Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs)
Abstract
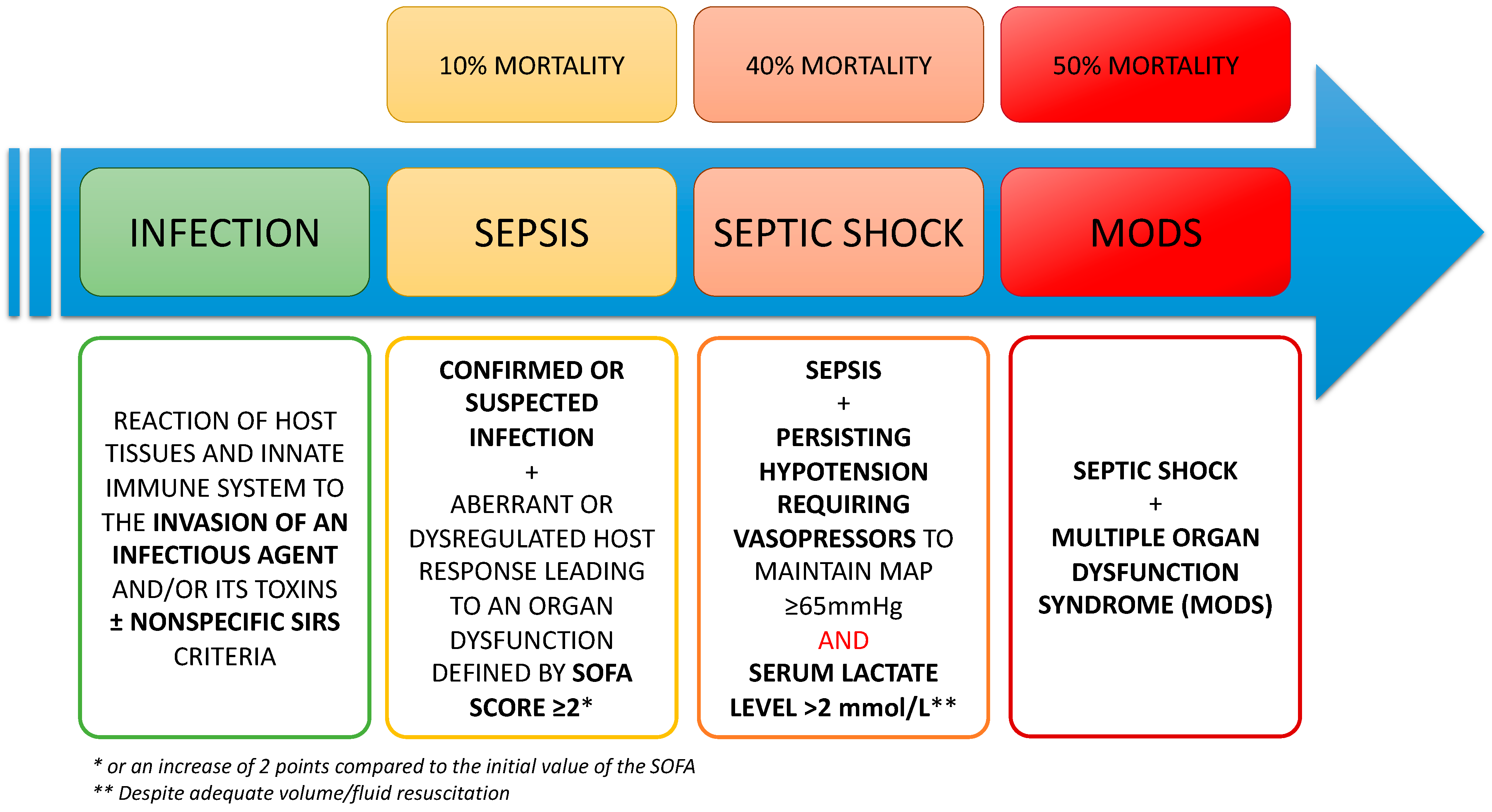
:1. Concept of Sepsis and Genesis of Septic Shock
1.1. Definitions
1.2. Physiopathology and Treatments of the Septic Shock
1.2.1. Cell-Mediated Innate and Adaptative Immune Responses
1.2.2. Peripheral Neuro-Immune Control of the Inflammatory Process in Sepsis
1.2.3. The Balance between Pro-Inflammatory and Anti-Inflammatory Processes
1.2.4. Endothelial Dysfunction and Hemostasis Troubles
1.2.5. Central Nervous System Involvement in Sepsis
1.2.6. Sepsis and Immunoparalysis
1.3. Therapeutics for the Treatment of Septic Shock
2. Origins and Roles of Mesenchymal Stem/Stromal Cells
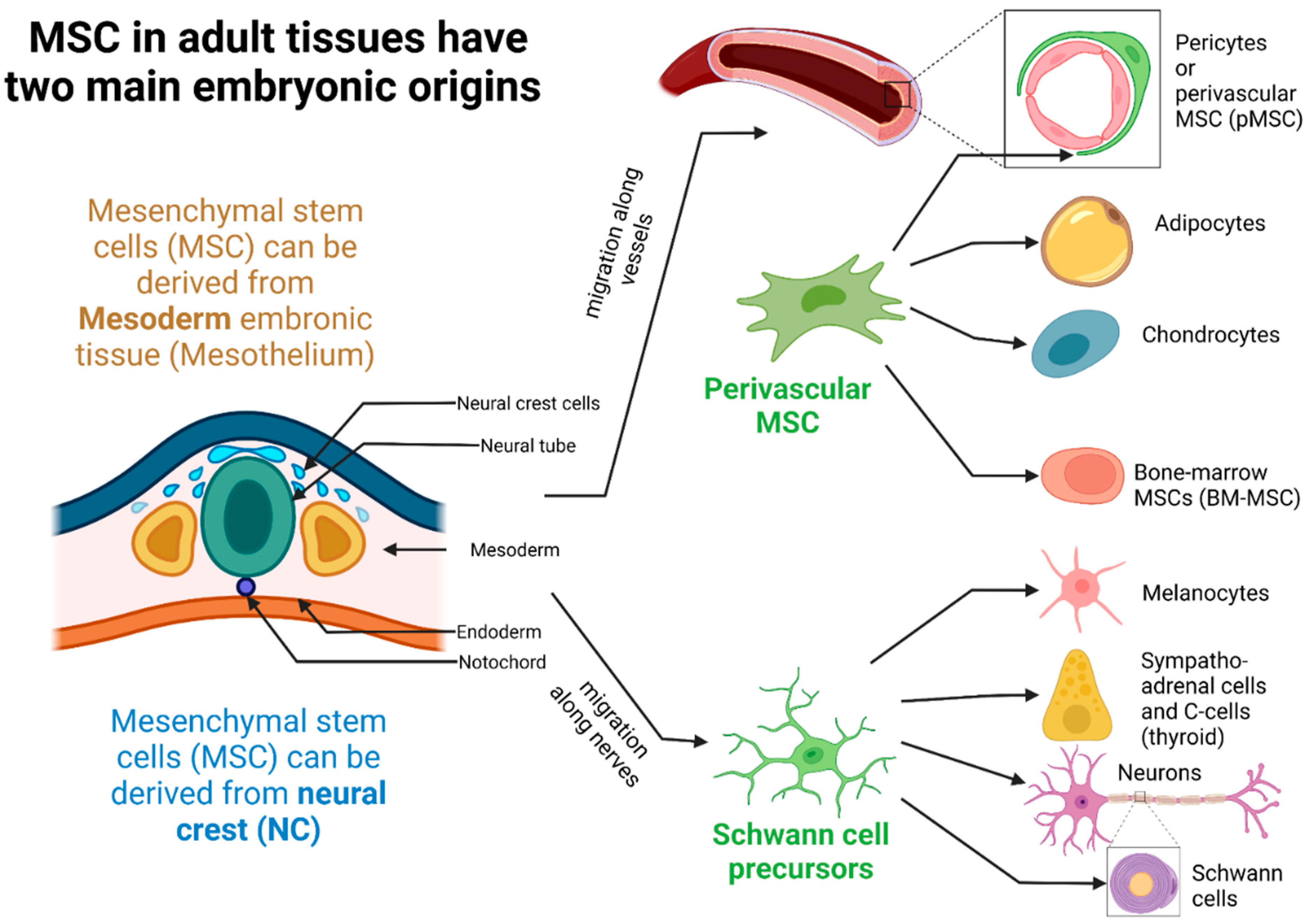
2.1. Ontogeny, Tissue Localization, and Different Populations of MSCs in Tissues
2.2. MSCs Characterization and Surface Markers Expression
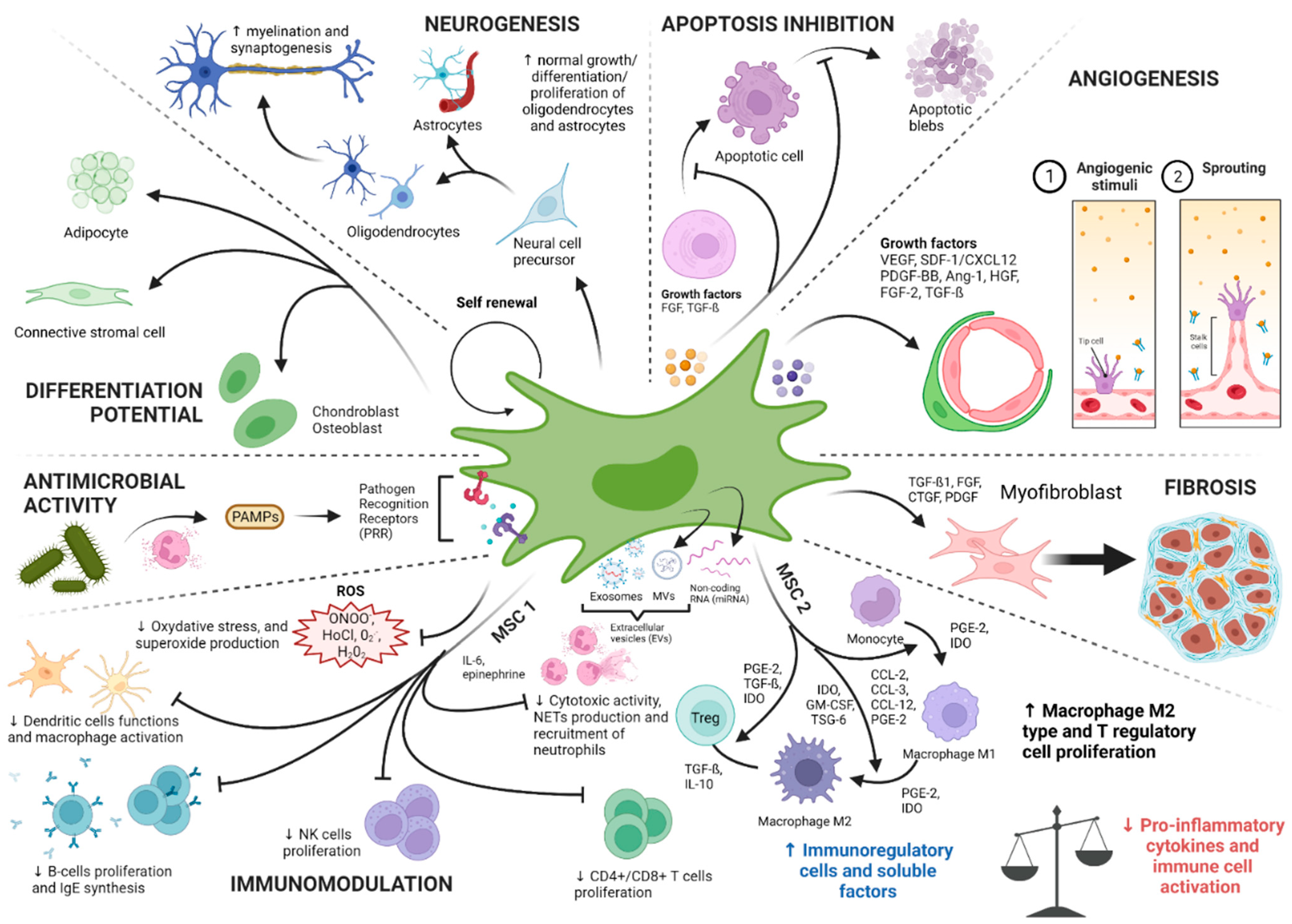
2.3. Physiological Roles of MSCs: A Niche for Stem Cells and Tissue Gatekeepers Alerted in Response to Tissue Injuries
