Cyclophilin A Promotes Osteoblast Differentiation by Regulating Runx2
Abstract
1. Introduction
2. Results
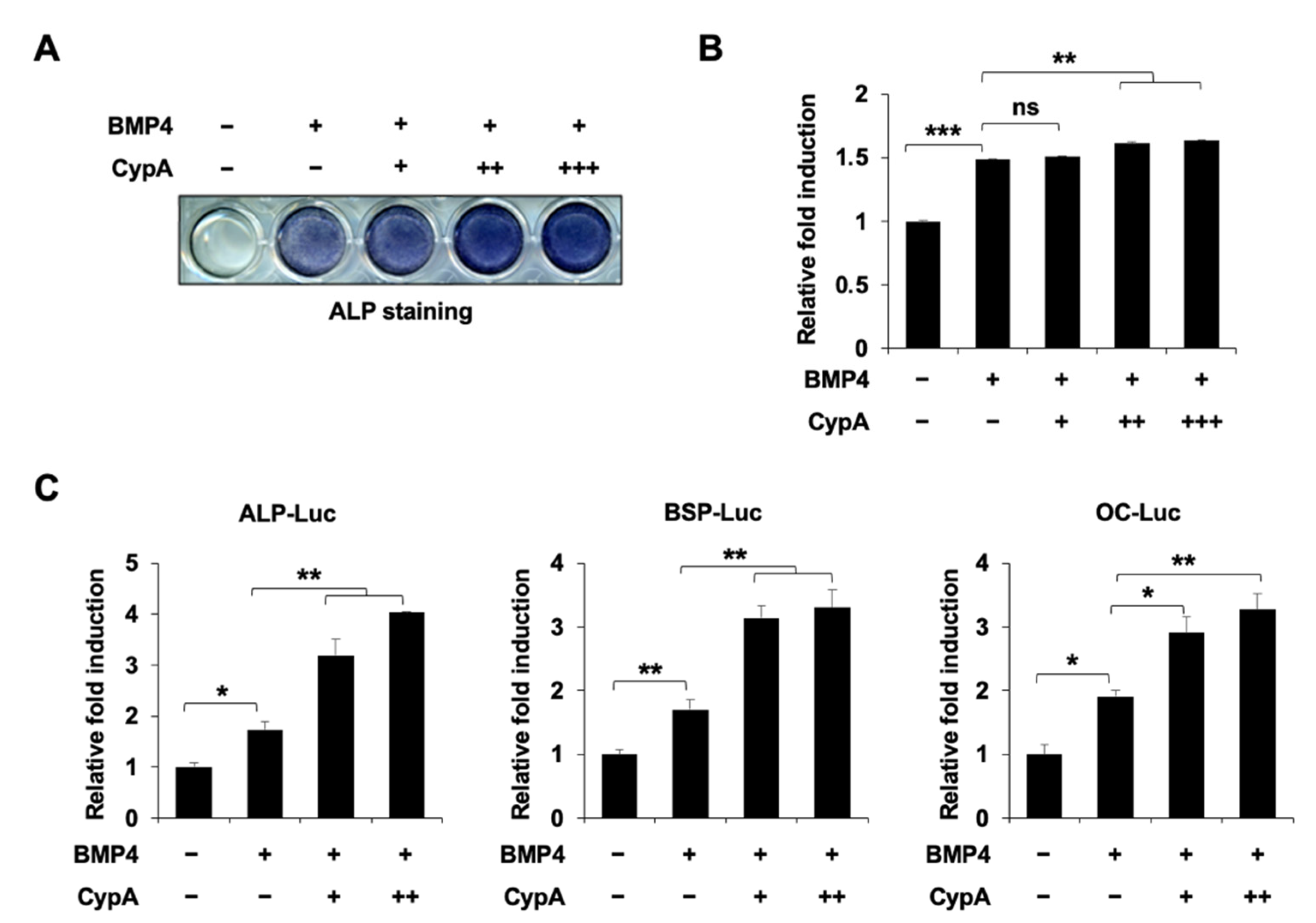
2.1. CypA Enhances BMP4-Induced Osteoblast Differentiation In Vitro
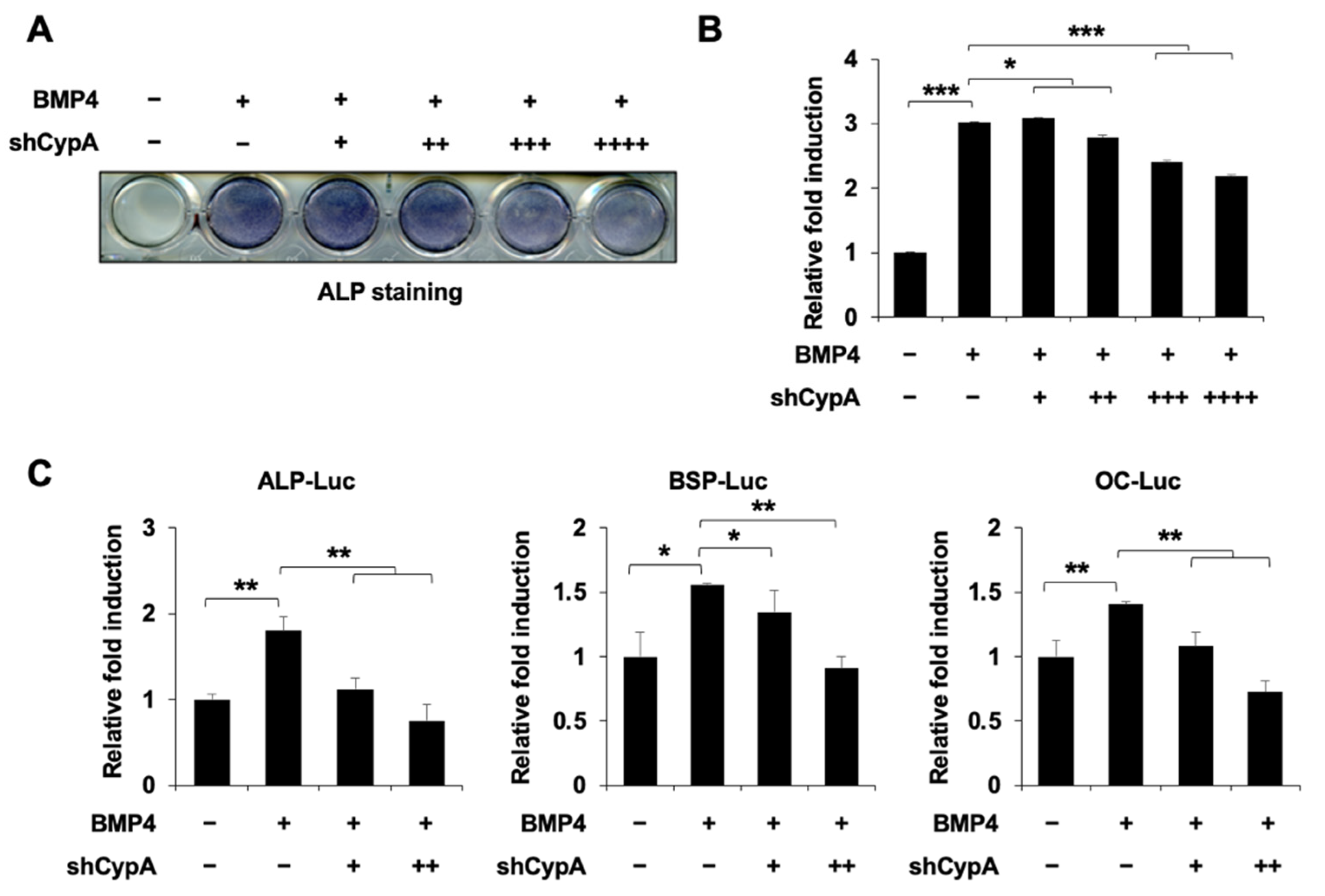
2.2. Knockdown of CypA Reduces Osteoblast Differentiation
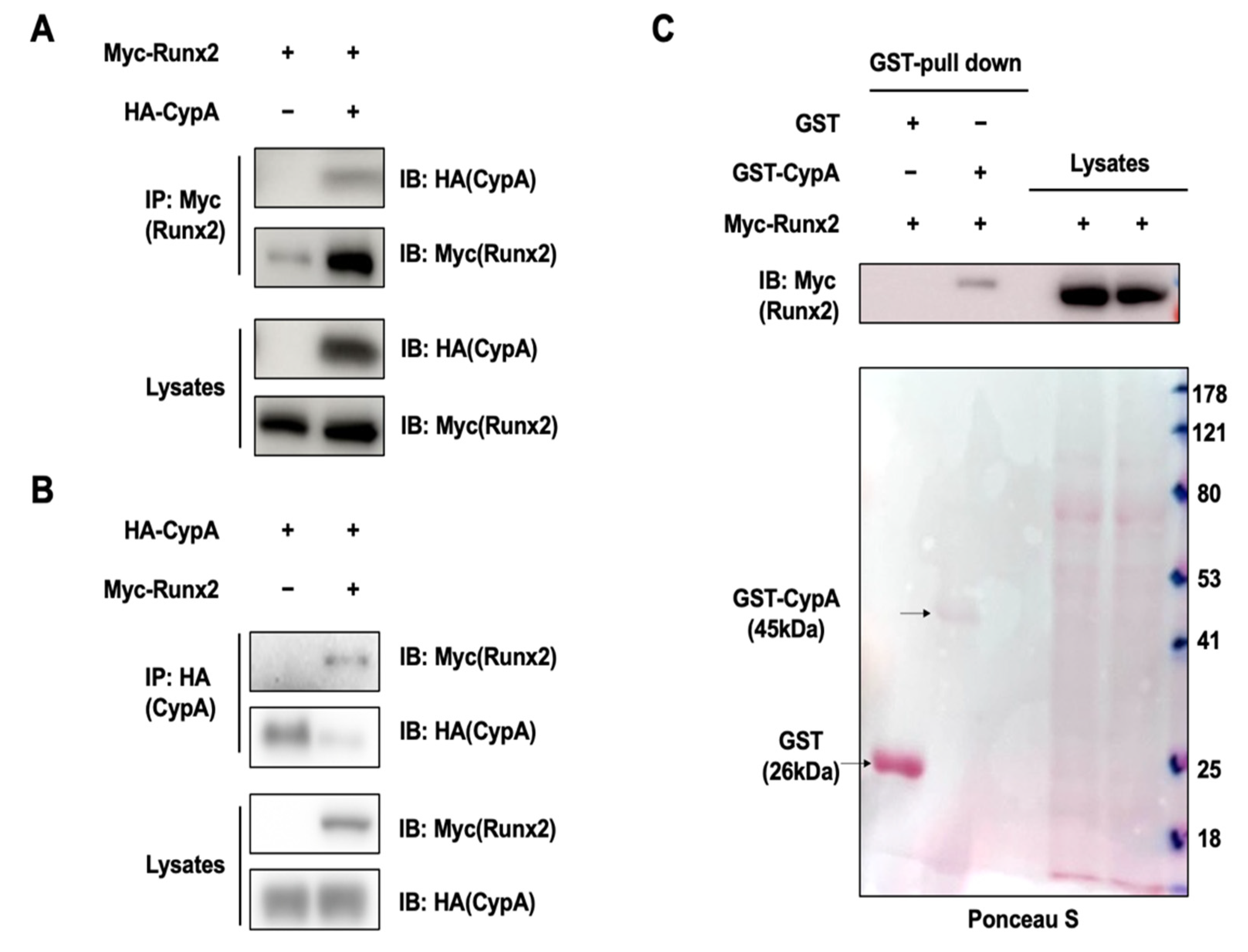
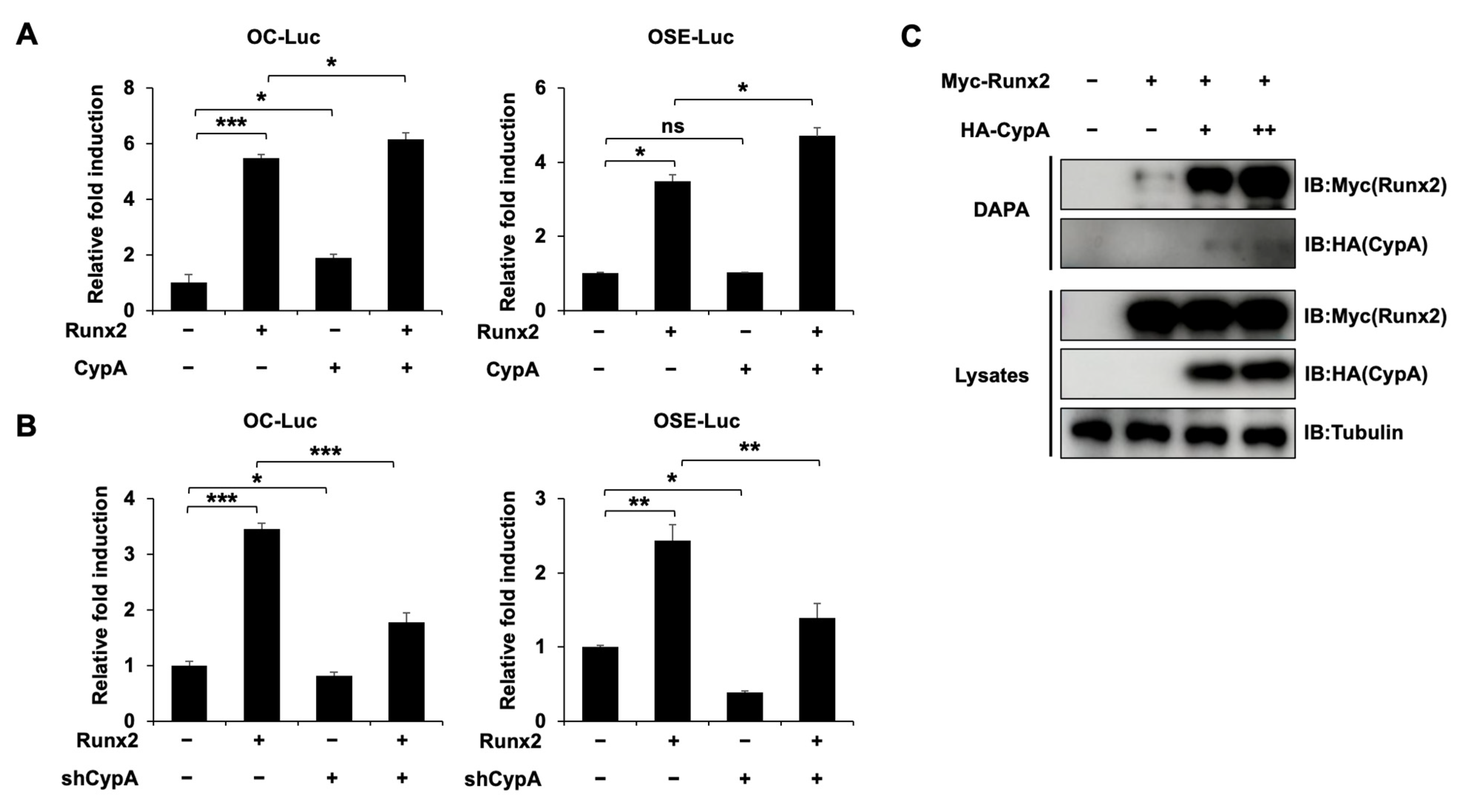
2.3. CypA Interacts with Runx2
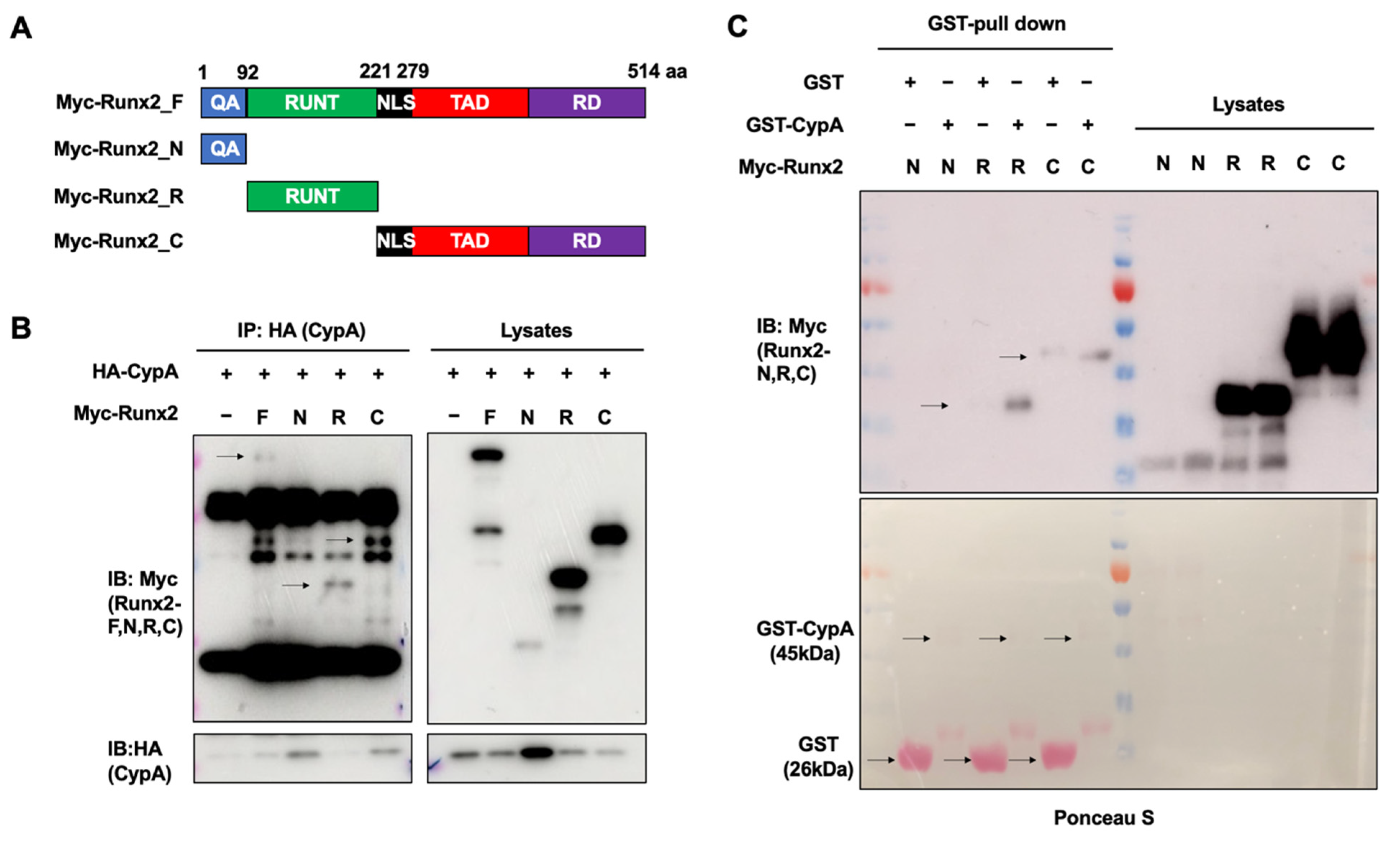
2.4. The Runt and C-terminal Domains of Runx2 Are Essential for Interacting with CypA
2.5. CypA Promotes the Osteogenic Activity of Runx2 and Enhances the Binding of Runx2 to Target DNA
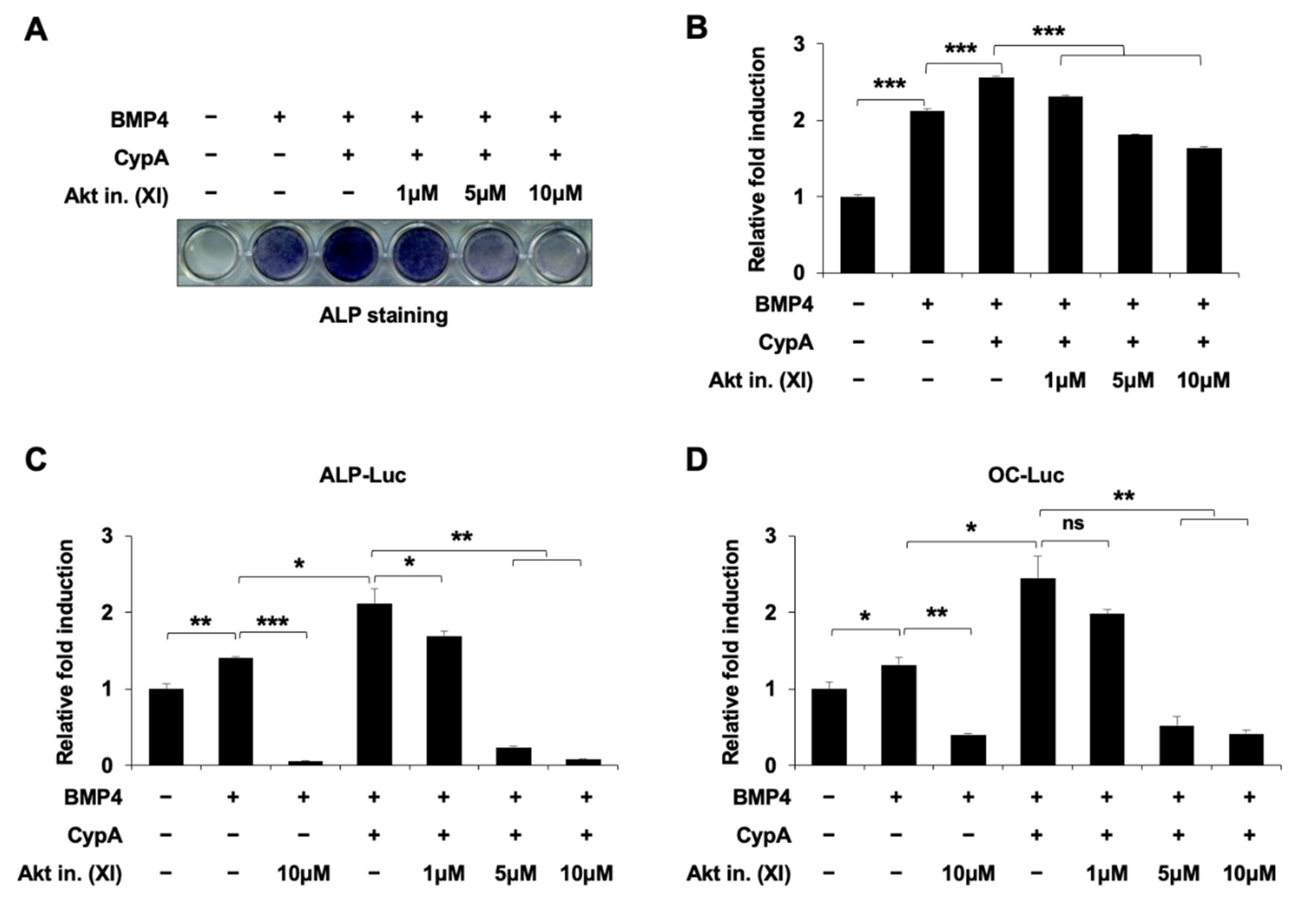
2.6. Akt Signaling Is Involved in CypA-Regulated Osteoblast Differentiation
3. Discussion
4. Materials and Methods
4.1. Plasmids and Chemicals
4.2. Cell Culture and Osteoblast Differentiation
4.3. Alkaline Phosphatase (ALP) Staining
4.4. Luciferase Assay and Transfection
4.5. Immunoblotting and Immunoprecipitation
4.6. GST Pulldown Assays
4.7. DNA Affinity Precipitation Assay (DAPA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galat, A. Peptidylproline cis-trans-isomerases: Immunophilins. Eur. J. Biochem. 1993, 216, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Colgan, J.; Asmal, M.; Yu, B.; Luban, J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. J. Immunol. 2005, 174, 6030–6038. [Google Scholar] [CrossRef]
- Lu, K.P.; Finn, G.; Lee, T.H.; Nicholson, L.K. Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 2007, 3, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; James, A.W.; Kwak, J.H.; Shen, J.; Yokoyama, K.K.; Ting, K.; Soo, C.B.; Chiu, R.H. Cyclophilin A (CypA) Plays Dual Roles in Regulation of Bone Anabolism and Resorption. Sci. Rep. 2016, 6, 22378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Xiao, G. Regulation of the osteoblast-specific transcription factor, Runx2: Responsiveness to multiple signal transduction pathways. J. Cell. Biochem. 2003, 88, 446–454. [Google Scholar] [CrossRef]
- Jonason, J.H.; Xiao, G.; Zhang, M.; Xing, L.; Chen, D. Post-translational Regulation of Runx2 in Bone and Cartilage. J. Dent. Res. 2009, 88, 693–703. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.J.; Ryoo, H.M. Post-Translational Regulations of Transcriptional Activity of RUNX2. Mol. Cells 2020, 43, 160–167. [Google Scholar] [CrossRef]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Salasznyk, R.M.; Klees, R.F.; Hughlock, M.K.; Plopper, G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun. Adhes. 2004, 11, 137–153. [Google Scholar] [CrossRef]
- Tascau, L.; Gardner, T.; Anan, H.; Yongpravat, C.; Cardozo, C.P.; Bauman, W.A.; Lee, F.Y.; Oh, D.S.; Tawfeek, H.A. Activation of Protein Kinase A in Mature Osteoblasts Promotes a Major Bone Anabolic Response. Endocrinology 2016, 157, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Jin, Y.H.; Choi, Y.H.; Yum, J.; Choi, J.K.; Yeo, C.Y.; Lee, K.Y. PKC signaling inhibits osteogenic differentiation through the regulation of Msx2 function. Biochim. Biophys. Acta 2012, 1823, 1225–1232. [Google Scholar] [CrossRef]
- Kawamura, N.; Kugimiya, F.; Oshima, Y.; Ohba, S.; Ikeda, T.; Saito, T.; Shinoda, Y.; Kawasaki, Y.; Ogata, N.; Hoshi, K.; et al. Akt1 in osteoblasts and osteoclasts controls bone remodeling. PLoS ONE 2007, 2, e1058. [Google Scholar] [CrossRef]
- Kugimiya, F.; Kawaguchi, H.; Ohba, S.; Kawamura, N.; Hirata, M.; Chikuda, H.; Chung, U.I. GSK-3β Controls Osteogenesis through Regulating Runx2 Activity. PLoS ONE 2007, 2, e837. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A.; Zaidi, S.K.; Young, D.W.; Choi, J.Y.; Pockwinse, S.M. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef]
- Jeon, E.J.; Lee, K.Y.; Choi, N.S.; Lee, M.H.; Kim, H.N.; Jin, Y.H.; Ryoo, H.M.; Choi, J.Y.; Yoshida, M.; Nishino, N.; et al. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J. Biol. Chem. 2006, 281, 16502–16511. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Chen, M.; Wang, Y.J.; Kaneki, H.; Xing, L.; O’keefe, R.J.; Chen, D. Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. J. Biol. Chem. 2006, 281, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Hayano, T.; Suzuki, M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 1989, 337, 473–475. [Google Scholar] [CrossRef]
- Guo, M.; Shen, J.; Kwak, J.H.; Choi, B.; Lee, M.; Hu, S.; Zhang, X.; Ting, K.; Soo, C.B.; Chiu, R.H. Novel role for cyclophilin A in regulation of chondrogenic commitment and endochondral ossification. Mol. Cell. Biol. 2015, 35, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Islam, R.; Cho, Y.D.; Woo, K.M.; Baek, J.H.; Uchida, T.; Komori, T.; van Wijnen, A.; Stein, J.L.; Lian, J.B.; et al. Pin1-mediated Runx2 modification is critical for skeletal development. J. Cell. Physiol. 2013, 228, 2377–2385. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, Y.H.; Kim, Y.J.; Choi, H.S.; Yeo, C.Y.; Lee, K.Y. Prolyl isomerase Pin1 enhances osteoblast differentiation through Runx2 regulation. FEBS Lett. 2013, 587, 3640–3647. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, H.M.; Han, Y.; Cheong, H.; Kang, B.Y.; Lee, K.Y. Prolyl isomerase Pin1 regulates the osteogenic activity of Osterix. Mol. Cell. Endocrinol. 2015, 400, 32–40. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, Y.J.; Jeong, H.M.; Jin, Y.H.; Yeo, C.Y.; Lee, K.Y. Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J. 2014, 281, 3656–3666. [Google Scholar] [CrossRef]
- Pande, S.; Browne, G.; Padmanabhan, S.; Zaidi, S.K.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Oncogenic cooperation between PI3K/Akt signaling and transcription factor Runx2 promotes the invasive properties of metastatic breast cancer cells. J. Cell. Physiol. 2013, 228, 1784–1792. [Google Scholar] [CrossRef]
