Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions
Abstract
1. Introduction
2. Results
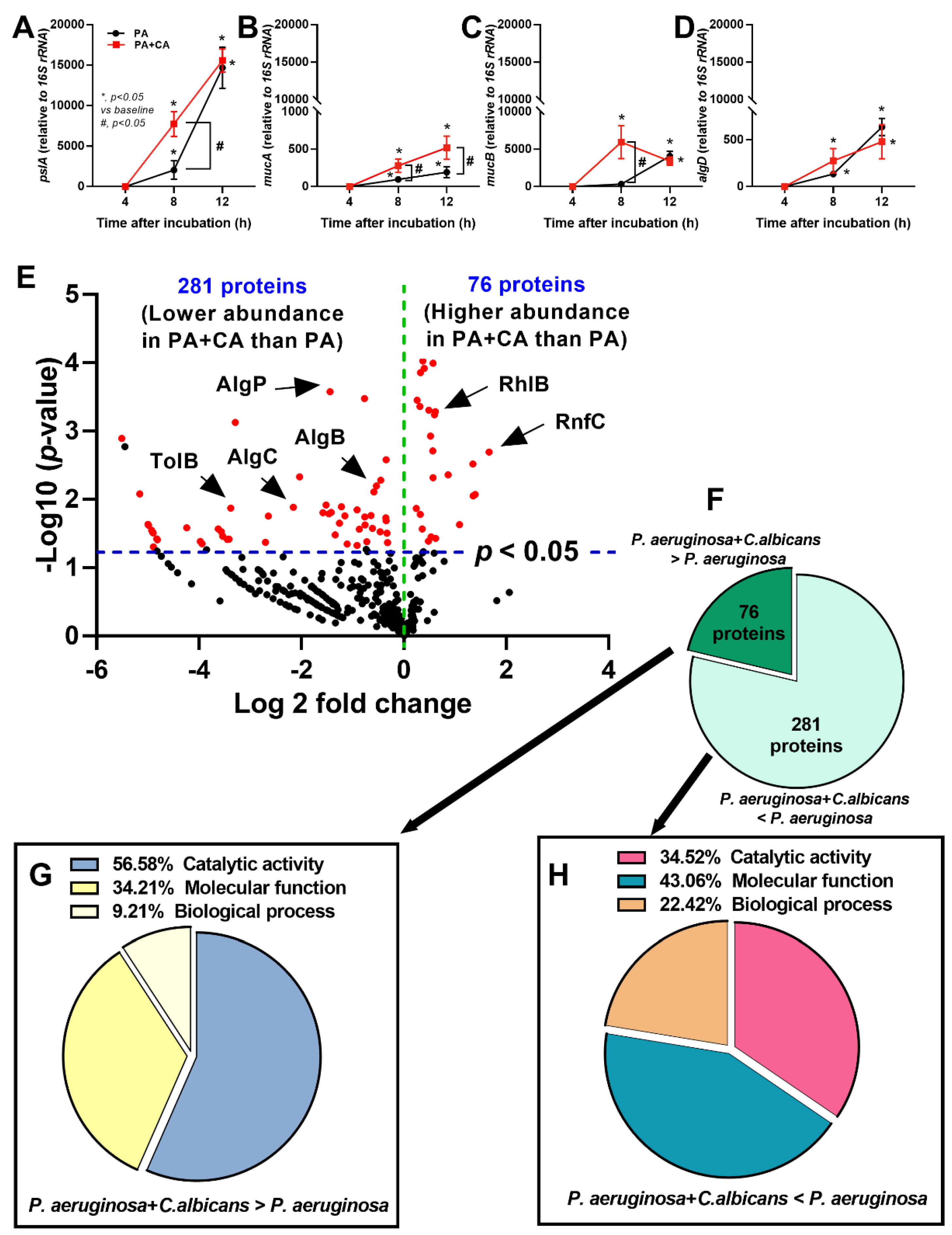
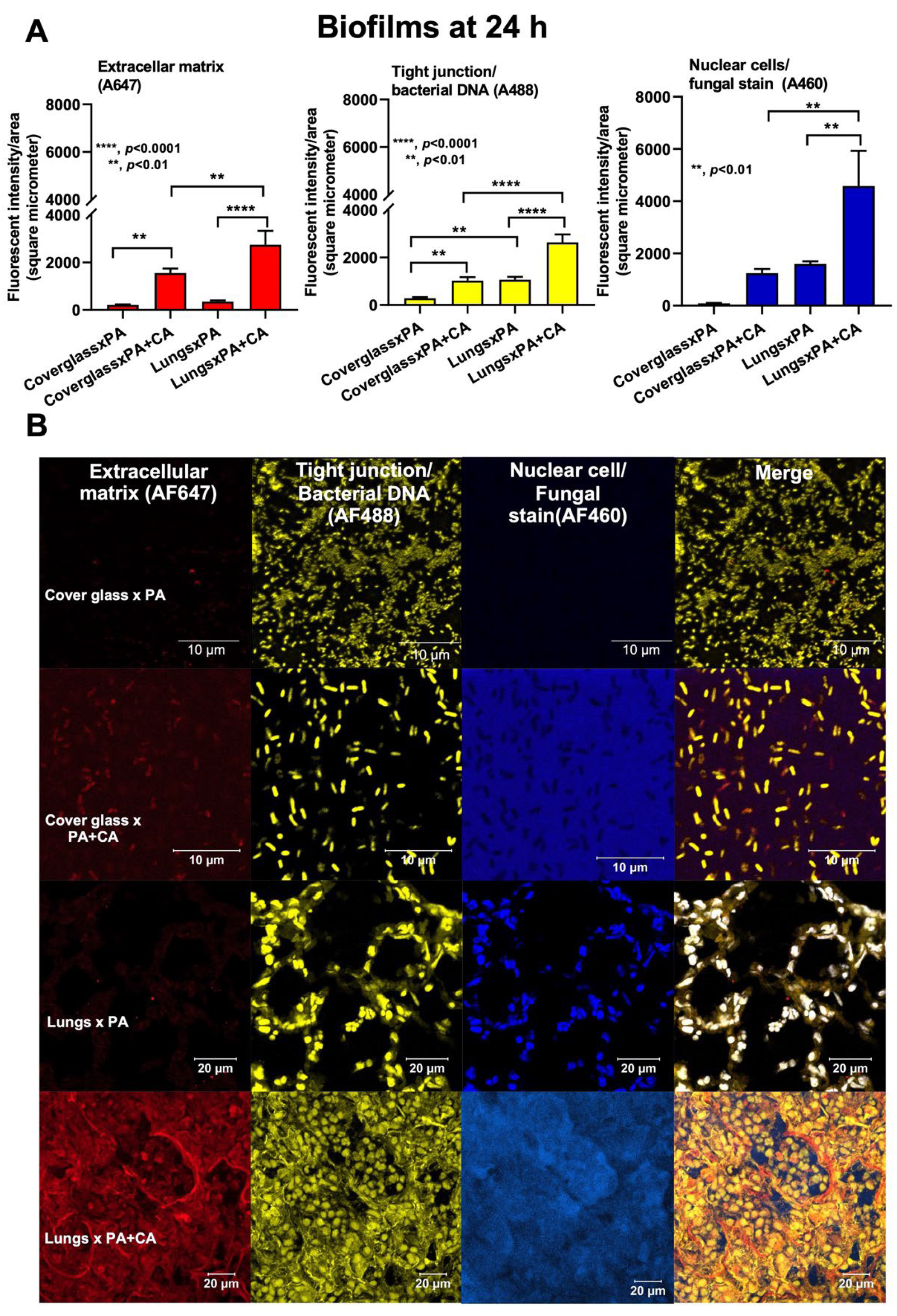
2.1. Prominent Mucoid Biofilms from Pseudomonas plus Candida on Human Pulmonary Cells
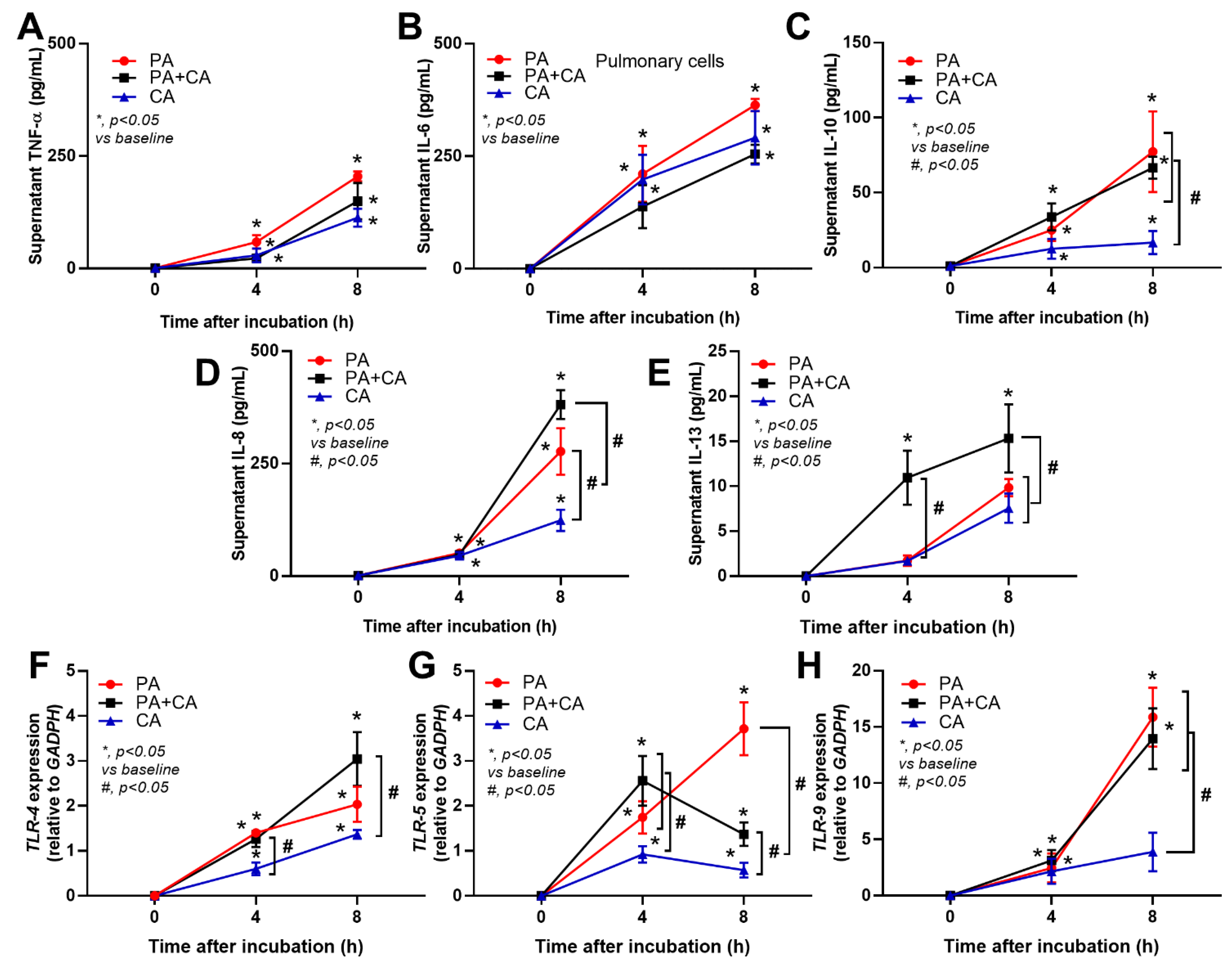
2.2. The Pulmonary Responses against Interkingdom Pseudomonas–Candida Biofilms
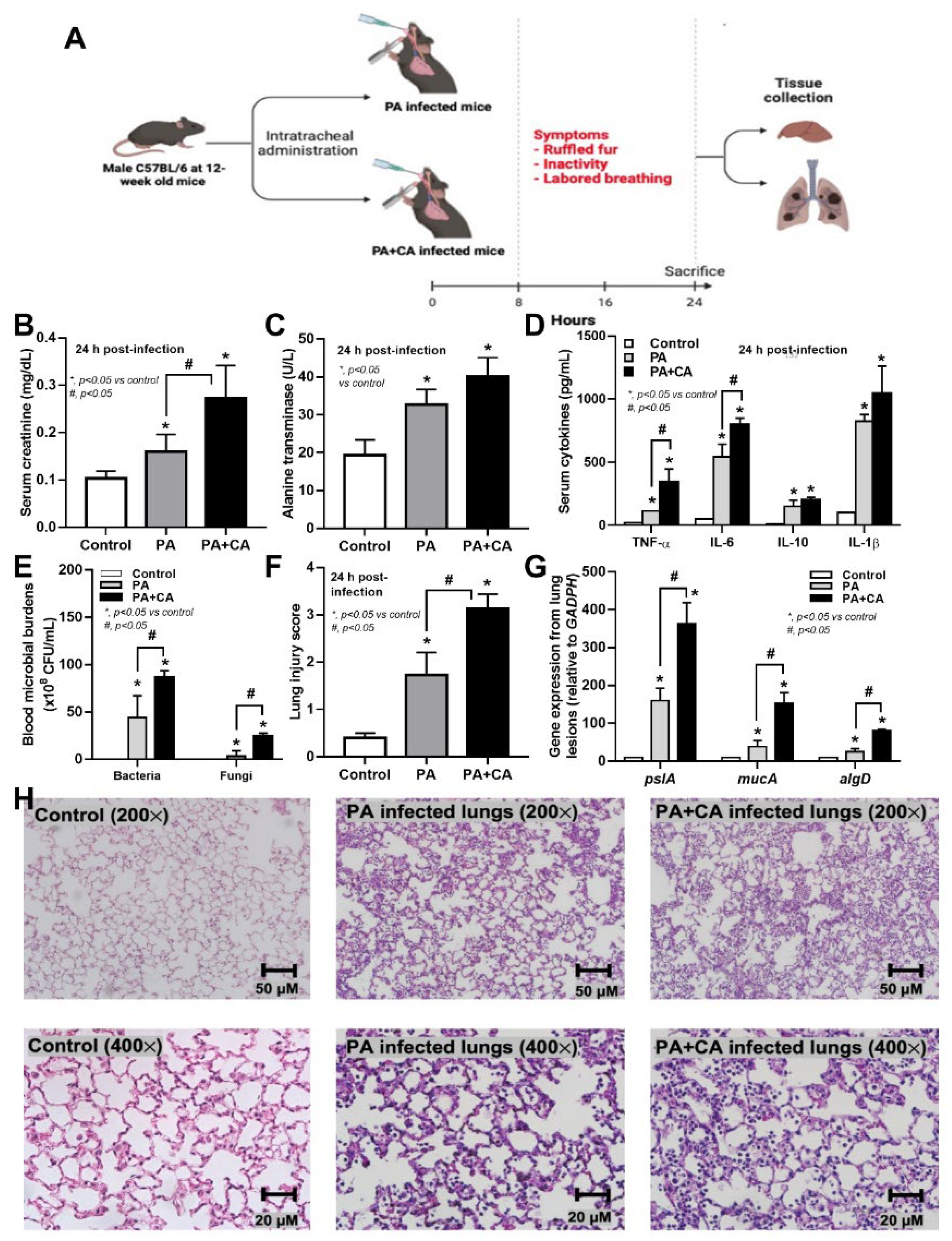
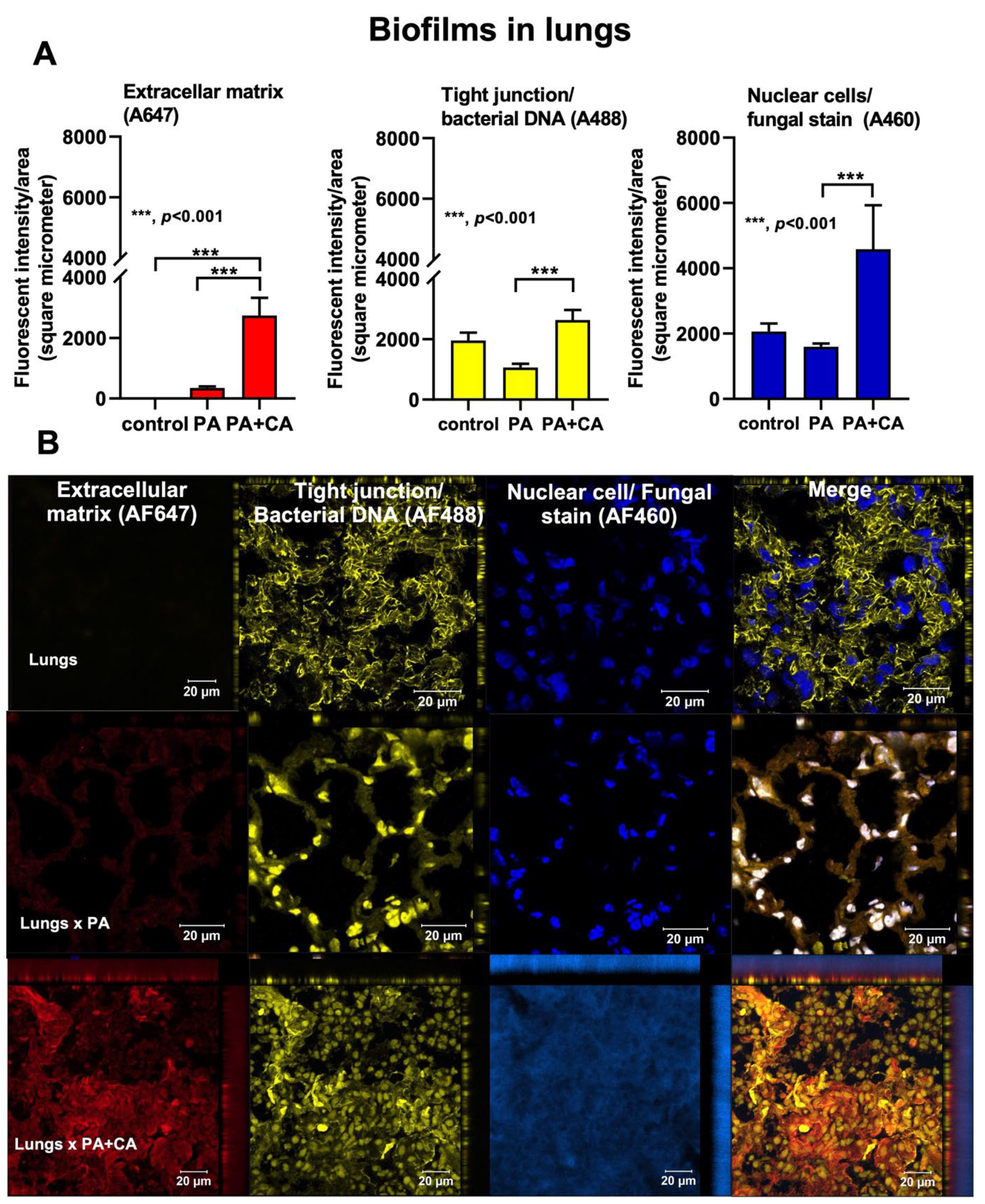
2.3. Biofilm Formation in the Lungs of Acute Pneumonia, a Possible Initiator of Chronic Lesions
3. Discussion
4. Materials and Methods
4.1. Microbial Preparation and Biofilms on Human Cells
4.2. Quantitative Real-Time Polymerase Chain Reaction
4.3. Proteomic Analysis
4.4. Animals and Animal Model
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.; Kim, D.Y.; Smalley, N.E.; Salukhe, I.; Greenberg, E.P.; Dandekar, A.A. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc. Natl. Acad. Sci. USA 2019, 116, 7027–7032. [Google Scholar] [CrossRef]
- Hossain, M.A.; Sattenapally, N.; Parikh, H.I.; Li, W.; Rumbaugh, K.P.; German, N.A. Design, synthesis, and evaluation of compounds capable of reducing Pseudomonas aeruginosa virulence. Eur. J. Med. Chem. 2020, 185, 111800. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis pathogenesis. Methods Mol. Biol. 2014, 1106, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Briaud, P.; Vandenesch, F.; Moreau, K. How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus. Front. Microbiol. 2021, 12, 617784. [Google Scholar] [CrossRef]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef]
- Phuengmaung, P.; Panpetch, W.; Singkham-In, U.; Chatsuwan, T.; Chirathaworn, C.; Leelahavanichkul, A. Presence of Candida tropicalis on Staphylococcus epidermidis biofilms facilitated biofilm production and Candida dissemination: An impact of fungi on bacterial biofilms. Front. Cell Infect. Microbiol. 2021, 11, 763239. [Google Scholar] [CrossRef]