3. Study of the Role of the MSCs in Sepsis/Septic Shock: What Do We Know?
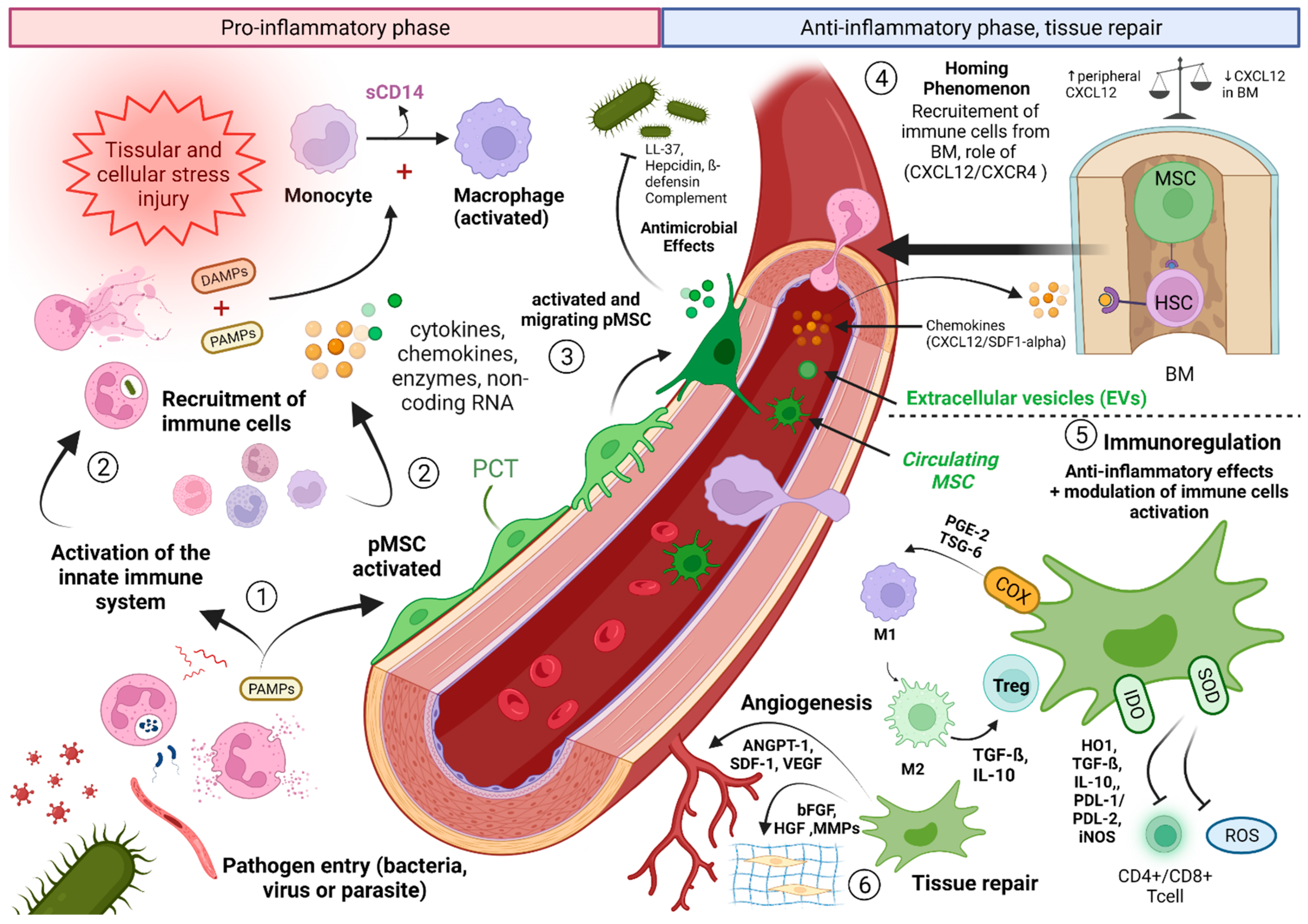
3.1. Contribution of Knowledge of the Role of MSCs in the Comprehension of the Septic Shock: Interactions between MSCs and the Immune System
- -
- IFN-γ, alone or combined with other pro-inflammatory cytokines such as TNF, IL-1α, or IL-1β are able to induce the secretion of chemokines responsible for the activation of iNOS and the attraction of T cells. MSCs are also able to control the proliferation and activation of macrophages, natural killer T (NKT) cells, and γδ T cells [103]. MSCs, after stimulation with the inflammatory cytokines IFN-γ, TNF-α, or IL-1, can express inducible (i) NOS, releasing NO. High NO concentrations can, in turn, inhibit the transcription of (STAT)-5 phosphorylation in T cells and decrease the apoptosis of immune cells, participating in immunomodulation [104].
- -
- Indoleamine 2,3-dioxygenase, also expressed by MSCs stimulated by IFN-γ, inhibits lymphocyte proliferation by depleting tryptophan in the microenvironment. IDO-secreting MSCs are also potent inhibitors of Th1 cells and NK activity with the help of PGE2.
- -
- Role of cyclooxygenase-2 (COX2) and prostaglandins expressed by MSCs: In the context of a severe infection associated with high levels of LPS and/or host-derived factors (e.g., TNF-α) or even in hypoxic conditions, MSCs will engage the stimulation of the NFκB pathway. Activation of NF-κB signaling can upregulate the expression of COX2 and the COX2-dependent increase of PGE2 synthesis. PGE2 in turn will bind to G-protein-coupled receptors EP2 and EP4 on macrophages to increase the expression of the canonical anti-inflammatory cytokine IL-10 chiefly involved in the control of an overt inflammatory response [96,97].
- -
- -
- HLA-G5 is secreted by MSCs stimulated by IL-10 and following contact between MSCs and activated T cells. HLA-G5 has an anti-proliferative action on T cells, NK cells, and cytotoxic T lymphocytes.
