The Role of Obesity and Diabetes in Dementia
Abstract
1. Introduction
2. Factors Affecting the Development of Obesity
2.1. Age and Obesity
2.2. Gender and Obesity
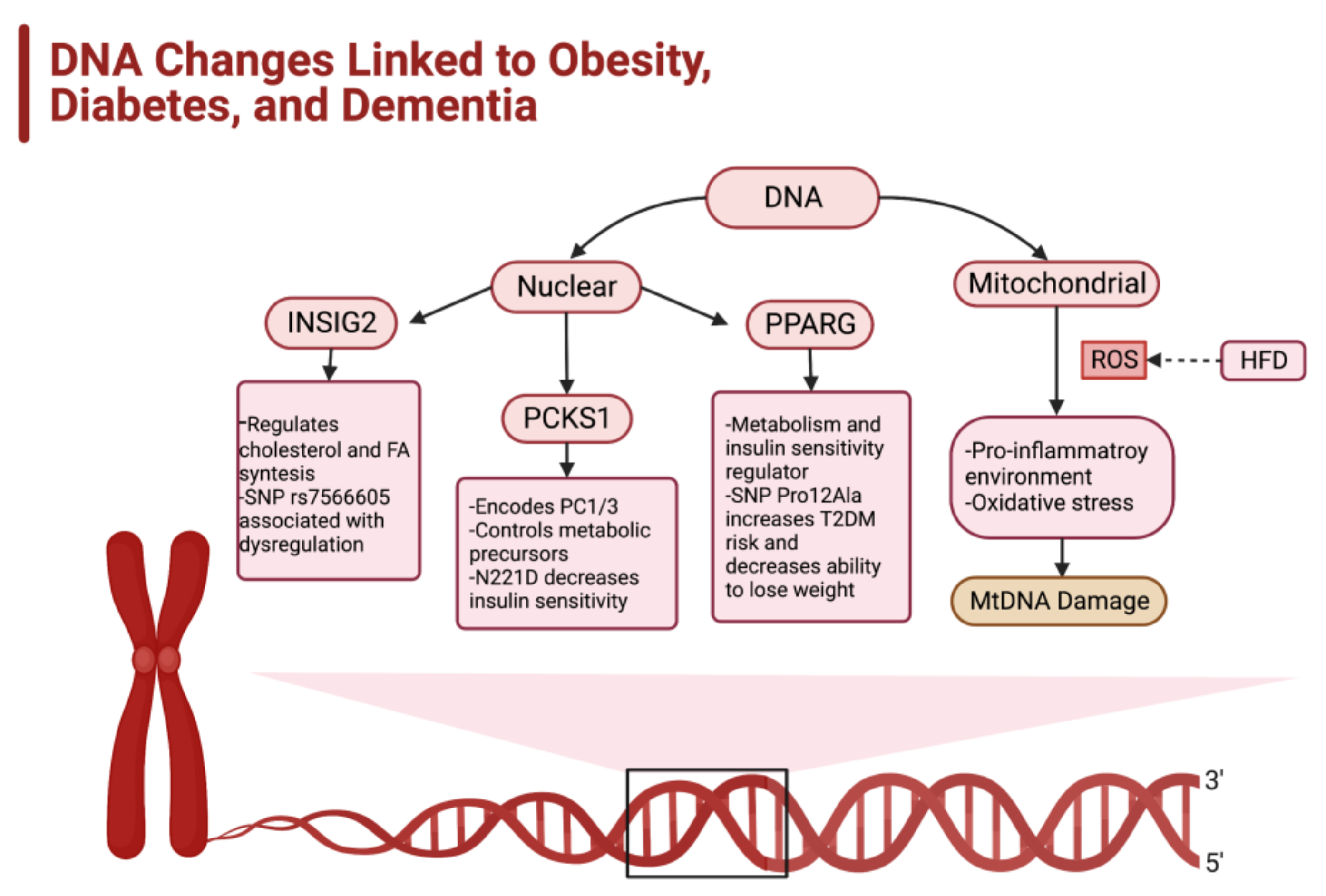
2.3. Genetic Factors Impacting the Development of Obesity
2.4. Epigenetics and Predisposition for Obesity
2.5. Socioeconomic Status and Obesity
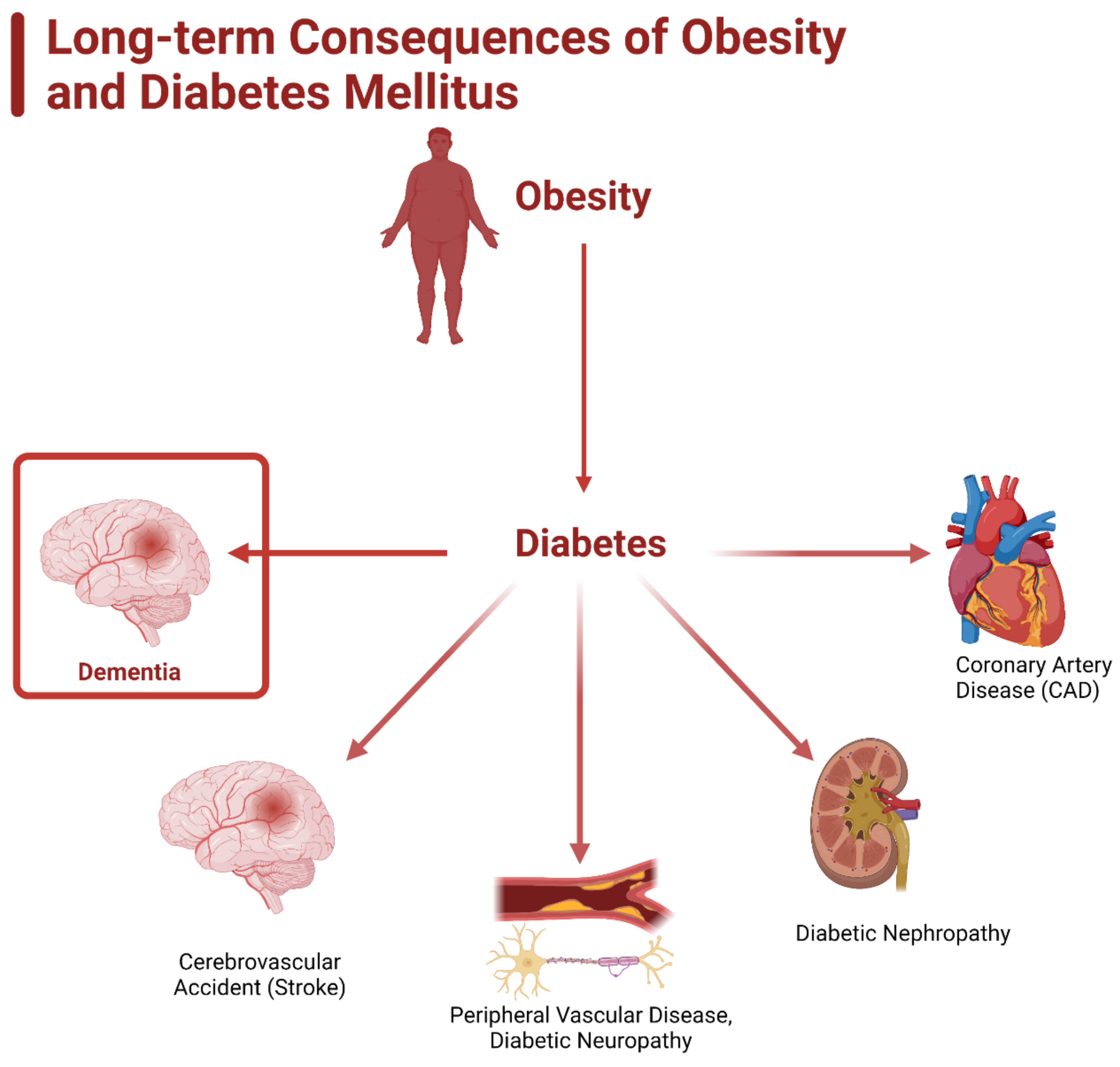
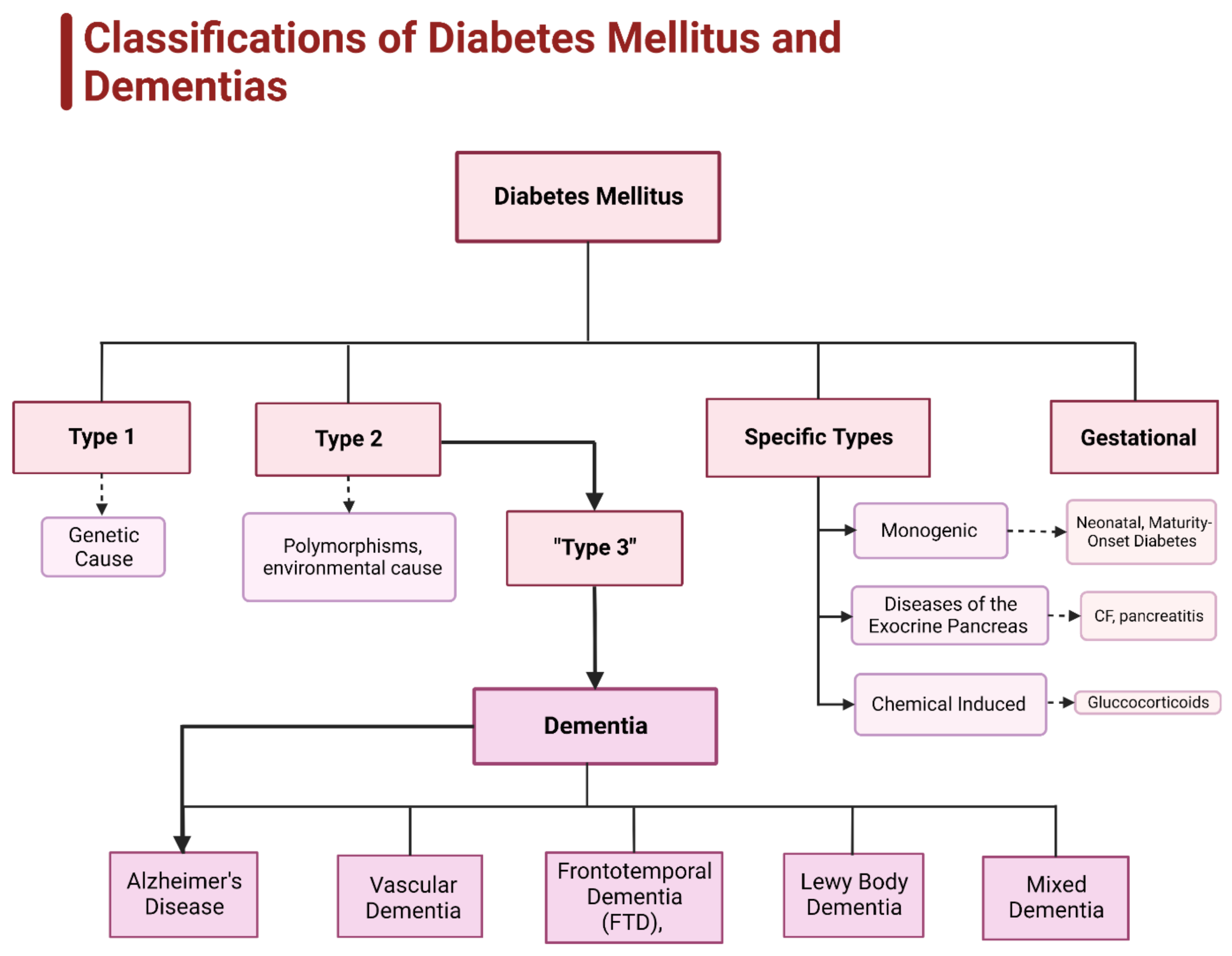
3. Diabetes and Obesity
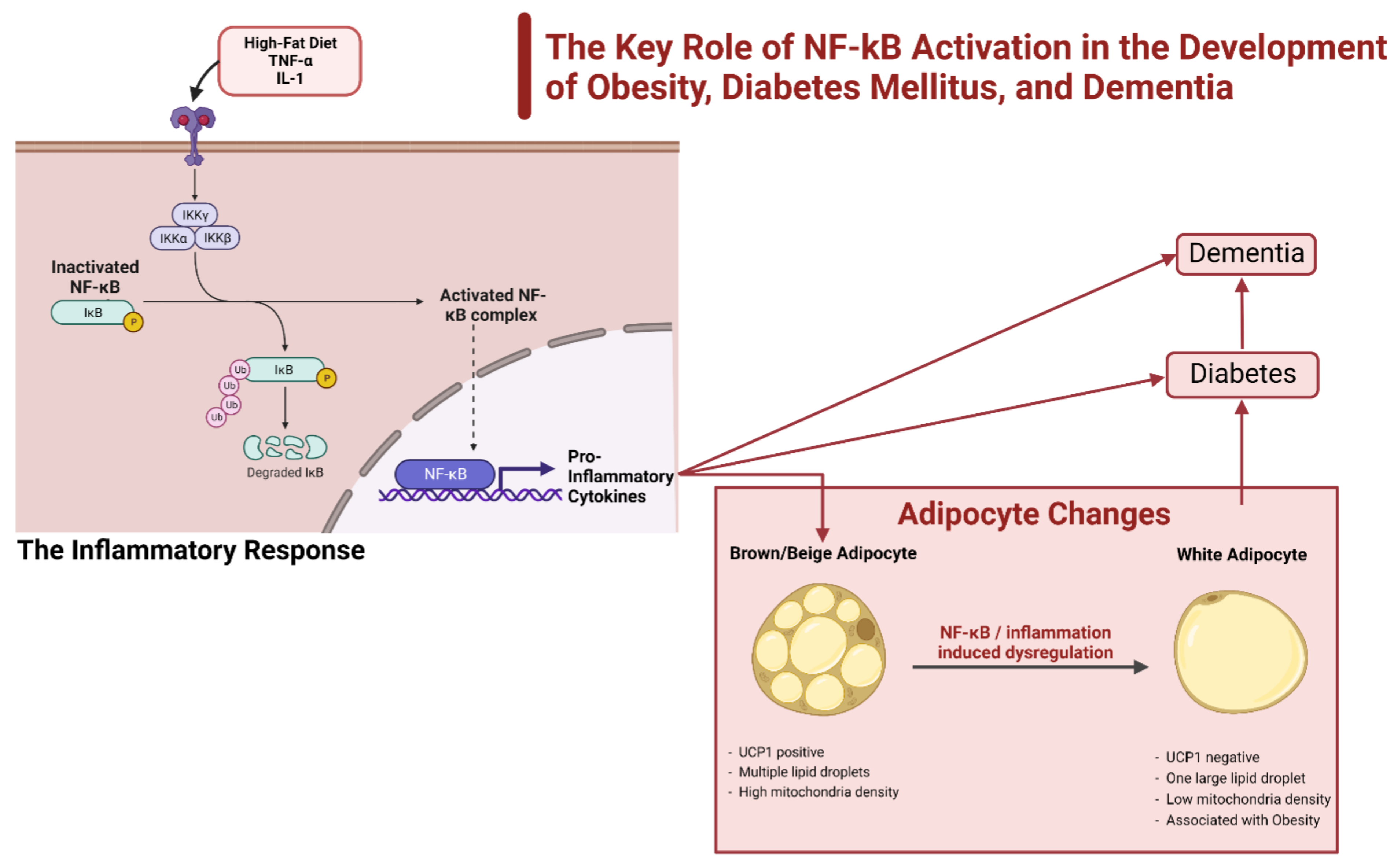
3.1. NF-κβ and the Impact of the Inflammatory Response
3.2. CNS Control and the Impact of Inflammation
3.3. Role of Inflammation in Diabetes and Dementia
4. Current Status of Research on Obesity, Diabetes, and Dementia
4.1. Studies on the POMC-MCR Pathway
Drugs Utilizing the POMC-MCR Pathway for Obesity Treatment
4.2. Effect of Metformin in the Treatment and Prevention of Dementia
4.3. Studies Utilizing Pdia4 to Treat Diabetes
5. Diets That Affect Obesity
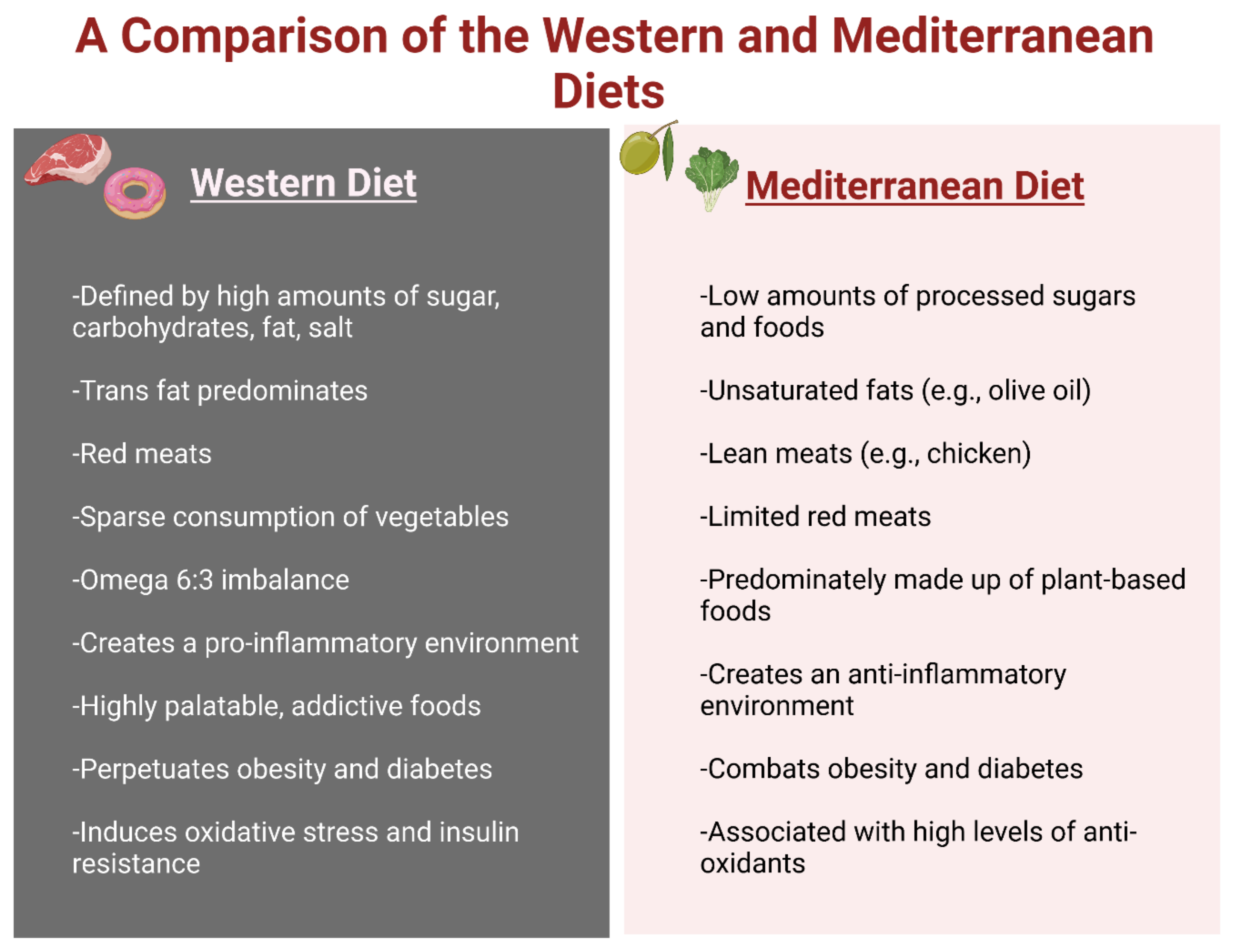
5.1. Western Diet (WD)
5.2. Mediterranean Diet (MD)
6. Current Therapeutics
6.1. Diet and Lifestyle Modifications
6.2. Pharmaceutical and Surgical Options for Obesity Treatment
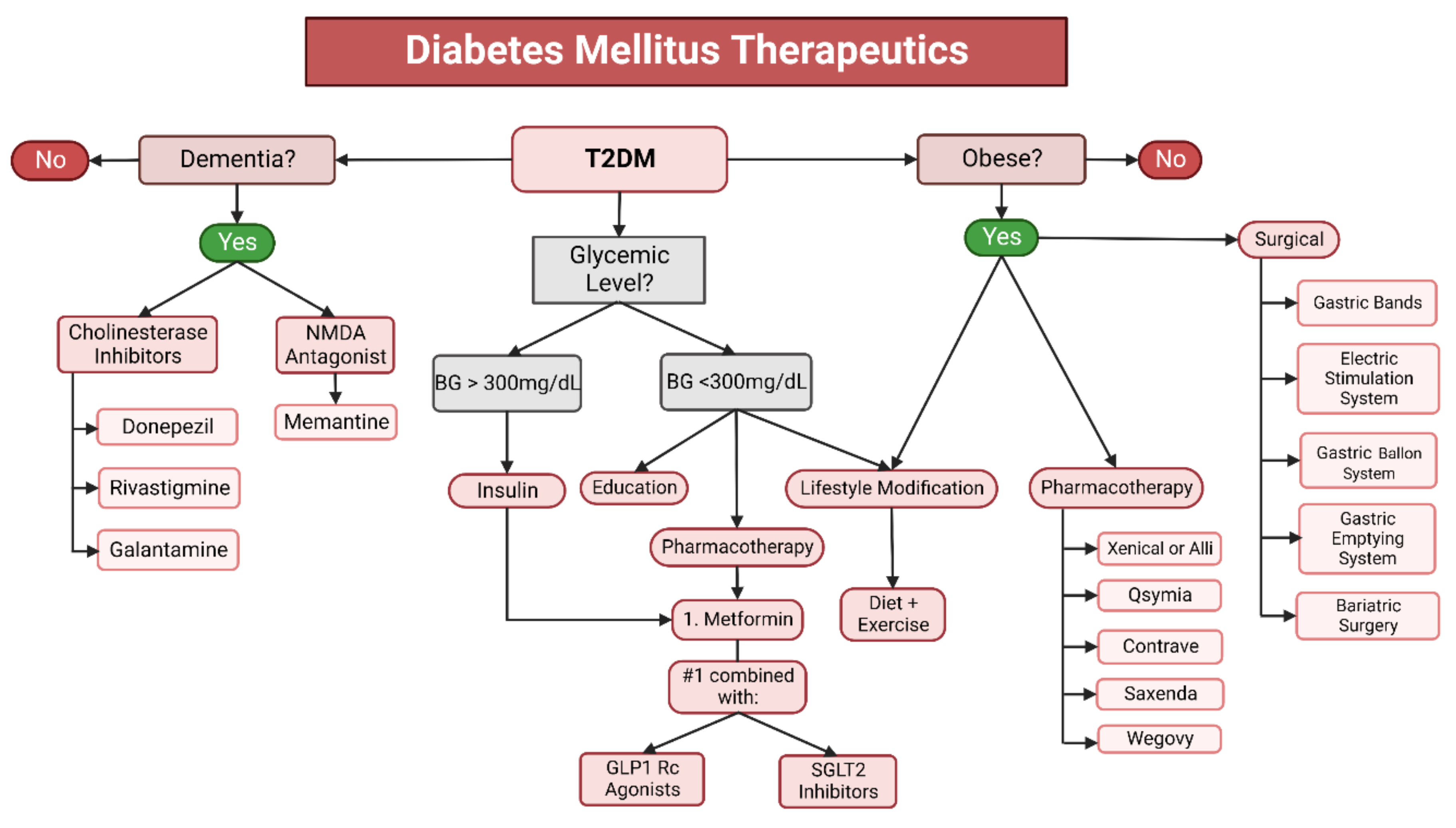
6.3. Therapeutics for Diabetes Mellitus
6.4. Therapeutics for Dementia
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| WD | Western diet |
| MD | Mediterranean diet |
| T2DM | Type 2 diabetes mellitus |
| T3DM | Type 3 diabetes mellitus |
| CDC | Centers for Disease Control and Prevention |
| BMI | Body mass index |
| AD | Alzheimer’s disease |
| FTD | Frontotemporal dementia |
| LBD | Lewy body dementia |
| VD | Vascular dementia |