- Panpetch, W.; Phuengmaung, P.; Hiengrach, P.; Issara-Amphorn, J.; Cheibchalard, T.; Somboonna, N.; Tumwasorn, S.; Leelahavanichkul, A. Candida worsens Klebsiella pneumoniae induced-sepsis in a mouse model with low dose dextran sulfate solution through gut dysbiosis and enhanced inflammation. Int. J. Mol. Sci. 2022, 23, 7050. [Google Scholar] [CrossRef]
- Fourie, R.; Pohl, C.H. Beyond Antagonism: The Interaction Between Candida Species and Pseudomonas aeruginosa. J. Fungi 2019, 5, 34. [Google Scholar] [CrossRef]
- Hattab, S.; Dagher, A.M.; Wheeler, R.T. Pseudomonas synergizes with fluconazole against Candida during treatment of polymicrobial Infection. Infect. Immun. 2022, 90, e0062621. [Google Scholar] [CrossRef] [PubMed]
- El-Azizi, M.A.; Starks, S.E.; Khardori, N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 2004, 96, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Phuengmaung, P.; Somparn, P.; Panpetch, W.; Singkham-In, U.; Wannigama, D.L.; Chatsuwan, T.; Leelahavanichkul, A. Coexistence of Pseudomonas aeruginosa with Candida albicans enhances biofilm thickness through alginate-related extracellular matrix but is attenuated by N-acetyl-l-cysteine. Front. Cell. Infect. Microbiol. 2020, 10, 594336. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.C.; Seman, B.G.; Hammond, J.H.; Archambault, L.S.; Hogan, D.A.; Wheeler, R.T. Candida albicans and Pseudomonas aeruginosa interact to enhance virulence of mucosal infection in transparent zebrafish. Infect. Immun. 2017, 85, e00475-17. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Shoseyov, D.; Polacheck, I.; Korem, M.; Kerem, E.; Cohen-Cymberknoh, M. Association of chronic Candida albicans respiratory infection with a more severe lung disease in patients with cystic fibrosis. Pediatr. Pulmonol. 2015, 50, 1082–1089. [Google Scholar] [CrossRef]
