Autoantibodies against Complement Classical Pathway Components C1q, C1r, C1s and C1-Inh in Patients with Lupus Nephritis
Abstract
1. Introduction
2. Results
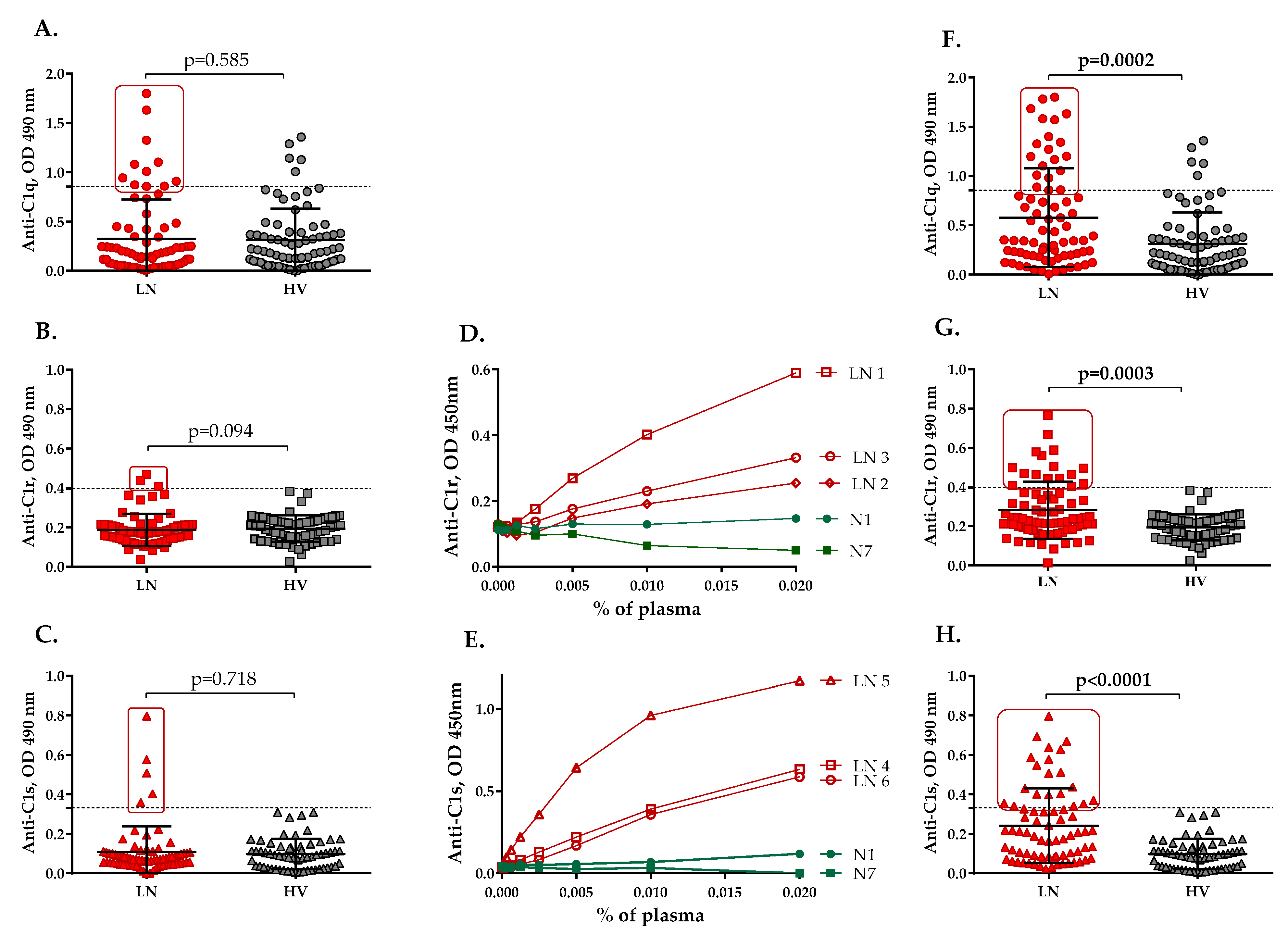
2.1. Autoantibodies Recognizing the Components of the C1 Complex Are Present in the Plasma of LN Patients
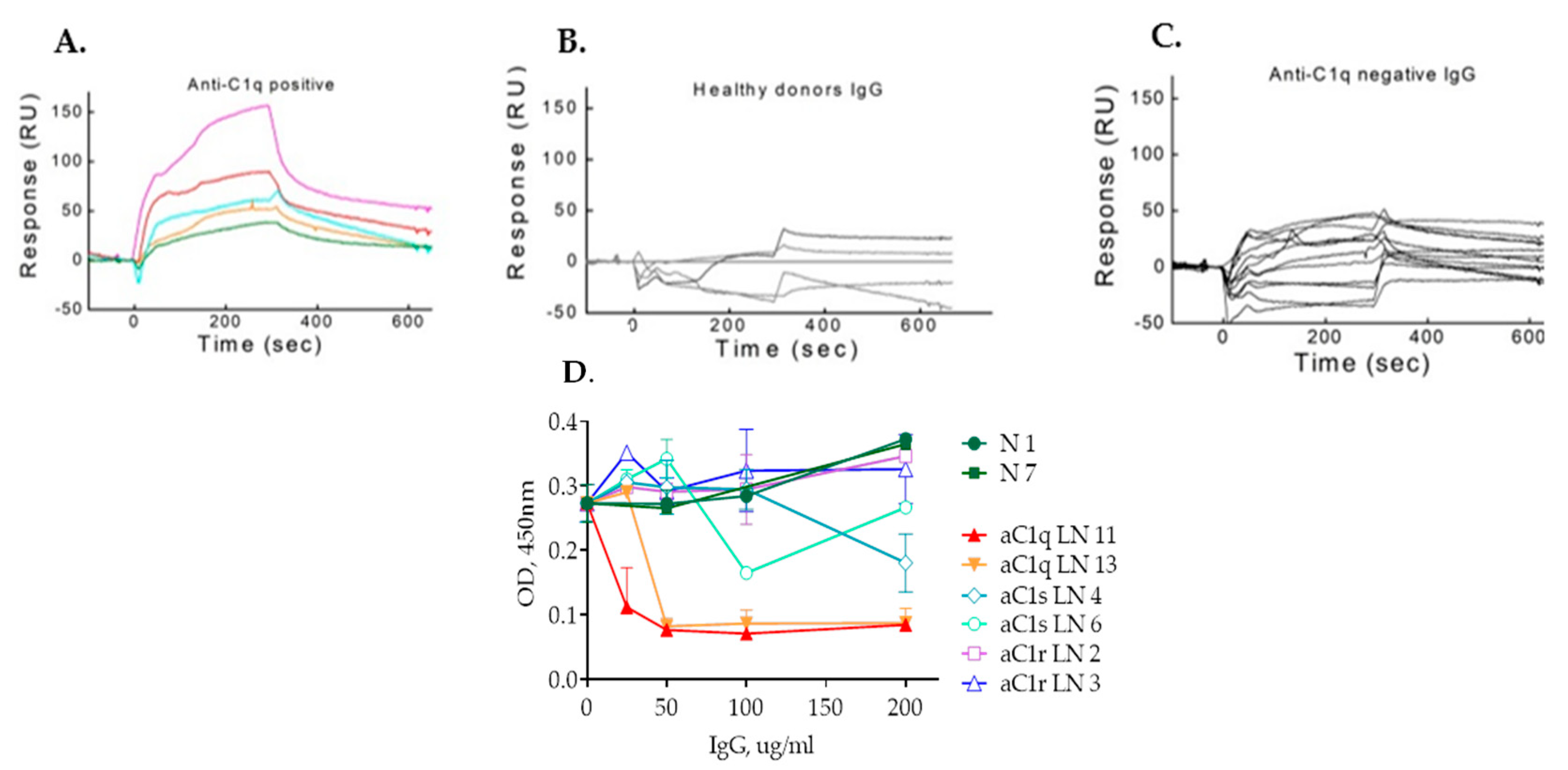
2.2. IgG Purified from LN Patients’ Plasma Show Binding Kinetics via SPR for C1q but Not for C1r and C1s
2.3. IgG Antibodies from Anti-C1r- and Anti-C1s-Positive Plasma Do Not Affect C1 Complex Formation as Opposed to Anti-C1q Autoantibodies
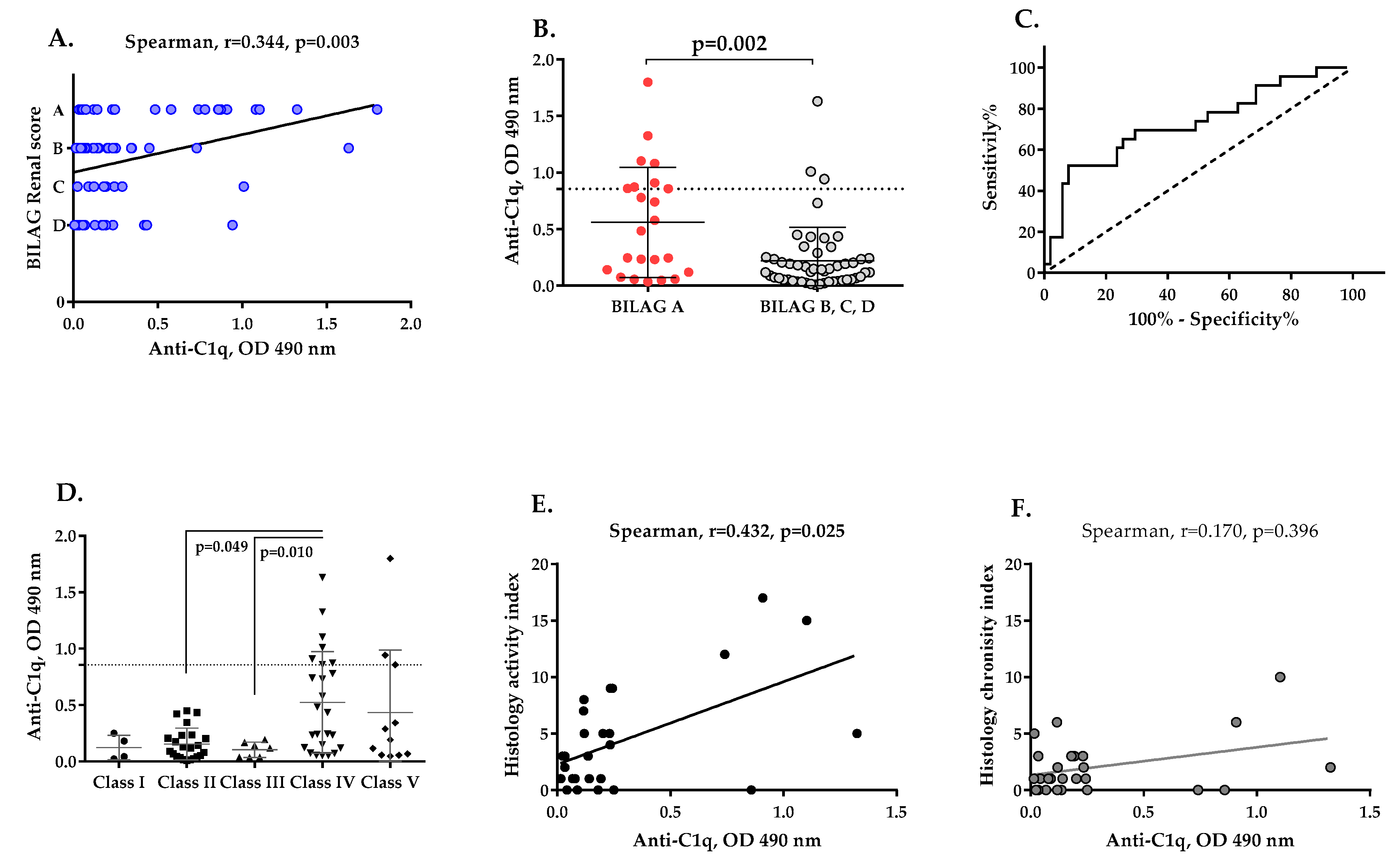
2.4. Association between Anti-C1q, Anti-C1r and Anti-C1s Autoantibodies and LN Severity
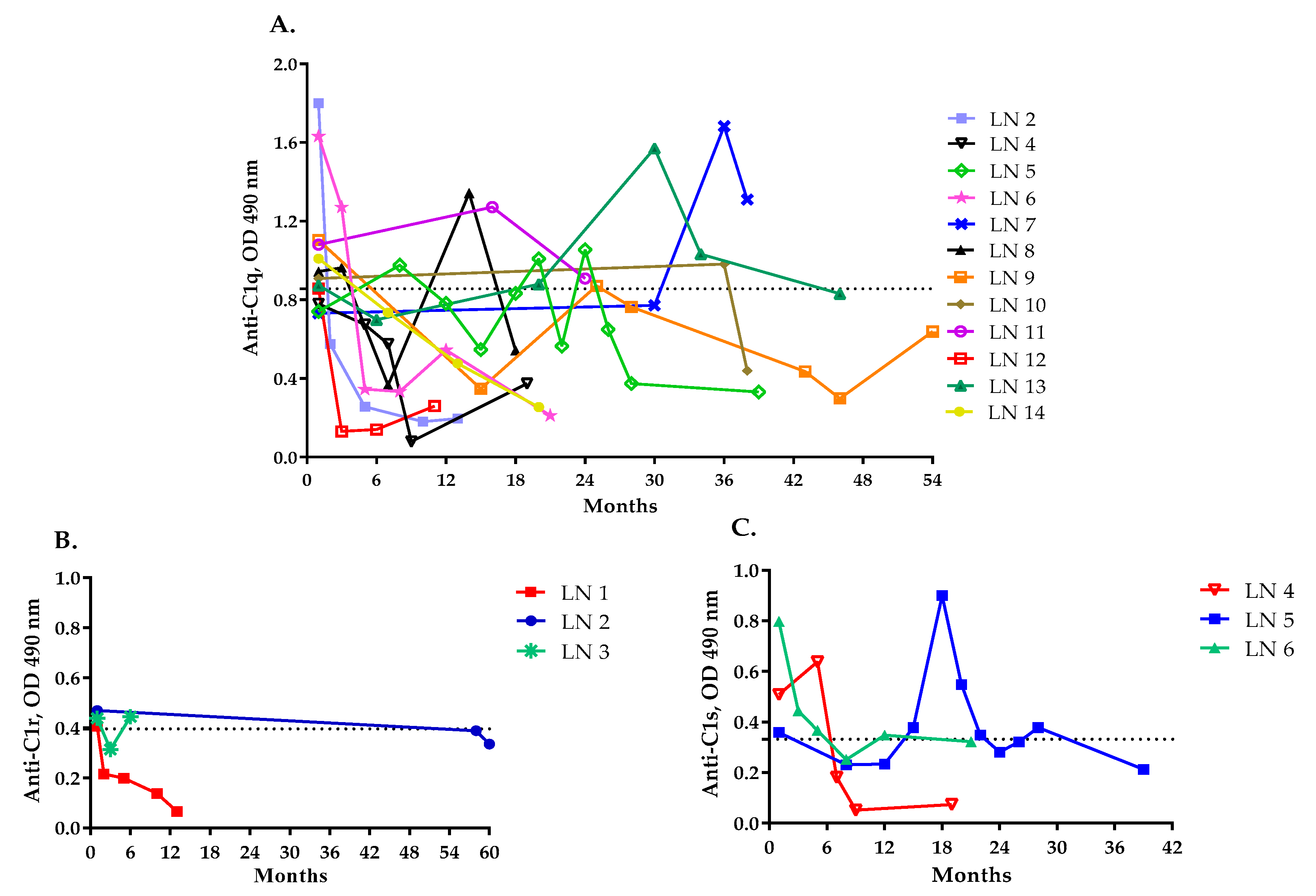
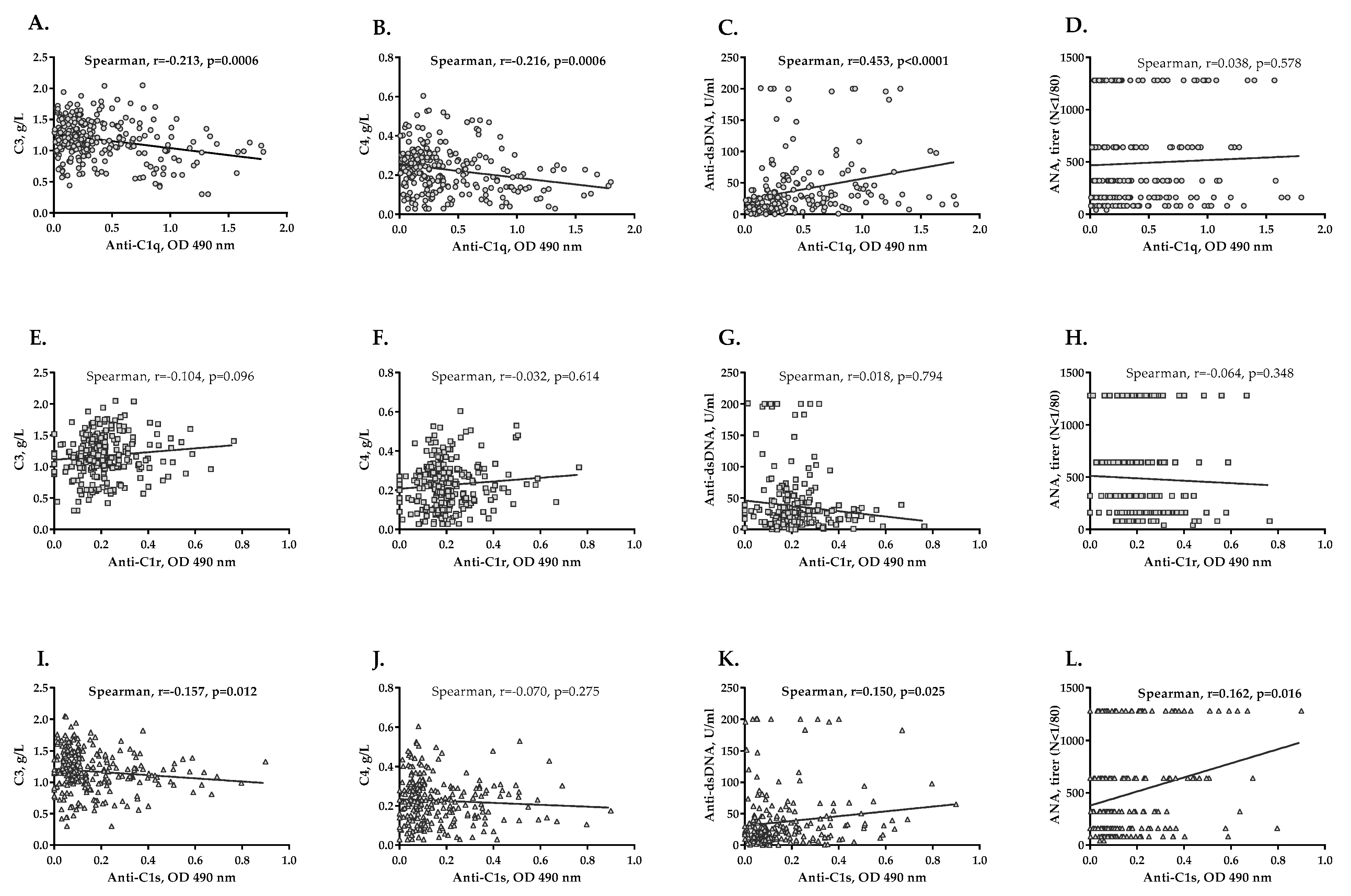
2.5. Levels of Anti-C1q, Anti-C1r and Anti-C1s Antibodies and LN Activity
2.6. Presence of Anti-C1q Autoantibodies Associates with Histological Features of LN
3. Discussion
4. Materials and Methods
4.1. LN Patient Cohort
4.2. ELISA for Detecting Anti-Complement Autoantibodies
4.3. IgG Purification
4.4. Characterization of Autoantigen-Autoantibody Interaction by Surface Plasmon Resonance (SPR)
4.5. Functional Test for C1 Complex Formation
4.6. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Truedsson, L.; Bengtsson, A.A.; Sturfelt, G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 2007, 40, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Solis, J.; Witte, T.; Hiepe, F.; Messer, G.; Chyderiotis, G.; Musset, L.; Pham, B.N.; Fabien, N.; Olsson, N.O.; Cervera, R. Systemic lupus erythematosus. In The General Practice Guide to Autoimmune Diseases; Shoenfeld, Y., Meroni, P., Eds.; Pabst Science Publishers: Lengerich, Germany, 2012; pp. 3–8. [Google Scholar]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Sène, D.; Radanova, M.; Blouin, J.; Halbwachs-Mecarelli, L.; Dragon-Durey, M.A.; Fridman, W.H.; Fremeaux-Bacchi, V. Functional complement C1q abnormality leads to impaired immune complexes and apoptotic cell clearance. J. Immunol. 2011, 187, 4369–4373. [Google Scholar] [CrossRef]
- Dragon-Durey, M.A.; Blanc, C.; Marinozzi, M.C.; van Schaarenburg, R.A.; Trouw, L.A. Autoantibodies against complement components and functional consequences. Mol. Immunol. 2013, 56, 213–221. [Google Scholar] [CrossRef]
- Siegert, C.; Daha, M.; Westedt, M.L.; van der Voort, E.; Breedveld, F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J. Rheumatol. 1991, 18, 230–234. [Google Scholar]