3.2. MSCs Interacting with PNN, and the Key Role of the SDF-1/CXCR4/CXCR7 Pathway
3.3. Circulating MSCs and Sepsis: Toward a Novel Entity?
3.4. Diagnostic and Therapeutic Consequences
4. Perspectives for the Study of MSCs in Sepsis Pathophysiology
4.1. Use of MSCs as In Vivo Immunomodulators
4.1.1. Preclinical Data and Control of Inflammatory Processes in Experimental Studies
4.1.2. Infusion of Derived-MSCs and Clinical Studies of Sepsis Models
4.1.3. Cell-Free Based Therapies: MSCs Extracellular Vesicles and Paracrine Factors Incriminated
4.2. Biomarkers of Disease States in Sepsis Including Those Related to Immune Cells and MSC Behaviors
4.3. Interest of the Study of miRNA in Septic States
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT-MSC | Adipose Tissue-Derived Mesenchymal Stem Cell |
| AIS | Adaptive Immune System |
| AKI | Acute Kidney Injury |
| ALI | Acute Lung Injury |
| ANS | Autonomic Nervous System |
| APC | Antigen Presenting Cell |
| ARDS | Acute Respiratory Distress Syndrome |
| BM | Bone Marrow |
| BM-MSC | Bone Marrow-Derived Mesenchymal Stem Cell |
| ChAT | Choline Acetyl Transferase |
| ChAT+ cells | Acetylcholine expressing T cells |
| CFU-Fs | Colony Forming Unit-Fibroblasts |
| CTLR | C-Type Lectin Receptor |
| DAMP | Damage Associated Molecular Pattern |
| DIC | Disseminated Intravascular Coagulation |
| EPC | Endothelial Progenitor Cell |
| EVs | Extracellular Vesicles |
| gMSC | glial Mesenchymal Stem Cell |
| HepSC | Hepatic Stellate Cell |
| HSC | Hematopoietic Stem Cell |
| ICU | Intensive Care Unit |
| IDO | InDoleamine 2,3-Dioxygenase |
| IIS | Innate Immune System |
| iNOS | inducible NO Synthase |
| IR | Immune Response |
| IIR | Innate Immune Response |
| LyB | B Lymphocyte |
| LTCD4+ | Lymphocyte CD4+ T Cell |
| LTCD8+ | Lymphocyte CD8+ T Cell |
| LyT | Lymphocyte T |
| MB-MSC | Menstrual Blood-Derived Mesenchymal Stem Cell |
| MC | Mesodermal Cell |
| mRNA | messenger ARN |
| miRNA | microRNA |
| MODS | Multiple Organ Dysfunction Syndrome |
| MSC | Mesenchymal Stem Cell |
| NC-MSC | Neural Crest-Derived Mesenchymal Stem Cell |
| NC-Pg | Neural Crest-Derived Progenitors |
| NK | Natural Killer Cell |
| NKT | Natural Killer “type T” cell |
| NLR | NOD-Like Receptor |
| NPV | Negative Predictive Value |
| NF-kB | Nuclear Factor kappa B |
| NO | Nitric Oxide |
| PAMP | Pathogen-Associated Molecular Pattern |
| pMSC | perivascular Mesenchymal Stem Cell |
| PRR | Pathogen Recognition Receptor |
| RLR | RIG-1-Like Receptor |
| ROS | Reactive Oxygen Species |
| SLO | Secondary Lymphoid Organs |
| SOFA | Sepsis-related Organ Failure Assessment |
| TH1 | T Helper 1 |
| TH2 | T Helper 2 |
| TLR | Toll Like Receptor |
| Treg cells | regulatory T Cells |
| UC-MSC | Umbilical Cord-Derived Mesenchymal Stem Cell |
References
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; for the Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775. [Google Scholar] [CrossRef] [PubMed]
- Muckart, D.J.; Bhagwanjee, S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Crit. Care Med. 1992, 20, 864–874. [Google Scholar]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States—An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef]
- Kaneki, M. Metabolic Inflammatory Complex in Sepsis: Septic Cachexia as a Novel Potential Therapeutic Target. Shock 2017, 48, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Zarbock, A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit. Rev. Immunol. 2015, 35, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.; Moldawer, L.L. Sepsis and the Dendritic Cell. Shock 2003, 20, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic Activators of Immune Responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-Beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Brown, M.A.; Jones, W.K. NF-KappaB Action in Sepsis: The Innate Immune System and the Heart. Front. Biosci. J. Virtual Libr. 2004, 9, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.; Oberholzer, C.; Moldawer, L.L. Sepsis Syndromes: Understanding the Role of Innate and Acquired Immunity. Shock 2001, 16, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the Adaptive Immune Response in Sepsis. Intensive Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- MacConmara, M.; Lederer, J.A. B Cells. Crit. Care Med. 2005, 33, S514–S516. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front. Immunol. 2017, 8, 1452. [Google Scholar] [CrossRef]
- Huston, J.M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Ashok, M.; Pavlov, V.; Czura, C.J.; Ulloa, L.; Goldstein, R.S.; et al. Vagus Nerve Stimulation Improves Survival in Experimental Sepsis.: 118-T. Crit. Care Med. 2005, 33, A137. [Google Scholar] [CrossRef]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Reardon, C.; Duncan, G.S.; Brüstle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.S.; Olofsson, P.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-Derived ACh Regulates Local Innate but Not Adaptive Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 1410–1415. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Steinberg, B.E.; Sobbi, R.; Cox, M.A.; Ahmed, M.N.; Oswald, M.; Szekeres, F.; Hanes, W.M.; Introini, A.; Liu, S.F.; et al. Blood Pressure Regulation by CD4+ Lymphocytes Expressing Choline Acetyltransferase. Nat. Biotechnol. 2016, 34, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in Patients Who Die of Sepsis and Multiple Organ Failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Venet, F.; Pachot, A.; Lepape, A. Monitoring Immune Dysfunctions in the Septic Patient: A New Skin for the Old Ceremony. Mol. Med. Camb. Mass 2008, 14, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The Sympathetic Nerve—An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Sanders, V.M.; Straub, R.H. Norepinephrine, the Beta-Adrenergic Receptor, and Immunity. Brain. Behav. Immun. 2002, 16, 290–332. [Google Scholar] [CrossRef]
- Nicholls, A.J.; Wen, S.W.; Hall, P.; Hickey, M.J.; Wong, C.H.Y. Activation of the Sympathetic Nervous System Modulates Neutrophil Function. J. Leukoc. Biol. 2018, 103, 295–309. [Google Scholar] [CrossRef]