- Leclair, L.W.; Hogan, D.A. Mixed bacterial-fungal infections in the CF respiratory tract. Med. Mycol. 2010, 48 (Suppl. S1), S125–S132. [Google Scholar] [CrossRef][Green Version]
- Garau, J.; Gomez, L. Pseudomonas aeruginosa pneumonia. Curr. Opin. Infect. Dis. 2003, 16, 135–143. [Google Scholar] [CrossRef]
- Roux, D.; Gaudry, S.; Khoy-Ear, L.; Aloulou, M.; Phillips-Houlbracq, M.; Bex, J.; Skurnik, D.; Denamur, E.; Monteiro, R.C.; Dreyfuss, D.; et al. Airway fungal colonization compromises the immune system allowing bacterial pneumonia to prevail. Crit. Care Med. 2013, 41, e191–e199. [Google Scholar] [CrossRef]
- Dhamgaye, S.; Qu, Y.; Peleg, A.Y. Polymicrobial infections involving clinically relevant Gram-negative bacteria and fungi. Cell. Microbiol. 2016, 18, 1716–1722. [Google Scholar] [CrossRef]
- Kong, E.F.; Kucharikova, S.; Van Dijck, P.; Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Clinical implications of oral candidiasis: Host tissue damage and disseminated bacterial disease. Infect. Immun. 2015, 83, 604–613. [Google Scholar] [CrossRef]
- Kruger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions in health and disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflug. Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Alonso, B.; Fernandez-Barat, L.; Di Domenico, E.G.; Marin, M.; Cercenado, E.; Merino, I.; de Pablos, M.; Munoz, P.; Guembe, M. Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect. Dis. 2020, 20, 909. [Google Scholar] [CrossRef]