- Horváth, L.; Czirják, L.; Fekete, B.; Jakab, L.; Pozsonyi, T.; Kalabay, L.; Romics, L.; Miklós, K.; Varga, L.; Prohászka, Z.; et al. High levels of antibodies against Clq are associated with disease activity and nephritis but not with other organ manifestations in SLE patients. Clin. Exp. Rheumatol. 2001, 19, 667–672. [Google Scholar]
- Marto, N.; Bertolaccini, M.L.; Calabuig, E.; Hughes, G.R.; Khamashta, M.A. Anti-C1q antibodies in nephritis: Correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann. Rheum. Dis. 2005, 64, 444–448. [Google Scholar] [CrossRef]
- Trendelenburg, M.; Marfurt, J.; Gerber, I.; Tyndall, A.; Schifferli, J.A. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 1999, 42, 187–188. [Google Scholar] [CrossRef]
- Liu, C.C.; Ahearn, J.M. The search for lupus biomarkers. Best Pract. Res. Clin. Rheumatol. 2009, 23, 507–523. [Google Scholar] [CrossRef][Green Version]
- Mok, C.C.; Ho, L.Y.; Leung, H.W.; Wong, L.G. Performance of anti-C1q, antinucleosome, and anti-dsDNA antibodies for detecting concurrent disease activity of systemic lupus erythematosus. Transl. Res. 2010, 156, 320–325. [Google Scholar] [CrossRef]
- Moroni, G.; Trendelenburg, M.; Del Papa, N.; Quaglini, S.; Raschi, E.; Panzeri, P.; Testoni, C.; Tincani, A.; Banfi, G.; Balestrieri, G.; et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am. J. Kidney Dis. 2001, 37, 490–498. [Google Scholar] [CrossRef]
- Sinico, R.A.; Radice, A.; Ikehata, M.; Giammarresi, G.; Corace, C.; Arrigo, G.; Bollini, B.; Li Vecchi, M. Anti-C1q autoantibodies in lupus nephritis: Prevalence and clinical significance. Ann. N. Y. Acad. Sci. 2005, 1050, 193–200. [Google Scholar] [CrossRef]
- Trendelenburg, M.; Lopez-Trascasa, M.; Potlukova, E.; Moll, S.; Regenass, S.; Frémeaux-Bacchi, V.; Martinez-Ara, J.; Jancova, E.; Picazo, M.L.; Honsova, E.; et al. High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol. Dial. Transplant. 2006, 21, 3115–3121. [Google Scholar] [CrossRef]
- Wu, F.Q.; Zhao, Q.; Cui, X.D.; Zhang, W. C1q and anti-C1q antibody levels are correlated with disease severity in Chinese pediatric systemic lupus erythematosus. Rheumatol. Int. 2011, 31, 501–505. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Shan, G.; Zhang, X. Diagnostic value of serum anti-C1q antibodies in patients with lupus nephritis: A meta-analysis. Lupus 2012, 21, 1088–1097. [Google Scholar] [CrossRef]