- Scanzano, A.; Schembri, L.; Rasini, E.; Luini, A.; Dallatorre, J.; Legnaro, M.; Bombelli, R.; Congiu, T.; Cosentino, M.; Marino, F. Adrenergic Modulation of Migration, CD11b and CD18 Expression, ROS and Interleukin-8 Production by Human Polymorphonuclear Leukocytes. Inflamm. Res. 2015, 64, 127–135. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annu. Rev. Immunol. 2011, 29, 139–162. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Maury, E.; Lehoux, S.; Guidet, B.; Offenstadt, G. The Endothelium: Physiological Functions and Role in Microcirculatory Failure during Severe Sepsis. Intensive Care Med. 2010, 36, 1286–1298. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Meshaka, P.; Pinton, P.; Vallet, B.; EPISEPSIS Study Group. EPISEPSIS: A Reappraisal of the Epidemiology and Outcome of Severe Sepsis in French Intensive Care Units. Intensive Care Med. 2004, 30, 580–588. [Google Scholar] [CrossRef]
- Torgersen, C.; Moser, P.; Luckner, G.; Mayr, V.; Jochberger, S.; Hasibeder, W.R.; Dünser, M.W. Macroscopic Postmortem Findings in 235 Surgical Intensive Care Patients with Sepsis. Anesth. Analg. 2009, 108, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.K.; Hayford, K.; Tran, V.; Al Kibria, G.M.; Baqui, A.; Manajjir, A.; Mahmud, A.; Begum, N.; Siddiquee, M.; Kain, K.C.; et al. Biomarkers of Endothelial Dysfunction Predict Sepsis Mortality in Young Infants: A Matched Case-Control Study. BMC Pediatr. 2018, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Connors, J.M.; Nagaoka, I.; Levy, J.H. Recent Advances in the Research and Management of Sepsis-Associated DIC. Int. J. Hematol. 2021, 113, 24–33. [Google Scholar] [CrossRef]
- Coletta, C.; Módis, K.; Oláh, G.; Brunyánszki, A.; Herzig, D.S.; Sherwood, E.R.; Ungvári, Z.; Szabo, C. Endothelial Dysfunction Is a Potential Contributor to Multiple Organ Failure and Mortality in Aged Mice Subjected to Septic Shock: Preclinical Studies in a Murine Model of Cecal Ligation and Puncture. Crit. Care 2014, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Roquer, J.; Segura, T.; Serena, J.; Castillo, J. Endothelial Dysfunction, Vascular Disease and Stroke: The ARTICO Study. Cerebrovasc. Dis. 2009, 27, 25–37. [Google Scholar] [CrossRef]
- Boisramé-Helms, J.; Kremer, H.; Schini-Kerth, V.; Meziani, F. Endothelial Dysfunction in Sepsis. Curr. Vasc. Pharmacol. 2013, 11, 150–160. [Google Scholar]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Berger, M.M.; Grocott, M.P.W. Facing Acute Hypoxia: From the Mountains to Critical Care Medicine. Br. J. Anaesth. 2017, 118, 283–286. [Google Scholar] [CrossRef]
- Kiers, H.D.; Scheffer, G.-J.; van der Hoeven, J.G.; Eltzschig, H.K.; Pickkers, P.; Kox, M. Immunologic Consequences of Hypoxia during Critical Illness. Anesthesiology 2016, 125, 237–249. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and Sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal Regulation of Immunity: Why, How and Where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, X.; Dong, C.; Zou, L.; Zhao, H.; Zhang, X.; Tian, M.; Jin, G. In Vitro Differentiation of Human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs), Derived from Wharton’s Jelly, into Choline Acetyltransferase (ChAT)-Positive Cells. Int. J. Dev. Neurosci. 2012, 30, 471–477. [Google Scholar] [CrossRef]
- Gao, Q.; Hernandes, M.S. Sepsis-Associated Encephalopathy and Blood-Brain Barrier Dysfunction. Inflammation 2021, 44, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Danielski, L.G.; Giustina, A.D.; Badawy, M.; Barichello, T.; Quevedo, J.; Dal-Pizzol, F.; Petronilho, F. Brain Barrier Breakdown as a Cause and Consequence of Neuroinflammation in Sepsis. Mol. Neurobiol. 2018, 55, 1045–1053. [Google Scholar] [CrossRef]
- Walton, A.H.; Muenzer, J.T.; Rasche, D.; Boomer, J.S.; Sato, B.; Brownstein, B.H.; Pachot, A.; Brooks, T.L.; Deych, E.; Shannon, W.D.; et al. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS ONE 2014, 9, e98819. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef]
- Ohkawa, T.; Seki, S.; Dobashi, H.; Koike, Y.; Habu, Y.; Ami, K.; Hiraide, H.; Sekine, I. Systematic Characterization of Human CD8+ T Cells with Natural Killer Cell Markers in Comparison with Natural Killer Cells and Normal CD8+ T Cells. Immunology 2001, 103, 281–290. [Google Scholar] [CrossRef]
- Etogo, A.O.; Nunez, J.; Lin, C.Y.; Toliver-Kinsky, T.E.; Sherwood, E.R. NK but Not CD1-Restricted NKT Cells Facilitate Systemic Inflammation during Polymicrobial Intra-Abdominal Sepsis. J. Immunol. 2008, 180, 6334–6345. [Google Scholar] [CrossRef]
- Condotta, S.A.; Rai, D.; James, B.R.; Griffith, T.S.; Badovinac, V.P. Sustained and Incomplete Recovery of Naive CD8+ T Cell Precursors after Sepsis Contributes to Impaired CD8+ T Cell Responses to Infection. J. Immunol. 2013, 190, 1991–2000. [Google Scholar] [CrossRef]
- Chen, C.-W.; Mittal, R.; Klingensmith, N.J.; Burd, E.M.; Terhorst, C.; Martin, G.S.; Coopersmith, C.M.; Ford, M.L. Cutting Edge: 2B4-Mediated Coinhibition of CD4+ T Cells Underlies Mortality in Experimental Sepsis. J. Immunol. 2017, 199, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Spec, A.; Shindo, Y.; Burnham, C.-A.D.; Wilson, S.; Ablordeppey, E.A.; Beiter, E.R.; Chang, K.; Drewry, A.M.; Hotchkiss, R.S. T Cells from Patients with Candida Sepsis Display a Suppressive Immunophenotype. Crit. Care 2016, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Laterre, P.F.; Garbino, J.; Pingleton, S.; Butler, T.; Dugernier, T.; Margolis, B.; Kudsk, K.; Zimmerli, W.; Anderson, P.; et al. Lenercept (P55 Tumor Necrosis Factor Receptor Fusion Protein) in Severe Sepsis and Early Septic Shock: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase III Trial with 1342 Patients. Crit. Care Med. 2001, 29, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Vincent, J.L.; Laterre, P.F.; LaRosa, S.P.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-Based Immunoglobulin G Therapy of Patients with Sepsis: The SBITS Study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of TAK-242 for the Treatment of Severe Sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- Opal, S.M.; Fisher, C.J.; Dhainaut, J.F.; Vincent, J.L.; Brase, R.; Lowry, S.F.; Sadoff, J.C.; Slotman, G.J.; Levy, H.; Balk, R.A.; et al. Confirmatory Interleukin-1 Receptor Antagonist Trial in Severe Sepsis: A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 1997, 25, 1115–1124. [Google Scholar] [CrossRef]