| WAT | White adipose tissue |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin 6 |
| SVF | Stromal vascular fraction |
| SREBP | Sterol regulatory element-binding protein |
| FTO | Fat mass and obesity-related |
| LEPR | Leptin receptor |
| MCR4 | Melanocontin 4 receptor |
| SNP | Single nucleotide polymorphism |
| PCOS | Polycystic ovary syndrome |
| INSIG2 | Insulin-induced gene 2 |
| PCKS1 | Proprotein convertase subtilisin/kexin type 1 |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| BAT | Brown adipose tissue |
| UPC1 | Uncoupling protein 1 |
| miRNA | Micro-RNA |
| HFD | High-fat diet |
| IGF | Insulin growth factor |
| RXRα | Retinoid X receptor alpha gene |
| CNS | Central nervous system |
| HDAC | Histone deacetylase |
| IGF2 | Insulin-like growth factor 2 |
| TLR4 | Toll-like receptor 4 |
| NEMO | NF-κB essential modulator |
| GSK-3β | Glycogen synthase kinase 3β |
| HAN | Hypothalamic arcuate nucleus |
| KCNQ1 | Potassium channel voltage gated KQT-like subfamily member 1 (KCNQ1) |
| KLF14 | Kruppel-like factor 14 |
| MSH | Melanocyte-stimulating hormone |
| MCRs | Melanocortin receptors |
| POMC | Proopio melanocortin |
| DNA | Deoxyribonucleic acid |
| ROS | Reactive oxygen species |
| IR | Insulin resistance |
| SNS | Sympathetic nervous system |
| GHTT | Glucopyranosyloxy1-hydroxytrideca |
| CRP | C-reactive protein |
| PDI | Protein disulfide isomerase |
| ER | Endoplasmic reticulum |
| MDD | Major depressive disorder |
| CFS | Cerebrospinal fluid |
| FDA | Federal Drug Administration |
| ARC | Arcuate nucleus |
| MRI | Magnetic resonance imaging |
| BBB | Blood–brain barrier |
| PUFA | Polyunsaturated fatty acids |
| AMPK | AMP activated protein kinase |