- Mahler, M.; van Schaarenburg, R.A.; Trouw, L.A. Anti-C1q autoantibodies, novel tests, and clinical consequences. Front. Immunol. 2013, 4, 117. [Google Scholar] [CrossRef]
- Siegert, C.E.; Daha, M.R.; Tseng, C.M.; Coremans, I.E.; van Es, L.A.; Breedveld, F.C. Predictive value of IgG autoantibodies against C1q for nephritis in systemic lupus erythematosus. Ann. Rheum. Dis. 1993, 52, 851–856. [Google Scholar] [CrossRef]
- He, S.; Lin, Y.L. In vitro stimulation of C1s proteolytic activities by C1s-presenting autoantibodies from patients with systemic lupus erythematosus. J. Immunol. 1998, 160, 4641–4647. [Google Scholar]
- Loos, M.; Martin, H.; Petry, F. The biosynthesis of C1q, the collagen-like and Fc-recognizing molecule of the complement system. Behring Inst. Mitt. 1989, 84, 32–41. [Google Scholar]
- Castellano, G.; Woltman, A.M.; Nauta, A.J.; Roos, A.; Trouw, L.A.; Seelen, M.A.; Schena, F.P.; Daha, M.R.; van Kooten, C. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood 2004, 103, 3813–3820. [Google Scholar] [CrossRef]
- Revel, M.; Sautès-Fridman, C.; Fridman, W.H.; Roumenina, L.T. C1q+ macrophages: Passengers or drivers of cancer progression. Trends Cancer 2022, 8, 517–526. [Google Scholar] [CrossRef]
- Tang, S.; Lui, S.L.; Lai, K.N. Pathogenesis of lupus nephritis: An update. Nephrology 2005, 10, 174–179. [Google Scholar] [CrossRef]
- Trendelenburg, M. Antibodies against C1q in patients with systemic lupus erythematosus. Springer Semin. Immunopathol. 2005, 27, 276–285. [Google Scholar] [CrossRef]
- Orbai, A.M.; Truedsson, L.; Sturfelt, G.; Nived, O.; Fang, H.; Alarcón, G.S.; Gordon, C.; Merrill, J.; Fortin, P.R.; Bruce, I.N.; et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus 2015, 24, 42–49. [Google Scholar] [CrossRef]
- Gargiulo, M.L.; Gómez, G.; Khoury, M.; Collado, M.V.; Suárez, L.; Álvarez, C.; Sarano, J. Association between the presence of anti-C1q antibodies and active nephritis in patients with systemic lupus erythematosus. Medicina 2015, 75, 23–28, Erratum in: Medicina 2015, 75, 261. [Google Scholar]
- Frémeaux-Bacchi, V.; Noël, L.H.; Schifferli, J.A. No lupus nephritis in the absence of antiC1q autoantibodies? Nephrol. Dial. Transplant. 2002, 17, 2041–2043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radanova, M.; Vasilev, V.; Deliyska, B.; Kishore, U.; Ikonomov, V.; Ivanova, D. Anti-C1q autoantibodies specific against the globular domain of the C1qB-chain from patient with lupus nephritis inhibit C1q binding to IgG and CRP. Immunobiology 2012, 217, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Tsacheva, I.; Radanova, M.; Todorova, N.; Argirova, T.; Kishore, U. Detection of autoantibodies against the globular domain of human C1q in the sera of systemic lupus erythematosus patients. Mol. Immunol. 2007, 44, 2147–2151. [Google Scholar] [CrossRef] [PubMed]