- Guntupalli, K.; Dean, N.; Morris, P.E.; Bandi, V.; Margolis, B.; Rivers, E.; Levy, M.; Lodato, R.F.; Ismail, P.M.; Reese, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Talactoferrin in Patients with Severe Sepsis. Crit. Care Med. 2013, 41, 706–716. [Google Scholar] [CrossRef]
- Angus, D.C.; Barnato, A.E.; Bell, D.; Bellomo, R.; Chong, C.-R.; Coats, T.J.; Davies, A.; Delaney, A.; Harrison, D.A.; Holdgate, A.; et al. A Systematic Review and Meta-Analysis of Early Goal-Directed Therapy for Septic Shock: The ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015, 41, 1549–1560. [Google Scholar] [CrossRef]
- Gupta, R.G.; Hartigan, S.M.; Kashiouris, M.G.; Sessler, C.N.; Bearman, G.M.L. Early Goal-Directed Resuscitation of Patients with Septic Shock: Current Evidence and Future Directions. Crit. Care 2015, 19, 286. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and Mortality of Septic Shock in Europe and North America: A Systematic Review and Meta-Analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone Marrow Osteogenic Stem Cells: In Vitro Cultivation and Transplantation in Diffusion Chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. New MSC: MSCs as Pericytes Are Sentinels and Gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In Search of the in Vivo Identity of Mesenchymal Stem Cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Creuzet, S.; Couly, G.; Dupin, E. Neural Crest Cell Plasticity and Its Limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Adameyko, I. Schwann Cell Precursor: A Neural Crest Cell in Disguise? Dev. Biol. 2018, 444, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Dupin, E.; Sommer, L. Neural Crest Progenitors and Stem Cells: From Early Development to Adulthood. Dev. Biol. 2012, 366, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Hirschi, K.K.; D’Amore, P.A. Pericytes in the Microvasculature. Cardiovasc. Res. 1996, 32, 687–698. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Bedoui, Y.; Lebeau, G.; Guillot, X.; Dargai, F.; Guiraud, P.; Neal, J.W.; Ralandison, S.; Gasque, P. Emerging Roles of Perivascular Mesenchymal Stem Cells in Synovial Joint Inflammation. J. Neuroimmune Pharmacol. 2020, 15, 838–851. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Götze, S.; Herebian, D.; Häussinger, D. Hepatic Stellate Cells Contribute to Progenitor Cells and Liver Regeneration. J. Clin. Investig. 2014, 124, 5503–5515. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent Mesenchymal Stromal Cells and the Innate Immune System. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.D.; Bonnet, D.; Janes, S.M. Stem Cells of the Alveolar Epithelium. Lancet 2005, 366, 249–260. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, J.-Z.; Xiang, L.-X.; Dong, X.-J.; Zhang, G.-R. Mesenchymal Stem Cells: A Promising Candidate in Regenerative Medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat Tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Wolbank, S.; van Griensven, M.; Grillari-Voglauer, R.; Peterbauer-Scherb, A. Alternative Sources of Adult Stem Cells: Human Amniotic Membrane. Adv. Biochem. Eng. Biotechnol. 2010, 123, 1–27. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, Cultivation, and Characterization of Human Mesenchymal Stem Cells. Cytom. Part J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical Parameters for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Caplan, A. Mesenchymal Stem-Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Isern, J.; García-García, A.; Martín, A.M.; Arranz, L.; Martín-Pérez, D.; Torroja, C.; Sánchez-Cabo, F.; Méndez-Ferrer, S. The Neural Crest Is a Source of Mesenchymal Stem Cells with Specialized Hematopoietic Stem Cell Niche Function. eLife 2014, 3, e03696. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How Mesenchymal Stem Cells Interact with Tissue Immune Responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kortesidis, A.; Zannettino, A.; Isenmann, S.; Shi, S.T.; Lapidot, T.; Gronthos, S. Stromal-Derived Factor-1 Promotes the Growth, Survival, and Development of Human Bone Marrow Stromal Stem Cells. Blood 2005, 105, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal Stem Cells for Treatment of Steroid-Resistant, Severe, Acute Graft-versus-Host Disease: A Phase II Study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef]
- Gasque, P.; Jaffar-Bandjee, M.C. The Immunology and Inflammatory Responses of Human Melanocytes in Infectious Diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef]
- Saint-Pastou Terrier, C.; Gasque, P. Bone Responses in Health and Infectious Diseases: A Focus on Osteoblasts. J. Infect. 2017, 75, 281–292. [Google Scholar] [CrossRef]
- Neal, J.W.; Gasque, P. The Role of Primary Infection of Schwann Cells in the Aetiology of Infective Inflammatory Neuropathies. J. Infect. 2016, 73, 402–418. [Google Scholar] [CrossRef]
- Vogel, S.; Börger, V.; Peters, C.; Förster, M.; Liebfried, P.; Metzger, K.; Meisel, R.; Däubener, W.; Trapp, T.; Fischer, J.C.; et al. Necrotic Cell-Derived High Mobility Group Box 1 Attracts Antigen-Presenting Cells but Inhibits Hepatocyte Growth Factor-Mediated Tropism of Mesenchymal Stem Cells for Apoptotic Cell Death. Cell Death Differ. 2015, 22, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal Stem Cells Ameliorate Experimental Autoimmune Encephalomyelitis Inducing T-Cell Anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Prigione, I.; Benvenuto, F.; Bocca, P.; Battistini, L.; Uccelli, A.; Pistoia, V. Reciprocal Interactions between Human Mesenchymal Stem Cells and γδ T Cells Or Invariant Natural Killer T Cells. Stem Cells 2009, 27, 693–702. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric Oxide Plays a Critical Role in Suppression of T-Cell Proliferation by Mesenchymal Stem Cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bouffi, C.; Bony, C.; Courties, G.; Jorgensen, C.; Noël, D. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS ONE 2010, 5, e14247. [Google Scholar] [CrossRef]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.M.; Silberstein, L.E. Human Bone Marrow Stromal Cells Express a Distinct Set of Biologically Functional Chemokine Receptors. Stem Cells 2006, 24, 1030–1041. [Google Scholar] [CrossRef]