| US | United States |
References
- Centers for Disease Control and Prevention (CDC). Obesity is a Common, Serious, and Costly Disease. Obesity Basics. 2020. Available online: https://www.cdc.gov/obesity/basics/index.html (accessed on 8 July 2021).
- World Health Organization (WHO). Obesity. Health Topics. 2021. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 8 July 2021).
- Ma, Y.; Ajnakina, O.; Steptoe, A.; Cadar, D. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol. 2020, 49, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Greenblat, C. Dementia. World Health Organization (WHO), 2 September 2021. Available online: www.who.int/news-room/fact-sheets/detail/dementia (accessed on 8 July 2021).
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated With Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Scherneck, S.; Joost, H.G.; Al-Hasani, H. Molecular links between obesity and diabetes:“diabesity”. In Endotext (Internet); MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.A.; Gannaban, R.B.; Khan, S.; Shah, H.; Siddik, A.B.; Hegde, V.K.; Reddy, P.H.; Shin, A.C. Neural Underpinnings of Obesity: The Role of Oxidative Stress and Inflammation in the Brain. Antioxidants 2020, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes, metabolic syndrome and obesity: Targets and therapy. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Lupsa, B. Clinical presentation, diagnosis, and initial evaluation of diabetes mellitus in adults. Aggiornamento 2021, 10. [Google Scholar]
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- Colino, S. What is Type 3 Diabetes? Semel Institute UCLA 26 2017. Available online: www.semel.ucla.edu/longevity/news/what-type-3-diabetes (accessed on 8 July 2021).