- Siegert, C.E.; Daha, M.R.; van der Voort, E.A.; Breedveld, F.C. IgG and IgA antibodies to the collagen-like region of C1q in rheumatoid vasculitis. Arthritis Rheum. 1990, 33, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Siegert, C.E.; Kazatchkine, M.D.; Sjöholm, A.; Würzner, R.; Loos, M.; Daha, M.R. Autoantibodies against C1q: View on clinical relevance and pathogenic role. Clin. Exp. Immunol. 1999, 116, 4–8. [Google Scholar] [CrossRef]
- Antes, U.; Heinz, H.P.; Loos, M. Evidence for the presence of autoantibodies to the collagen-like portion of C1q in systemic lupus erythematosus. Arthritis Rheum. 1988, 31, 457–464. [Google Scholar] [CrossRef]
- Uwatoko, S.; Mannik, M. Low-molecular weight C1q-binding immunoglobulin G in patients with systemic lupus erythematosus consists of autoantibodies to the collagen-like region of C1q. J. Clin. Investig. 1988, 82, 816–824. [Google Scholar] [CrossRef]
- Trendelenburg, M. Autoantibodies against complement component C1q in systemic lupus erythematosus. Clin. Transl. Immunol. 2021, 10, e1279. [Google Scholar] [CrossRef]
- Golan, M.D.; Burger, R.; Loos, M. Conformational changes in C1q after binding to immune complexes: Detection of neoantigens with monoclonal antibodies. J. Immunol. 1982, 129, 445–447. [Google Scholar]
- Vanhecke, D.; Roumenina, L.T.; Wan, H.; Osthoff, M.; Schaller, M.; Trendelenburg, M. Identification of a major linear C1q epitope allows detection of systemic lupus erythematosus anti-C1q antibodies by a specific peptide-based enzyme-linked immunosorbent assay. Arthritis Rheum. 2012, 64, 3706–3714. [Google Scholar] [CrossRef]
- Uwatoko, S.; Gauthier, V.J.; Mannik, M. Autoantibodies to the collagen-like region of C1Q deposit in glomeruli via C1Q in immune deposits. Clin. Immunol. Immunopathol. 1991, 61 Pt 1, 268–273. [Google Scholar] [CrossRef]
- Bigler, C.; Schaller, M.; Perahud, I.; Osthoff, M.; Trendelenburg, M. Autoantibodies against complement C1q specifically target C1q bound on early apoptotic cells. J. Immunol. 2009, 183, 3512–3521. [Google Scholar] [CrossRef]