- Yang, J.; Zou, M.; Pezoldt, J.; Zhou, X.; Huehn, J. Thymus-Derived Foxp3+ Regulatory T Cells Upregulate RORγt Expression under Inflammatory Conditions. J. Mol. Med. 2018, 96, 1387–1394. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+ CD25+ CD4+ Natural Regulatory T Cells in Dominant Self-Tolerance and Autoimmune Disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Kim, S.-J.; Lee, S.-M. Overexpression of HO-1 Contributes to Sepsis-Induced Immunosuppression by Modulating the Th1/Th2 Balance and Regulatory T-Cell Function. J. Infect. Dis. 2017, 215, 1608–1618. [Google Scholar] [CrossRef]
- Jeremias, I.C.; Victorino, V.J.; Barbeiro, H.V.; Kubo, S.A.; Prado, C.M.; Lima, T.M.; Soriano, F.G. The Role of Acetylcholine in the Inflammatory Response in Animals Surviving Sepsis Induced by Cecal Ligation and Puncture. Mol. Neurobiol. 2016, 53, 6635–6643. [Google Scholar] [CrossRef]
- Carvelli, J.; Piperoglou, C.; Bourenne, J.; Farnarier, C.; Banzet, N.; Demerlé, C.; Gainnier, M.; Vély, F. Imbalance of Circulating Innate Lymphoid Cell Subpopulations in Patients with Septic Shock. Front. Immunol. 2019, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal Stem Cell Effects on T-Cell Effector Pathways. Stem Cell Res. Ther. 2011, 2, 34. [Google Scholar] [CrossRef]
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.; Pham, S.; Li, S.; Vazquez-Padron, R.I.; Mathew, J.; Ruiz, P.; Salgar, S.K. Interleukin-10 Delivery via Mesenchymal Stem Cells: A Novel Gene Therapy Approach to Prevent Lung Ischemia-Reperfusion Injury. Hum. Gene Ther. 2010, 21, 713–727. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The Dynamic in Vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef] [PubMed]
- de Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC by Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; Rajagopal, J.; Prockop, D. Stem Cells and Cell Therapies in Lung Biology and Diseases: Conference Report. Ann. Am. Thorac. Soc. 2013, 10, S25–S44. [Google Scholar] [CrossRef]
- Matthay, M.A.; Anversa, P.; Bhattacharya, J.; Burnett, B.K.; Chapman, H.A.; Hare, J.M.; Hei, D.J.; Hoffman, A.M.; Kourembanas, S.; McKenna, D.H.; et al. Cell Therapy for Lung Diseases. Report from an NIH-NHLBI Workshop, November 13–14, 2012. Am. J. Respir. Crit. Care Med. 2013, 188, 370–375. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-Resolution Proteomic and Lipidomic Analysis of Exosomes and Microvesicles from Different Cell Sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen Enrichment in Exosomes Secreted by a Nematode Parasite versus Those Derived from Its Mouse Host: Implications for Exosome Stability and Biology. J. Extracell. Vesicles 2016, 5, 30741. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Yang, K.; Liang, Z.; Wan, Y.; Cheng, Y.; Ma, D.; Zhang, H.; Hou, W.; Fu, P. Sphingosine-1-Phosphate Mediates the Therapeutic Effects of Bone Marrow Mesenchymal Stem Cell-Derived Microvesicles on Articular Cartilage Defect. Transl. Res. J. Lab. Clin. Med. 2018, 193, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Zhu, X.-J.; Zou, P. Research progress of mesenchymal stem cell-derived microvesicle. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 227–230. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, R.; Qiu, G.; Ge, M.; Wang, J.; Shu, Q.; Xu, J. Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Regenerative and Immunomodulatory Effects and Potential Applications in Sepsis. Cell Tissue Res. 2018, 374, 1–15. [Google Scholar] [CrossRef]
- Fotuhi, S.N.; Khalaj-Kondori, M.; Hoseinpour Feizi, M.A.; Talebi, M. Long Non-Coding RNA BACE1-AS May Serve as an Alzheimer’s Disease Blood-Based Biomarker. J. Mol. Neurosci. 2019, 69, 351–359. [Google Scholar] [CrossRef]
- Pan, C.; Stevic, I.; Müller, V.; Ni, Q.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Exosomal MicroRNAs as Tumor Markers in Epithelial Ovarian Cancer. Mol. Oncol. 2018, 12, 1935–1948. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Z.; Yu, A.M.; Wang, W.; Wei, Z.; Akhter, E.; Maurer, K.; Costa Reis, P.; Song, L.; Petri, M.; et al. The SLE Transcriptome Exhibits Evidence of Chronic Endotoxin Exposure and Has Widespread Dysregulation of Non-Coding and Coding RNAs. PLoS ONE 2014, 9, e93846. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional Proteins of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.-A.; Mardani, K. Microvesicles Derived from Mesenchymal Stem Cells: Potent Organelles for Induction of Tolerogenic Signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Gong, Y.-Z.; Wu, P.; Liao, D.-F.; Zheng, X.-L. Mesenchymal Stem Cell-Derived Microparticles: A Promising Therapeutic Strategy. Int. J. Mol. Sci. 2014, 15, 14348–14363. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.S.; Apcher, G.S.; Comber, J.D.; Eisenlohr, L.C. Exosome-Driven Antigen Transfer for MHC Class II Presentation Facilitated by the Receptor Binding Activity of Influenza Hemagglutinin. J. Immunol. 2010, 185, 6608–6616. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic Cell-Derived Exosomes as Maintenance Immunotherapy after First Line Chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434–445. [Google Scholar] [CrossRef]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and Transferable Modulatory Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles on T, B and NK Cell Functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef]