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Van Giau, V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging (NIA). What Is Dementia? Alzheimers.gov. 2022. Available online: https://www.alzheimers.gov/alzheimers-dementias/what-is-dementia#what-causes-dementia (accessed on 8 July 2021).
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The Critical Role of Metabolic Pathways in Aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-α) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef]
- Wueest, S.; Konrad, D. The controversial role of IL-6 in adipose tissue on obesity-induced dysregulation of glucose metabolism. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E607–E613. [Google Scholar] [CrossRef]
- Li, N.; Zhao, S.; Zhang, Z.; Zhu, Y.; Gliniak, C.M.; Vishvanath, L.; An, Y.A.; Wang, M.-Y.; Deng, Y.; Zhu, Q.; et al. Adiponectin preserves metabolic fitness during aging. Elife 2021, 10, e65108. [Google Scholar] [CrossRef]
- Kim, J.H.; Bachmann, R.A.; Chen, J. Chapter 21 Interleukin-6 and Insulin Resistance. Vitam. Horm. 2009, 80, 613–633. [Google Scholar] [CrossRef]
- Gabriely, I.; Ma, X.H.; Yang, X.M.; Rossetti, L.; Barzilai, N. Leptin Resistance During Aging Is Independent of Fat Mass. Diabetes 2002, 51, 1016–1021. [Google Scholar] [CrossRef]
- Isidori, A.M.; Strollo, F.; Morè, M.; Caprio, M.; Aversa, A.; Moretti, C.; Frajese, G.; Riondino, G.; Fabbri, A. Leptin and Aging: Correlation with Endocrine Changes in Male and Female Healthy Adult Populations of Different Body Weights. J. Clin. Endocrinol. Metab. 2000, 85, 1954–1962. [Google Scholar] [CrossRef]