- Pendleton, K.M.; Huffnagle, G.B.; Dickson, R.P. The significance of Candida in the human respiratory tract: Our evolving understanding. Pathog. Dis. 2017, 75, ftx029. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yang, Z.; Wang, J.; Liu, X.; Cao, Y.; Pan, Y.; Han, L.; Zhan, S. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Wozniak, D.J. Psl Produced by mucoid Pseudomonas aeruginosa contributes to the establishment of biofilms and immune evasion. mBio 2017, 8, e00864-17. [Google Scholar] [CrossRef] [PubMed]
- Stoner, S.N.; Baty, J.J.; Scoffield, J.A. Pseudomonas aeruginosa polysaccharide Psl supports airway microbial community development. ISME J. 2022, 16, 1730–1739. [Google Scholar] [CrossRef]
- Vaschetto, R.; Kuiper, J.W.; Chiang, S.R.; Haitsma, J.J.; Juco, J.W.; Uhlig, S.; Plotz, F.B.; Della Corte, F.; Zhang, H.; Slutsky, A.S. Inhibition of poly(adenosine diphosphate-ribose) polymerase attenuates ventilator-induced lung injury. Anesthesiology 2008, 108, 261–268. [Google Scholar] [CrossRef]
- Overhage, J.; Schemionek, M.; Webb, J.S.; Rehm, B.H. Expression of the psl operon in Pseudomonas aeruginosa PAO1 biofilms: PslA performs an essential function in biofilm formation. Appl. Environ. Microbiol. 2005, 71, 4407–4413. [Google Scholar] [CrossRef]
- Grainha, T.; Jorge, P.; Alves, D.; Lopes, S.P.; Pereira, M.O. Unraveling Pseudomonas aeruginosa and Candida albicans communication in coinfection scenarios: Insights through network analysis. Front. Cell. Infect. Microbiol. 2020, 10, 550505. [Google Scholar] [CrossRef]
- Byrd, M.S.; Pang, B.; Mishra, M.; Swords, W.E.; Wozniak, D.J. The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-κB activation in A549 cells. mBio 2010, 1, e00140-10. [Google Scholar] [CrossRef] [PubMed]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The role of interleukin-8 in lung inflammation and injury: Implications for the management of COVID-19 and hyperinflammatory acute respiratory distress syndrome. Front. Pharmacol. 2021, 12, 808797. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, Y.; Yu, K.; Zhang, D.; Shi, Y.; Li, Y.; Sun, L.; Qian, F. Akt1 regulates pulmonary fibrosis via modulating IL-13 expression in macrophages. Innate Immun. 2019, 25, 451–461. [Google Scholar] [CrossRef]