- Thanei, S.; Trendelenburg, M. Anti-C1q Autoantibodies from Systemic Lupus Erythematosus Patients Induce a Proinflammatory Phenotype in Macrophages. J. Immunol. 2016, 196, 2063–2074. [Google Scholar] [CrossRef]
- Rabatscher, P.A.; Trendelenburg, M. Anti-C1q autoantibodies from systemic lupus erythematosus patients enhance CD40-CD154-mediated inflammation in peripheral blood mononuclear cells in vitro. Clin. Transl. Immunol. 2022, 11, e1408. [Google Scholar] [CrossRef]
- Moura, C.G.; Lima, I.; Barbosa, L.; Athanazio, D.; Reis, E.; Reis, M.; Burlingame, R.W.; Santiago, M.B. Anti-C1q antibodies: Association with nephritis and disease activity in systemic lupus erythematosus. J. Clin. Lab. Anal. 2009, 23, 19–23. [Google Scholar] [CrossRef]
- Emad, G.; Al-Barshomy, S.M. Anti-C1q antibodies in lupus nephritis and their correlation with the disease activity. Saudi J. Kidney Dis. Transpl. 2020, 31, 342–352. [Google Scholar]
- Chen, Z.; Wang, G.S.; Wang, G.H.; Li, X.P. Anti-C1q antibody is a valuable biological marker for prediction of renal pathological characteristics in lupus nephritis. Clin. Rheumatol. 2012, 31, 1323–1329. [Google Scholar] [CrossRef]
- Moroni, G.; Quaglini, S.; Radice, A.; Trezzi, B.; Raffiotta, F.; Messa, P.; Sinico, R.A. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J. Immunol. Res. 2015, 2015, 106904. [Google Scholar] [CrossRef]
- Sterner, R.M.; Hartono, S.P.; Grande, J.P. The Pathogenesis of Lupus Nephritis. J. Clin. Cell Immunol. 2014, 5, 205. [Google Scholar]
- Kościelska-Kasprzak, K.; Bartoszek, D.; Myszka, M.; Zabińska, M.; Klinger, M. The complement cascade and renal disease. Arch. Immunol. Ther. Exp. 2014, 62, 47–57. [Google Scholar] [CrossRef]
- Cook, H.T.; Botto, M. Mechanisms of Disease: The complement system and the pathogenesis of systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2006, 2, 330–337. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Gupta, N.; Pulukool, S.; Mohan, H.; Mahasampath, G.; Danda, D. Anti-C1q Antibody is Associated with Renal and Cutaneous Manifestations in Asian Indian Patients with Systemic Lupus Erythematosus. J. Clin. Diagn. Res. 2017, 11, OC39–OC42. [Google Scholar] [CrossRef]
- Pradhan, V.; Rajadhyaksha, A.; Mahant, G.; Surve, P.; Patwardhan, M.; Dighe, S.; Ghosh, K. Anti-C1q antibodies and their association with complement components in Indian systemic lupus erythematosus patients. Indian J. Nephrol. 2012, 22, 353–357. [Google Scholar] [CrossRef]