- Liu, J.; Kuwabara, A.; Kamio, Y.; Hu, S.; Park, J.; Hashimoto, T.; Lee, J.-W. Human Mesenchymal Stem Cell-Derived Microvesicles Prevent the Rupture of Intracranial Aneurysm in Part by Suppression of Mast Cell Activation via a PGE2-Dependent Mechanism. Stem Cells 2016, 34, 2943–2955. [Google Scholar] [CrossRef]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human Mesenchymal Stem Cells and Derived Extracellular Vesicles Induce Regulatory Dendritic Cells in Type 1 Diabetic Patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes Derived from Mesenchymal Stem Cells Attenuate the Progression of Atherosclerosis in ApoE-/- Mice via MiR-Let7 Mediated Infiltration and Polarization of M2 Macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.H.; Lim, S.K. Mesenchymal Stem Cells Secrete Immunologically Active Exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, W.; Lim, T.H.; Cho, Y.; Choi, K.-S.; Jang, B.-H. The Delta Neutrophil Index (DNI) as a Prognostic Marker for Mortality in Adults with Sepsis: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 6621. [Google Scholar] [CrossRef]
- Shen, X.-F.; Cao, K.; Jiang, J.-P.; Guan, W.-X.; Du, J.-F. Neutrophil Dysregulation during Sepsis: An Overview and Update. J. Cell. Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef]
- Liu, L.; Sun, B. Neutrophil Pyroptosis: New Perspectives on Sepsis. Cell. Mol. Life Sci. CMLS 2019, 76, 2031–2042. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Demaret, J.; Venet, F.; Friggeri, A.; Cazalis, M.-A.; Plassais, J.; Jallades, L.; Malcus, C.; Poitevin-Later, F.; Textoris, J.; Lepape, A.; et al. Marked Alterations of Neutrophil Functions during Sepsis-Induced Immunosuppression. J. Leukoc. Biol. 2015, 98, 1081–1090. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency Granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Reca, R.; Wojakowski, W.; Ratajczak, J. The Pleiotropic Effects of the SDF-1-CXCR4 Axis in Organogenesis, Regeneration and Tumorigenesis. Leukemia 2006, 20, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; von Ungern-Sternberg, S.N.I.; Seizer, P.; Schlegel, F.; Büttcher, M.; Sindhu, N.A.; Müller, S.; Mack, A.; Gawaz, M. Platelet-Derived CXCL12 Regulates Monocyte Function, Survival, Differentiation into Macrophages and Foam Cells through Differential Involvement of CXCR4-CXCR7. Cell Death Dis. 2015, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Suratt, B.T.; Petty, J.M.; Young, S.K.; Malcolm, K.C.; Lieber, J.G.; Nick, J.A.; Gonzalo, J.-A.; Henson, P.M.; Worthen, G.S. Role of the CXCR4/SDF-1 Chemokine Axis in Circulating Neutrophil Homeostasis. Blood 2004, 104, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 Antagonistically Regulate Neutrophil Trafficking from Murine Bone Marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Wang, Y.; Chew, W.K.; Lima, R.; A-González, N.; Mattar, C.N.Z.; Chong, S.Z.; Schlitzer, A.; Bakocevic, N.; Chew, S.; et al. Neutrophil Mobilization via Plerixafor-Mediated CXCR4 Inhibition Arises from Lung Demargination and Blockade of Neutrophil Homing to the Bone Marrow. J. Exp. Med. 2013, 210, 2321–2336. [Google Scholar] [CrossRef]
- Strydom, N.; Rankin, S.M. Regulation of Circulating Neutrophil Numbers under Homeostasis and in Disease. J. Innate Immun. 2013, 5, 304–314. [Google Scholar] [CrossRef]
- Delano, M.J.; Kelly-Scumpia, K.M.; Thayer, T.C.; Winfield, R.D.; Scumpia, P.O.; Cuenca, A.G.; Harrington, P.B.; O’Malley, K.A.; Warner, E.; Gabrilovich, S.; et al. Neutrophil Mobilization from the Bone Marrow during Polymicrobial Sepsis Is Dependent on CXCL12 Signaling. J. Immunol. 2011, 187, 911–918. [Google Scholar] [CrossRef]
- Ngamsri, K.-C.; Jans, C.; Putri, R.A.; Schindler, K.; Gamper-Tsigaras, J.; Eggstein, C.; Köhler, D.; Konrad, F.M. Inhibition of CXCR4 and CXCR7 Is Protective in Acute Peritoneal Inflammation. Front. Immunol. 2020, 11, 407. [Google Scholar] [CrossRef]
- Ngamsri, K.-C.; Müller, A.; Bösmüller, H.; Gamper-Tsigaras, J.; Reutershan, J.; Konrad, F.M. The Pivotal Role of CXCR7 in Stabilization of the Pulmonary Epithelial Barrier in Acute Pulmonary Inflammation. J. Immunol. 2017, 198, 2403–2413. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, H.; Yuan, F.; Shen, M. Lipopolysaccharide Promotes Choroidal Neovascularization by Up-Regulation of CXCR4 and CXCR7 Expression in Choroid Endothelial Cell. PLoS ONE 2015, 10, e0136175. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.S.; El-Gendy, F.M.; Helwa, M.A. Serum Stromal-Derived-Factor-1 (CXCL12) and Its Alpha Chemokine Receptor (CXCR4) as Biomarkers in Neonatal Sepsis. J. Matern.-Fetal Neonatal Med. 2018, 31, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Guo, C.; Zingarelli, B.; Wang, L.; Halushka, P.V.; Cook, J.A.; Fan, H. Combined Treatment with a CXCL12 Analogue and Antibiotics Improves Survival and Neutrophil Recruitment and Function in Murine Sepsis. Immunology 2014, 144, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ramonell, K.M.; Zhang, W.; Hadley, A.; Chen, C.-W.; Fay, K.T.; Lyons, J.D.; Klingensmith, N.J.; McConnell, K.W.; Coopersmith, C.M.; Ford, M.L. CXCR4 Blockade Decreases CD4+ T Cell Exhaustion and Improves Survival in a Murine Model of Polymicrobial Sepsis. PLoS ONE 2017, 12, e0188882. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.C.; Makena, P.S.; Gorantla, V.; Sinclair, S.E.; Waters, C.M. CXCR4 Regulates Migration of Lung Alveolar Epithelial Cells through Activation of Rac1 and Matrix Metalloproteinase-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L846–L856. [Google Scholar] [CrossRef]
- Kwon, M.-Y.; Ghanta, S.; Ng, J.; Tsoyi, K.; Lederer, J.A.; Bronson, R.T.; El-Chemaly, S.; Chung, S.W.; Liu, X.; Perrella, M.A. Expression of Stromal Cell-Derived Factor-1 by Mesenchymal Stromal Cells Impacts Neutrophil Function During Sepsis. Crit. Care Med. 2020, 48, e409–e417. [Google Scholar] [CrossRef]
- Drifte, G.; Dunn-Siegrist, I.; Tissières, P.; Pugin, J. Innate Immune Functions of Immature Neutrophils in Patients with Sepsis and Severe Systemic Inflammatory Response Syndrome. Crit. Care Med. 2013, 41, 820–832. [Google Scholar] [CrossRef]
- Yano, T.; Torisawa, T.; Oiwa, K.; Tsukita, S. AMPK-Dependent Phosphorylation of Cingulin Reversibly Regulates Its Binding to Actin Filaments and Microtubules. Sci. Rep. 2018, 8, 15550. [Google Scholar] [CrossRef]
- He, Q.; Wan, C.; Li, G. Concise Review: Multipotent Mesenchymal Stromal Cells in Blood. Stem Cells 2007, 25, 69–77. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P.G. Circulating Skeletal Stem Cells. J. Cell Biol. 2001, 153, 1133–1140. [Google Scholar] [CrossRef]
- Tondreau, T.; Meuleman, N.; Delforge, A.; