. Med. 2018, 2018, 2767208. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D., Jr. Studies of Atomic Bomb Survivors Understanding Radiation Effects. JAMA 1990, 264, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, C.; Xi, S.; Qian, F.; Peng, X.; Huang, J.; Tang, F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules 2020, 25, 5719. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov. Today 2010, 15, 907–918. [Google Scholar] [CrossRef]
- Kim, W.; Seong, K.M.; Youn, B. Phenylpropanoids in radioregulation: Double edged sword. Exp. Mol. Med. 2011, 43, 323–333. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, K.P. Flavonoids sensitize tumor cells to radiation: Molecular mechanisms and relevance to cancer radiotherapy. Int. J. Radiat. Biol. 2020, 96, 360–369. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.R.; El-Esawy, R.O.; El-Sakaa, M.H. Troxerutin downregulates C/EBP-β gene expression via modulating the IFNγ-ERK1/2 signaling pathway to ameliorate rotenone-induced retinal neurodegeneration. J. Biochem. Mol. Toxicol. 2020, 34, e22482. [Google Scholar] [CrossRef]
- Kale, A.; Pişkin, Ö.; Baş, Y.; Aydın, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood–brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord Grape Juice Supplementation and Neurocognitive Function in Human Aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- McRobb, L.S.; McKay, M.J.; Gamble, J.R.; Grace, M.; Moutrie, V.; Santos, E.D.; Lee, V.S.; Zhao, Z.; Molloy, M.P.; Stoodley, M.A. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging 2017, 9, 1248–1268. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Huang, W. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Prieto, P.; De La Hoz, A. A Critical Overview on the Effect of Microwave Irradiation in Organic Synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Gye, M.C.; Park, C.J. Effect of electromagnetic field exposure on the reproductive system. Clin. Exp. Reprod. Med. 2012, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Weinberg, U.; Palti, Y. Tumor treating fields: A new frontier in cancer therapy. Ann. N. Y. Acad. Sci. 2013, 1291, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Pless, M.; Weinberg, U. Tumor treating fields: Concept, evidence and future. Expert Opin. Investig. Drugs 2011, 20, 1099–1106. [Google Scholar] [CrossRef]
- Kim, E.H.; Song, H.S.; Yoo, S.H.; Yoon, M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 2016, 7, 65125–65136. [Google Scholar] [CrossRef]
- Meggyeshazi, N.; Andocs, G.; Balogh, L.; Balla, P.; Kiszner, G.; Teleki, I.; Jeney, A.; Krenacs, T. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther. Und Onkol. 2014, 190, 815–822. [Google Scholar] [CrossRef]
- van der Horst, A.; Versteijne, E.; Besselink, M.G.H.; Daams, J.G.; Bulle, E.B.; Bijlsma, M.F.; Wilmink, J.W.; van Delden, O.M.; van Hooft, J.E.; Franken, N.A.P.; et al. The clinical benefit of hyperthermia in pancreatic cancer: A systematic review. Int. J. Hyperth. 2018, 34, 969–979. [Google Scholar] [CrossRef]
- Baronzio, G.; Gramaglia, A.; Fiorentini, G. Hyperthermia and Immunity. A Brief Overview. In Vivo 2006, 20, 689–695. [Google Scholar]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Falk, R.E.; Moffat, F.L.; Lawler, M.; Heine, J.; Makowka, L.; Falk, J.A. Combination therapy for resectable and unresectable adenocarcinoma of the pancreas. Cancer 1986, 57, 685–688. [Google Scholar] [CrossRef]
- Brüningk, S.C.; Ijaz, J.; Rivens, I.; Nill, S.; ter Haar, G.; Oelfke, U. A comprehensive model for heat-induced radio-sensitisation. Int. J. Hyperth. 2018, 34, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Nani, R.; Fiorentini, C.; Guadagni, S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr. Cancer Ther. 2019, 18, 1534735419878505. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.; Simeni Simeni, M.; Bruggeman, P.J. Electric field dynamics in an atmospheric pressure helium plasma jet impinging on a substrate. Phys. Plasmas 2020, 27, 123505. [Google Scholar] [CrossRef]
- Wu, Q. Effect of high-power microwave on indicator bacteria for sterilization. IEEE Trans. Biomed. Eng. 1996, 43, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, O.; Petrova, M.; Smolensky, A.; Osmonov, B.; Toimatov, S.; Kharybina, T.; Karbainov, S.; Ovchinnikov, L.; Vesnin, S.; Tarakanov, A.; et al. Using medical microwave radiometry for brain temperature measurements. Drug Discov. Today 2022, 27, 881–889. [Google Scholar] [CrossRef]
- Saied, I.M.; Arslan, T.; Chandran, S. Classification of Alzheimer’s Disease Using RF Signals and Machine Learning. IEEE J. Electromagn. RF Microw. Med. Biol. 2022, 6, 77–85. [Google Scholar] [CrossRef]
- Ullah, R.; Saied, I.; Arslan, T. Big Data-Machine Learning Processing of Recorded Radiofrequency Physiological and Pathological Measurements to Predict the Progression of Alzheimer’s Disease. In Proceedings of the 2021 IEEE Asia-Pacific Microwave Conference (APMC), Brisbane, Australia, 28 November–1 December 2021; pp. 223–225. [Google Scholar]
- Albuquerque, H.M.T.; Pinto, D.C.G.A.; Silva, A.M.S. Microwave Irradiation: Alternative Heating Process for the Synthesis of Biologically Applicable Chromones, Quinolones, and Their Precursors. Molecules 2021, 26, 6293. [Google Scholar] [CrossRef]
- Pall, L.M. Low Intensity Electromagnetic Fields Act via Voltage-Gated Calcium Channel (VGCC) Activation to Cause Very Early Onset Alzheimer’s Disease: 18 Distinct Types of Evidence. Curr. Alzheimer Res. 2022, 19, 119–132. [Google Scholar] [CrossRef]
- Cura, O.K.; Yilmaz, G.C.; Ture, H.S.; Akan, A. Classification of Alzheimers’ Dementia by Using Various Signal Decomposition Methods. In Proceedings of the 2021 Medical Technologies Congress (TIPTEKNO), Antalya, Turkey, 4–6 November 2021; pp. 1–4. [Google Scholar]
- Aguilar-Barrientos, J.P.; Moo-Puc, R.E.; Villanueva-Toledo, J.R.; Murillo, F.; Cáceres-Castillo, D.; Mirón-López, G.; De los Santos, M.G.; Sandoval-Ramírez, J.; Zeferino-Díaz, R.; Fernández-Herrera, M.A. Microwave-enhanced synthesis of 26-amino-22-oxocholestanes and their cytotoxic activity. Steroids 2022, 183, 109030. [Google Scholar] [CrossRef]
- Chen, X.; Fu, C.; Wang, Y.; Wu, Q.; Meng, X.; Xu, K. Mitochondria-targeting nanoparticles for enhanced microwave ablation of cancer. Nanoscale 2018, 10, 15677–15685. [Google Scholar] [CrossRef] [PubMed]
- Peña Eguiluz, R.; López-Callejas, R.; González-Arciniega, E.; Rodríguez-Méndez, B.G.; Mercado-Cabrera, A.; Guakil-Haber, A.; Kuri García, A.; Espinosa Mancilla, A.E.; Valencia-Alvarado, R. Non-thermal plasma wound healing after removal of a neck tumor in a patient with HIV: A case report. Otolaryngol. Case Rep. 2022, 22, 100391. [Google Scholar] [CrossRef]
- Khandagale, A.; Lindahl, B.; Lind, S.B.; Shevchenko, G.; Siegbahn, A.; Christersson, C. Plasma-derived extracellular vesicles from myocardial infarction patients inhibits tumor necrosis factor-alpha induced cardiac cell death. Curr. Res. Transl. Med. 2022, 70, 103323. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Bae, J.H.; Yoon, Y.J.; Chung, T.H.; Lee, E.-W.; Kim, Y.-H.; Joh, H.M.; Chung, J.W. Plasma-activated medium induces ferroptosis by depleting FSP1 in human lung cancer cells. Cell Death Dis. 2022, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, C.; Kong, S.; Jia, T.; Chu, Z.; Yang, L.; Wu, J.; Geng, S.; Guo, K. Biosafety and differentially expressed genes analysis of melanoma cells treated with cold atmospheric plasma. J. Biophotonics 2022, 15, e202100403. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. https://doi.org/10.3390/ijms23169288
Mumtaz S, Rana JN, Choi EH, Han I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. International Journal of Molecular Sciences. 2022; 23(16):9288. https://doi.org/10.3390/ijms23169288
Chicago/Turabian StyleMumtaz, Sohail, Juie Nahushkumar Rana, Eun Ha Choi, and Ihn Han. 2022. "Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects" International Journal of Molecular Sciences 23, no. 16: 9288. https://doi.org/10.3390/ijms23169288
APA StyleMumtaz, S., Rana, J. N., Choi, E. H., & Han, I. (2022). Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. International Journal of Molecular Sciences, 23(16), 9288. https://doi.org/10.3390/ijms23169288