- Bock, M.; Heijnen, I.; Trendelenburg, M. Anti-C1q antibodies as a follow-up marker in SLE patients. PLoS ONE 2015, 10, e0123572. [Google Scholar] [CrossRef]
- Fang, Q.Y.; Yu, F.; Tan, Y.; Xu, L.X.; Wu, L.H.; Liu, G.; Shao, F.M.; Zhao, M.H. Anti-C1q antibodies and IgG subclass distribution in sera from Chinese patients with lupus nephritis. Nephrol. Dial. Transplant. 2009, 24, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Tan, Y.; Yu, F.; Zhao, M.H. Combination of anti-C1q and anti-dsDNA antibodies is associated with higher renal disease activity and predicts renal prognosis of patients with lupus nephritis. Nephrol. Dial. Transplant. 2012, 27, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Trouw, L.A.; Groeneveld, T.W.; Seelen, M.A.; Duijs, J.M.; Bajema, I.M.; Prins, F.A.; Kishore, U.; Salant, D.J.; Verbeek, J.S.; van Kooten, C.; et al. Anti-C1q autoantibodies deposit in glomeruli but are only pathogenic in combination with glomerular C1q-containing immune complexes. J. Clin. Investig. 2004, 114, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, T.; Füst, G.; Farkas, H.; Jakab, L.; Temesszentandrási, G.; Nagy, G.; Kiss, E.; Gergely, P.; Zeher, M.; Griger, Z.; et al. C1-inhibitor autoantibodies in SLE. Lupus 2010, 19, 634–638. [Google Scholar] [CrossRef]
- Xavier, S.; Sahu, R.K.; Landes, S.G.; Yu, J.; Taylor, R.P.; Ayyadevara, S.; Megyesi, J.; Stallcup, W.B.; Duffield, J.S.; Reis, E.S.; et al. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2017, 312, F516–F532. [Google Scholar] [CrossRef]
- Daugan, M.V.; Revel, M.; Russick, J.; Dragon-Durey, M.A.; Gaboriaud, C.; Robe-Rybkine, T.; Poillerat, V.; Grunenwald, A.; Lacroix, G.; Bougouin, A.; et al. Complement C1s and C4d as Prognostic Biomarkers in Renal Cancer: Emergence of Noncanonical Functions of C1s. Cancer Immunol. Res. 2021, 9, 891–908. [Google Scholar] [CrossRef]
- Eriksson, H.; Nissen, M.H. Proteolysis of the heavy chain of major histocompatibility complex class I antigens by complement component C1s. Biochim. Biophys. Acta 1990, 1037, 209–215. [Google Scholar] [CrossRef]
- Nissen, M.H.; Roepstorff, P.; Thim, L.; Dunbar, B.; Fothergill, J.E. Limited proteolysis of beta 2-microglobulin at Lys-58 by complement component C1s. Eur. J. Biochem. 1990, 189, 423–429. [Google Scholar] [CrossRef]
- Busby, W.H., Jr.; Nam, T.J.; Moralez, A.; Smith, C.; Jennings, M.; Clemmons, D.R. The complement component C1s is the protease that accounts for cleavage of insulin-like growth factor-binding protein-5 in fibroblast medium. J. Biol. Chem. 2000, 275, 37638–37644. [Google Scholar] [CrossRef]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef]
- Cai, Y.; Teo, B.H.; Yeo, J.G.; Lu, J. C1q protein binds to the apoptotic nucleolus and causes C1 protease degradation of nucleolar proteins. J. Biol. Chem. 2015, 290, 22570–22580. [Google Scholar] [CrossRef]
- Kerr, F.K.; O’Brien, G.; Quinsey, N.S.; Whisstock, J.C.; Boyd, S.; de la Banda, M.G.; Kaiserman, D.; Matthews, A.Y.; Bird, P.I.; Pike, R.N. Elucidation of the substrate specificity of the C1s protease of the classical complement pathway. J. Biol. Chem. 2005, 280, 39510–39514. [Google Scholar] [CrossRef]
- Lu, J.; Kishore, U. C1 Complex: An Adaptable Proteolytic Module for Complement and Non-Complement Functions. Front. Immunol. 2017, 8, 592. [Google Scholar] [CrossRef]
- Kapferer-Seebacher, I.; Pepin, M.; Werner, R.; Aitman, T.J.; Nordgren, A.; Stoiber, H.; Thielens, N.; Gaboriaud, C.; Amberger, A.; Schossig, A.; et al. Periodontal Ehlers-Danlos Syndrome Is Caused by Mutations in C1R and C1S, which Encode Subcomponents C1r and C1s of Complement. Am. J. Hum. Genet. 2016, 99, 1005–1014. [Google Scholar] [CrossRef]
- Hay, E.M.; Bacon, P.A.; Gordon, C.; Isenberg, D.A.; Maddison, P.; Snaith, M.L.; Symmons, D.P.; Viner, N.; Zoma, A. The BILAG index: A reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. QJM 1993, 86, 447–458. [Google Scholar]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Noé, R.; Petitprez, F.; Vano, Y.A.; Sanchez-Salas, R.; Becht, E.; Meilleroux, J.; Clec’h, B.L.; Giraldo, N.A.; et al. Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer Immunol. Res. 2019, 7, 1091–1105. [Google Scholar] [CrossRef]
| Histologic Features * | Anti-C1q Levels, OD 490 nm Median (from–to) | Anti-C1r Levels, OD 490 nm Median (from–to) | Anti-C1s Levels, OD 490 nm Median (from–to) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Presence | Absence | p-Value | Presence | Absence | p-Value | Presence | Absence | p-Value | |
| Endocapillary proliferation | 0.139 (0.015–1.325) | 0.180 (0.043–0.858) | 0.846 | 0.165 (0.013–0.470) | 0.180 (0.114–0.198) | 0.698 | 0.071 (0.042–0.359) | 0.092 (0.043–0.123) | 0.194 |
| “Wire loop” deposits | 0.740 (0.12–1.325) | 0.127 (0.015–1.103) | 0.019 | 0.116 (0.084–0.47) | 0.174 (0.013–0.438) | 0.417 | 0.070 (0.054–0.359 | 0.073 (0.042–0.175) | 0.975 |
| Fibrinoid necrosis/karyorrhexis | 0.235 (0.024–1.103) | 0.136 (0.015–1.325) | 0.269 | 0.174 (0.103–0.254) | 0.148 (0.013–0.47) | 0.581 | 0.072 (0.055–0.359) | 0.074 (0.042–0.123) | 0.616 |
| Cellular crescents | 0.909 (0.116–1.325) | 0.128 (0.015–0.858) | 0.012 | 0.116 (0.084–0.182) | 0.171 (0.013–0.47) | 0.248 | 0.061 (0.054–0.359) | 0.076 (0.042–0.175) | 0.640 |
| Interstitial inflammation | 0.198 (0.116–1.103) | 0.089 (0.015–1.325) | 0.167 | 0.175 (0.013–0.470) | 0.159 (0.084–0.438) | 0.744 | 0.070 (0.052–0.111) | 0.085 (0.042–0.359) | 0.303 |
| Glomerular sclerosis | 0.180 (0.015–1.325) | 0.128 (0.024–0.858) | 1.000 | 0.177 (0.084–0.215) | 0.154 (0.013–0.470) | 0.884 | 0.071 (0.043–0.175) | 0.077 (0.042–0.359) | 0.407 |
| Fibrous crescents | 0.909 (0.116–1.325) | 0.128 (0.015–0.858) | 0.037 | 0.179 (0.084–0.198) | 0.165 (0.013–0.470) | 0.731 | 0.061 (0.054–0.111) | 0.076 (0.042–0.359) | 0.473 |
| Tubular atrophy | 0.187 (0.016–1.103) | 0.136 (0.015–1.325) | 0.880 | 0.181 (0.103–0.438) | 0.148 (0.013–0.470) | 0.228 | 0.066 (0.042–0.175) | 0.077 (0.047–0.359) | 0.280 |
| Interstitial fibrosis | 0.231 (0.016–1.103) | 0.127 (0.015–1.325) | 0.382 | 0.171 (0.103–0.47) | 0.165 (0.013–0.438) | 0.662 | 0.071 (0.043–0.175) | 0.081 (0.042–0.359) | 0.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radanova, M.; Vasilev, V.; Mihaylova, G.; Kosturkova, M.; Kishore, U.; Roumenina, L. Autoantibodies against Complement Classical Pathway Components C1q, C1r, C1s and C1-Inh in Patients with Lupus Nephritis. Int. J. Mol. Sci. 2022, 23, 9281. https://doi.org/10.3390/ijms23169281
Radanova M, Vasilev V, Mihaylova G, Kosturkova M, Kishore U, Roumenina L. Autoantibodies against Complement Classical Pathway Components C1q, C1r, C1s and C1-Inh in Patients with Lupus Nephritis. International Journal of Molecular Sciences. 2022; 23(16):9281. https://doi.org/10.3390/ijms23169281
Chicago/Turabian StyleRadanova, Maria, Vasil Vasilev, Galya Mihaylova, Mariya Kosturkova, Uday Kishore, and Lubka Roumenina. 2022. "Autoantibodies against Complement Classical Pathway Components C1q, C1r, C1s and C1-Inh in Patients with Lupus Nephritis" International Journal of Molecular Sciences 23, no. 16: 9281. https://doi.org/10.3390/ijms23169281
APA StyleRadanova, M., Vasilev, V., Mihaylova, G., Kosturkova, M., Kishore, U., & Roumenina, L. (2022). Autoantibodies against Complement Classical Pathway Components C1q, C1r, C1s and C1-Inh in Patients with Lupus Nephritis. International Journal of Molecular Sciences, 23(16), 9281. https://doi.org/10.3390/ijms23169281