- Van Andel, M.; Van Schoor, N.M.; Korten, N.C.; Comijs, H.C.; Heijboer, A.C.; Drent, M.L. The Association Between High-Molecular-Weight Adiponectin, Ghrelin and Leptin and Age-Related Cognitive Decline: Results From Longitudinal Aging Study Amsterdam. J. Gerontol. Ser. A 2020, 76, 131–140. [Google Scholar] [CrossRef]
- Kapoor, N.; Jiwanmall, S.A.; Nandyal, M.B.; Kattula, D.; Paravathareddy, S.; Paul, T.V.; Furler, J.; Oldenburg, B.; Thomas, N. Metabolic Score for Visceral Fat (METS-VF) Estimation—A Novel Cost-Effective Obesity Indicator for Visceral Adipose Tissue Estimation. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3261–3267. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Kabir, Z.; Devi, K.R.; Peter, P.S.; Das, B. Gender differences in central obesity: Implications for cardiometabolic health in South Asians. Indian Heart J. 2020, 72, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Sagui-Henson, S.J.; Radin, R.M.; Jhaveri, K.; Brewer, J.A.; Cohn, M.; Hartogensis, W.; Mason, A.E. Negative Mood and Food Craving Strength Among Women with Overweight: Implications for Targeting Mechanisms Using a Mindful Eating Intervention. Mindfulness 2021, 12, 2997–3010. [Google Scholar] [CrossRef]
- Hallam, J.; Boswell, R.G.; DeVito, E.E.; Kober, H. Gender-related Differences in Food Craving and Obesity. Yale J. Biol. Med. 2016, 89, 161–173. [Google Scholar] [PubMed]
- Tirthani, E.; Said, M.S.; Rehman, A. Genetics and Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Genes and Obesity. Genomics and Precision Health 2011. Available online: https://www.cdc.gov/genomics/resources/diseases/obesity/obesedit.htm30 (accessed on 8 July 2021).
- Vettori, A.; Pompucci, G.; Paolini, B.; Del Ciondolo, I.; Bressan, S.; Dundar, M.; Kenanoğlu, S.; Unfer, V.; Bertelli, M.; Geneob Project. Genetic background, nutrition and obesity: A review. Eur. Rev. Med. Pharm. Sci. 2019, 23, 1751–1761. [Google Scholar] [CrossRef]
- Pirini, F.; Rodriguez-Torres, S.; Ayandibu, B.G.; Orera-Clemente, M.; La Vega, A.G.; Lawson, F.; Thorpe, R.J., Jr.; Sidransky, D.; Guerrero-Preston, R. INSIG2 rs7566605 single nucleotide variant and global DNA methylation index levels are associated with weight loss in a personalized weight reduction program. Mol. Med. Rep. 2017, 17, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Talbert, M.E.; Langefeld, C.D.; Ziegler, J.T.; Haffner, S.M.; Norris, J.M.; Bowden, D.W. INSIG2 SNPs Associated With Obesity and Glucose Homeostasis Traits in Hispanics: The IRAS Family Study. Obesity 2009, 17, 1554–1562. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Martin, M.; Lindberg, I. PCSK1 Variants and Human Obesity. Prog. Mol. Biol. Transl. Sci. 2016, 140, 47–74. [Google Scholar] [CrossRef]
- Sarhangi, N.; Sharifi, F.; Hashemian, L.; Doabsari, M.H.; Heshmatzad, K.; Rahbaran, M.; Jamaldini, S.H.; Meybodi, H.R.A.; Hasanzad, M. PPARG (Pro12Ala) genetic variant and risk of T2DM: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12764. [Google Scholar] [CrossRef]
- Ricquier, D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: Historical perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef]
- Garaulet, M.; Smith, C.E.; Hernández-González, T.; Lee, Y.-C.; Ordovás, J.M. PPAR γ Pro12Ala interacts with fat intake for obesity and weight loss in a behavioural treatment based on the Mediterranean diet. Mol. Nutr. Food Res. 2011, 55, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.M.; Keildson, S.; Lindgren, C.M. Genetics and epigenetics of obesity. Maturitas 2011, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Guénard, F.; Deshaies, Y.; Cianflone, K.; Kral, J.G.; Marceau, P.; Vohl, M.-C. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc. Natl. Acad. Sci. USA 2013, 110, 11439–11444. [Google Scholar] [CrossRef] [PubMed]
- Perkins, E.; Murphy, S.K.; Murtha, A.P.; Schildkraut, J.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J. Pediatr. 2012, 161, 31–39. [Google Scholar] [CrossRef]
- Rasmussen, L.; Knorr, S.; Antoniussen, C.S.; Bruun, J.M.; Ovesen, P.G.; Fuglsang, J.; Kampmann, U. The Impact of Lifestyle, Diet and Physical Activity on Epigenetic Changes in the Offspring—A Systematic Review. Nutrients 2021, 13, 2821. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Akil, L.; Ahmad, H.A. Effects of socioeconomic factors on obesity rates in four southern states and Colorado. Ethn. Dis. 2011, 21, 58–62. [Google Scholar]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Benzler, J.; Ganjam, G.K.; Pretz, D.; Oelkrug, R.; Koch, C.E.; Legler, K.; Stöhr, S.; Culmsee, C.; Williams, L.M.; Tups, A. Central Inhibition of IKKβ/NF-κB Signaling Attenuates High-Fat Diet–Induced Obesity and Glucose Intolerance. Diabetes 2015, 64, 2015–2027. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-kappa B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Meyerovich, K.; Ortis, F.; Cardozo, A.K. The non-canonical NF-κB pathway and its contribution to β-cell failure in diabetes. J. Mol. Endocrinol. 2018, 61, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-H.; Bazuine, M.; Lumeng, C.N.; Geletka, L.M.; Mowers, J.; White, N.M.; Ma, J.-T.; Zhou, J.; Qi, N.; Westcott, D.; et al. The Protein Kinase IKKε Regulates Energy Balance in Obese Mice. Cell 2009, 138, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhou, Y.; Chen, Z.; Ren, D.; Cho, K.W.; Jiang, L.; Shen, H.; Sasaki, Y.; Rui, L. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 2012, 18, 943–949. [Google Scholar] [CrossRef]
- Benomar, Y.; Taouis, M. Molecular Mechanisms Underlying Obesity-Induced Hypothalamic Inflammation and Insulin Resistance: Pivotal Role of Resistin/TLR4 Pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Reis, W.L.; Yi, C.-X.; Gao, Y.; Tschöp, M.H.; Stern, J.E. Brain Innate Immunity Regulates Hypothalamic Arcuate Neuronal Activity and Feeding Behavior. Endocrinology 2015, 156, 1303–1315. [Google Scholar] [CrossRef]
- Méquinion, M.; Foldi, C.J.; Andrews, Z.B. The ghrelin-AgRP neuron nexus in anorexia nervosa: Implications for metabolic and behavioral adaptations. Front. Nutr. 2020, 6, 190. [Google Scholar] [CrossRef]
- Pereira, J.A.D.S.; Da Silva, F.C.; De Moraes-Vieira, P.M.M. The Impact of Ghrelin in Metabolic Diseases: An Immune Perspective. J. Diabetes Res. 2017, 2017, 4527980. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Alzheimer’s Disease & Related Dementias: Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 8 July 2021).
- Monte, S. Type 3 diabetes is sporadic Alzheimer’s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Millington, G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Alsina, R.; Trotta, M.; Bumaschny, V.F. Hypothalamic Proopiomelanocortin Is Necessary for Normal Glucose Homeostasis in Female Mice. Front. Endocrinol. 2018, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.L.; Piper, S.J.; Yeung, D.; O’Rahilly, S.; Coll, A.P. A Comparative Study of the Central Effects of Specific Proopiomelancortin (POMC)-Derived Melanocortin Peptides on Food Intake and Body Weight in Pomc Null Mice. Endocrinology 2006, 147, 5940–5947. [Google Scholar] [CrossRef] [PubMed]
- Raffan, E.; Dennis, R.J.; O’Donovan, C.J.; Becker, J.M.; Scott, R.A.; Smith, S.P.; Withers, D.J.; Wood, C.J.; Conci, E.; Clements, D.N.; et al. A Deletion in the Canine POMC Gene Is Associated with Weight and Appetite in Obesity-Prone Labrador Retriever Dogs. Cell Metab. 2016, 23, 893–900. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Miller, J.W.; Keogh, J.M.; Henning, E.; Satterwhite, J.H.; Cameron, G.S.; Astruc, B.; Mayer, J.P.; Brage, S.; See, T.C.; et al. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N. Engl. J. Med. 2009, 360, 44–52. [Google Scholar] [CrossRef]
- Kühnen, P.; Clément, K.; Wiegand, S.; Blankenstein, O.; Gottesdiener, K.; Martini, L.L.; Mai, K.; Blume-Peytavi, U.; Grüters, A.; Krude, H. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N. Engl. J. Med. 2016, 375, 240–246. [Google Scholar] [CrossRef]
- Collet, T.H.; Dubern, B.; Mokrosinski, J.; Connors, H.; Keogh, J.M.; de Oliveira, E.M.; Henning, E.; Poitou-Bernert, C.; Oppert, J.M.; Tounian, P.; et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 2017, 6, 1321–1329. [Google Scholar] [CrossRef]
- Kievit, P.; Halem, H.; Marks, D.L.; Dong, J.Z.; Glavas, M.M.; Sinnayah, P.; Pranger, L.; Cowley, M.A.; Grove, K.L.; Culler, M.D. Chronic Treatment With a Melanocortin-4 Receptor Agonist Causes Weight Loss, Reduces Insulin Resistance, and Improves Cardiovascular Function in Diet-Induced Obese Rhesus Macaques. Diabetes 2013, 62, 490–497. [Google Scholar] [CrossRef]
- Low, M.J. New hormone treatment for obesity caused by POMC-deficiency. Nat. Rev. Endocrinol. 2016, 12, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xie, F.; Xiao, Y.; Lu, C.; Zhong, J.; Huang, D.; Chen, J.; Yongwei, X.; Jiang, Y.; Zhong, T. Metformin: A Potential Candidate for Targeting Aging Mechanisms. Aging Dis. 2021, 12, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Attaluri, S.; Madhu, L.N.; Shuai, B.; Upadhya, R.; Gonzalez, J.J.; Rao, X.; Shetty, A.K. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 2021, 20, e13277. [Google Scholar] [CrossRef]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Shetty, A.K.; Kodali, M.; Upadhya, R.; Madhu, L.N. Emerging Anti-Aging Strategies—Scientific Basis and Efficacy. Aging Dis. 2018, 9, 1165–1184. [Google Scholar] [CrossRef]
- Teng, Z.; Feng, J.; Qi, Q.; Dong, Y.; Xiao, Y.; Xie, X.; Meng, N.; Chen, H.; Zhang, W.; Lv, P. Long-Term Use of Metformin Is Associated With Reduced Risk of Cognitive Impairment With Alleviation of Cerebral Small Vessel Disease Burden in Patients With Type 2 Diabetes. Front. Aging Neurosci. 2021, 13, 773797. [Google Scholar] [CrossRef]
- Kuo, T.; Hsu, S.; Huang, S.; Chang, C.L.; Feng, C.; Huang, M.; Chen, T.; Yang, M.; Jiang, S.; Wen, T.; et al. Pdia4 regulates β-cell pathogenesis in diabetes: Molecular mechanism and targeted therapy. EMBO Mol. Med. 2021, 13, e11668. [Google Scholar] [CrossRef]
- Su, S.; Chien, C.; Chen, Y.; Chiang, C.; Lin, F.; Kuo, F.; Huang, C.; Li, P.; Liu, J.; Lu, C.; et al. PDIA4, a novel ER stress chaperone, modulates adiponectin expression and inflammation in adipose tissue. BioFactors 2022. [Google Scholar] [CrossRef]
- Bherer, L. A pro-inflammatory diet increases the risk of dementia. Prev. Watch. 2021. [Google Scholar]
- Khan, S.H.; Hegde, V. Obesity and Diabetes Mediated Chronic Inflammation: A Potential Biomarker in Alzheimer’s Disease. J. Pers. Med. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes, Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- CDC; USDA. Dietary Guidelines for Americans 2020–2025, CDC 2020. Available online: www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf#page=31 (accessed on 8 July 2021).