- Fujita, T.; Azuma, Y.; Fukuyama, R.; Hattori, Y.; Yoshida, C.; Koida, M.; Ogita, K.; Komori, T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell. Biol. 2004, 166, 85–95. [Google Scholar] [CrossRef]
- Komori, T. Mechanism of transcriptional regulation by Runx2 in osteoblasts. Clin. Calcium. 2006, 16, 801–807. [Google Scholar]
- Jeong, H.M.; Choi, Y.H.; Lee, S.H.; Lee, K.Y. YY1 represses the transcriptional activity of Runx2 in C2C12 cells. Mol. Cell. Endocrinol. 2014, 383, 103–110. [Google Scholar] [CrossRef] [PubMed]

| Inhibitor | Product Name | Catalog # |
|---|---|---|
| Mitogen-activated protein kinase kinase (MEK) inhibitor | U0126 | 662005 |
| Protein kinase A (PKA) inhibitor | H89 | 371963 |
| Protein kinase C (PKC) inhibitor | Go6976 | 365250 |
| p38 MAPK inhibitor | SB203580 | 559386 |
| Akt inhibitor | XI | 124028 |
| Glycogen synthase kinase 3 (GSK3) inhibitor | LiCl | 438002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, M.; Lee, S.H.; Kim, M.J.; Choi, J.-K.; Yeo, C.-Y.; Lee, K.Y. Cyclophilin A Promotes Osteoblast Differentiation by Regulating Runx2. Int. J. Mol. Sci. 2022, 23, 9244. https://doi.org/10.3390/ijms23169244
Piao M, Lee SH, Kim MJ, Choi J-K, Yeo C-Y, Lee KY. Cyclophilin A Promotes Osteoblast Differentiation by Regulating Runx2. International Journal of Molecular Sciences. 2022; 23(16):9244. https://doi.org/10.3390/ijms23169244
Chicago/Turabian StylePiao, Meiyu, Sung Ho Lee, Myeong Ji Kim, Joong-Kook Choi, Chang-Yeol Yeo, and Kwang Youl Lee. 2022. "Cyclophilin A Promotes Osteoblast Differentiation by Regulating Runx2" International Journal of Molecular Sciences 23, no. 16: 9244. https://doi.org/10.3390/ijms23169244
APA StylePiao, M., Lee, S. H., Kim, M. J., Choi, J.-K., Yeo, C.-Y., & Lee, K. Y. (2022). Cyclophilin A Promotes Osteoblast Differentiation by Regulating Runx2. International Journal of Molecular Sciences, 23(16), 9244. https://doi.org/10.3390/ijms23169244