- Gasparoto, T.H.; Tessarolli, V.; Garlet, T.P.; Torres, S.A.; Garlet, G.P.; da Silva, J.S.; Campanelli, A.P. Absence of functional TLR4 impairs response of macrophages after Candida albicans infection. Med. Mycol. 2010, 48, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kumar, A.; Guo, H.; Wu, X.; Wheater, M.; Yu, F.S. Topical flagellin-mediated innate defense against Candida albicans keratitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ortiz, Z.G.; Specht, C.A.; Wang, J.P.; Lee, C.K.; Bartholomeu, D.C.; Gazzinelli, R.T.; Levitz, S.M. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect. Immun. 2008, 76, 2123–2129. [Google Scholar] [CrossRef]
- Mear, J.B.; Kipnis, E.; Faure, E.; Dessein, R.; Schurtz, G.; Faure, K.; Guery, B. Candida albicans and Pseudomonas aeruginosa interactions: More than an opportunistic criminal association? Med. Mal. Infect. 2013, 43, 146–151. [Google Scholar] [CrossRef]
- Bisht, K.; Baishya, J.; Wakeman, C.A. Pseudomonas aeruginosa polymicrobial interactions during lung infection. Curr. Opin. Microbiol. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 1973, 116, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Smith, S.E.; Dean, R.T. Alginate inhibition of the uptake of Pseudomonas aeruginosa by macrophages. Microbiology 1988, 134, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.J.; Wyckoff, T.J.; Starkey, M.; Keyser, R.; Azadi, P.; O’Toole, G.A.; Parsek, M.R. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 2003, 100, 7907–7912. [Google Scholar] [CrossRef] [PubMed]
- Stapper, A.P.; Narasimhan, G.; Ohman, D.E.; Barakat, J.; Hentzer, M.; Molin, S.; Kharazmi, A.; Hoiby, N.; Mathee, K. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J. Med. Microbiol. 2004, 53, 679–690. [Google Scholar] [CrossRef]
- Ma, L.; Lu, H.; Sprinkle, A.; Parsek, M.R.; Wozniak, D.J. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. J. Bacteriol. 2007, 189, 8353–8356. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.; Parsek, M.R. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K. Candida Biofilms: Development, architecture, and resistance. Microbiol. Spectr. 2015, 3, MB-0020-2015. [Google Scholar] [CrossRef]
- Padder, S.A.; Prasad, R.; Shah, A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018, 210, 51–58. [Google Scholar] [CrossRef]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef]
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D’Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, M.; Brochiero, E. Repair process impairment by Pseudomonas aeruginosa in epithelial tissues: Major features and potential therapeutic avenues. Front. Cell Infect. Microbiol. 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Saatian, B.; Rezaee, F.; Desando, S.; Emo, J.; Chapman, T.; Knowlden, S.; Georas, S.N. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013, 1, e24333. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Thomas, L.K.; Azghani, A.O. Inhibition of protein kinase C attenuates Pseudomonas aeruginosa elastase-induced epithelial barrier disruption. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Choi, Y.H.; Song, K.S. The PDZ motif peptide of ZO-1 attenuates Pseudomonas aeruginosa LPS-induced airway inflammation. Sci. Rep. 2020, 10, 19644. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Hoiby, N.; Jensen, P.O. Biofilms and host response—Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Nakamoto, K.; Watanabe, M.; Sada, M.; Inui, T.; Nakamura, M.; Honda, K.; Wada, H.; Ishii, H.; Takizawa, H. Pseudomonas aeruginosa-derived flagellin stimulates IL-6 and IL-8 production in human bronchial epithelial cells: A potential mechanism for progression and exacerbation of COPD. Exp. Lung Res. 2019, 45, 255–266. [Google Scholar] [CrossRef]
- Xia, P.; Wu, Y.; Lian, S.; Yan, L.; Meng, X.; Duan, Q.; Zhu, G. Research progress on Toll-like receptor signal transduction and its roles in antimicrobial immune responses. Appl. Microbiol. Biotechnol. 2021, 105, 5341–5355. [Google Scholar] [CrossRef]
- Cobo, F.; Cortes, J.L.; Cabrera, C.; Nieto, A.; Concha, A. Microbiological contamination in stem cell cultures. Cell Biol. Int. 2007, 31, 991–995. [Google Scholar] [CrossRef]
- Roszak, M.; Dotegowska, B.; Cecerska-Heryc, E.; Serwin, N.; Jabtonska, J.; Grygorcewicz, B. Bacteriophage-Ciprofloxacin combination effectiveness depends on Staphylococcus aureus-Candida albicans dual-species communities’ growth model. Microb. Drug Resist. 2022, 28, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ader, F.; Jawhara, S.; Nseir, S.; Kipnis, E.; Faure, K.; Vuotto, F.; Chemani, C.; Sendid, B.; Poulain, D.; Guery, B. Short term Candida albicans colonization reduces Pseudomonas aeruginosa-related lung injury and bacterial burden in a murine model. Crit. Care. 2011, 15, R150. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.K.; Hogan, D.A. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010, 6, e1000886. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Oligonucleotide Sequence (5-3) |
|---|---|
| Anti-sigma factor MucA (mucA) | F-GCGGATGAACTCGAGTTG R-CACTGACGGCGGATTGTT |
| Negative regulator for alginate biosynthesis MucB (mucB) | F-CTCCCTGTTGCTTTTGCTTG R-GATCTCATGGGTGGAGAAGC |
| GDP-mannose-6-dehydrogenase AlgD (algD) | F-AGGGCAACTGGACGGCTATC R-TGTGGTCGGCAATGAAGAAGA |
| Exopolysaccharide Psl (pslA) | F-ATGAACGCTCTGTTCGATTGTCCAC R-TCAAGCACTTGCACAGCAGACCT |
| 16S rRNA | F-ACGCAACTGACGAGTGTGAC R-GATCGCGACACCGAACTAAT |
| Toll-like receptor 4 (TLR4) | F-CACAGACTTGCGGGTTCTAC R-AGGACCGACACACCAATGATG |
| Toll-like receptor 5 (TLR5) | F-ATTGCGTGTACCCTGACTCG R-TTGAACACCAGTCTCTGGGC |
| Toll-like receptor 9 (TLR9) | F-GTGACAGATCCAAGGTGAAGT R-CTTCCTCTACAAATGCATCACT |
| Interleukin-8 (IL-8) | F-TAGCAAAATTGAGGCCAAGG R-GGACTTGTGGATCCTGGCTA |
| Interleukin-13 (IL-13) | F-GCAATGGCAGCATGGTATGG R-AAGGAATTTTACCCCTCCCTAACC |
| Glyceroldehyde-3-phosphate dehydrogenase (GADPH) | F-GTGAAGGTCGGTGTCAACGGATTT R-CACAGTCTTCTGAGTGGCAGTGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuengmaung, P.; Mekjaroen, J.; Saisorn, W.; Chatsuwan, T.; Somparn, P.; Leelahavanichkul, A. Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions. Int. J. Mol. Sci. 2022, 23, 9202. https://doi.org/10.3390/ijms23169202
Phuengmaung P, Mekjaroen J, Saisorn W, Chatsuwan T, Somparn P, Leelahavanichkul A. Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions. International Journal of Molecular Sciences. 2022; 23(16):9202. https://doi.org/10.3390/ijms23169202
Chicago/Turabian StylePhuengmaung, Pornpimol, Jiradej Mekjaroen, Wilasinee Saisorn, Tanittha Chatsuwan, Poorichaya Somparn, and Asada Leelahavanichkul. 2022. "Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions" International Journal of Molecular Sciences 23, no. 16: 9202. https://doi.org/10.3390/ijms23169202
APA StylePhuengmaung, P., Mekjaroen, J., Saisorn, W., Chatsuwan, T., Somparn, P., & Leelahavanichkul, A. (2022). Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions. International Journal of Molecular Sciences, 23(16), 9202. https://doi.org/10.3390/ijms23169202