- Wartella, A.H.; Boon, C.S. Examination of Front-of-Package Nutrition Rating Systems and Symbols: Phase I Report; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef] [PubMed]
- Erdelyi, I.; Levenkova, N.; Lin, E.Y.; Pinto, J.T.; Lipkin, M.; Quimby, F.W.; Holt, P.R. Western-Style Diets Induce Oxidative Stress and Dysregulate Immune Responses in the Colon in a Mouse Model of Sporadic Colon Cancer. J. Nutr. 2009, 139, 2072–2078. [Google Scholar] [CrossRef][Green Version]
- Merat, S.; Casanada, F.; Sutphin, M.; Palinski, W.; Reaven, P.D. Western-Type Diets Induce Insulin Resistance and Hyperinsulinemia in LDL Receptor-Deficient Mice But Do Not Increase Aortic Atherosclerosis Compared With Normoinsulinemic Mice in Which Similar Plasma Cholesterol Levels Are Achieved by a Fructose-Rich Diet. Arter. Thromb. Vasc. Biol. 1999, 19, 1223–1230. [Google Scholar] [CrossRef]
- Studzinski, C.M.; Li, F.; Bruce-Keller, A.J.; Fernandez-Kim, S.O.; Zhang, L.; Weidner, A.M.; Markesbery, W.R.; Murphy, M.P.; Keller, J.N. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP × PS1 knock-in mice. J. Neurochem. 2009, 108, 860–866. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Black, P.H. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med. Hypotheses 2006, 67, 879–891. [Google Scholar] [CrossRef]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1863, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.C.D.S.; Pinto, I.S.D.J.; Mourão, A.A.; Fajemiroye, J.O.; Colombari, E.; Reis, A.D.S.; Freiria-Oliveira, A.H.; Ferreira-Neto, M.L.; Pedrino, G.R. Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front. Physiol. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Cabandugama, P.K.; Gardner, M.J.; Sowers, J.R. The Renin Angiotensin Aldosterone System in Obesity and Hypertension. Med Clin. N. Am. 2016, 101, 129–137. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Blundell, J.E.; Mennella, J.A.; Batterham, R.L. Biological control of appetite: A daunting complexity. Obesity 2017, 25, S8–S16. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Poulimeneas, D.; Anastasiou, C.A.; Santos, I.; Hill, J.O.; Panagiotakos, D.B.; Yannakoulia, M. Exploring the relationship between the Mediterranean diet and weight loss maintenance: The MedWeight study. Br. J. Nutr. 2020, 124, 874–880. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Choosing a Safe and Successful Weight-Loss Program. National Institute of Health (NIH) 2018. Available online: www.niddk.nih.gov/health-information/weight-management/choosing-a-safe-successful-weight-loss-program (accessed on 8 July 2021).
- Centers for Disease Control and Prevention (CDC). Prevalence of Childhood Obesity in the United States. Childhood Obesity Facts 2022. Available online: www.cdc.gov/obesity/data/childhood.html (accessed on 8 July 2021).
- Bhatti, G.K.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 11, 369. [Google Scholar] [CrossRef]
- Balasundaram, P.; Krishna, S. Obesity Effects on Child Health; National Library of Medicine: Bethesda, MA, USA, 2022. [Google Scholar]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: A complex association. Nat. Rev. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Russell, A.P.; Foletta, V.C.; Snow, R.J.; Wadley, G.D. Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1276–1284. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Sun, Y.; Li, R.; Brana, C.; Feldon, J.; Yee, B.K. The impact of voluntary exercise on mental health in rodents: A neuroplasticity perspective. Behav. Brain Res. 2008, 192, 42–60. [Google Scholar] [CrossRef]
- National Institute on Aging (NIA). Four Types of Exercise Can Improve Your Health and Physical Ability. Exercise and Physical Activity. 2021. Available online: www.nia.nih.gov/health/four-types-exercise-can-improve-your-health-and-physical-ability (accessed on 8 July 2021).
- National Institute of Health (NIH). Prescription Medications to Treat Overweight & Obesity. National Institute of Diabetes and Digestive and Kidney Diseases 2021. Available online: www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 8 July 2021).
- FDA. Weight-Loss and Weight-Management Devices. FDA. 2020. Available online: www.fda.gov/medical-devices/products-and-medical-procedures/weight-loss-and-weight-management-devices (accessed on 8 July 2021).
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- ASMBS. Bariatric Surgery Procedures, ASMBS May 2021. Available online: https://asmbs.org/patients/bariatric-surgery-procedures (accessed on 8 July 2021).
- American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Matthews, D.R.; Paldánius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019, 394, 1519–1529. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Dementia. Mayo Clinic 2021. Published 17 June 2021. Available online: www.mayoclinic.org/diseases-conditions/dementia/diagnosis-treatment/drc-20352019 (accessed on 8 July 2021).
- Tampi, R.R.; Forester, B.P.; Agronin, M. Aducanumab: Evidence from clinical trial data and controversies. Drugs Context 2021, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duce, J.; Zhu, X.; Jacobson, L.; Beart, P. Therapeutics for dementia and Alzheimer’s disease: New directions for precision medicine. J. Cereb. Blood Flow Metab. 2019, 176, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Lalli, G.; Schott, J.M.; Hardy, J.; De Strooper, B. Aducanumab: A new phase in therapeutic development for Alzheimer’s disease? EMBO Mol. Med. 2021, 13, e14781. [Google Scholar] [CrossRef]

| Year Range | Obesity Prevalence Percentage | Diabetes Mellitus Prevalence Percentage |
|---|---|---|
| 1988–1994 | 20.2 | 8.8 |
| 1999–2002 | 27.6 | 10.8 |
| 2011–2014 | 34.25 | 12.9 |
| 2015–2018 | 40.45 | 14.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selman, A.; Burns, S.; Reddy, A.P.; Culberson, J.; Reddy, P.H. The Role of Obesity and Diabetes in Dementia. Int. J. Mol. Sci. 2022, 23, 9267. https://doi.org/10.3390/ijms23169267
Selman A, Burns S, Reddy AP, Culberson J, Reddy PH. The Role of Obesity and Diabetes in Dementia. International Journal of Molecular Sciences. 2022; 23(16):9267. https://doi.org/10.3390/ijms23169267
Chicago/Turabian StyleSelman, Ashley, Scott Burns, Arubala P. Reddy, John Culberson, and P. Hemachandra Reddy. 2022. "The Role of Obesity and Diabetes in Dementia" International Journal of Molecular Sciences 23, no. 16: 9267. https://doi.org/10.3390/ijms23169267
APA StyleSelman, A., Burns, S., Reddy, A. P., Culberson, J., & Reddy, P. H. (2022). The Role of Obesity and Diabetes in Dementia. International Journal of Molecular Sciences, 23(16), 9267. https://doi.org/10.3390/ijms23169267







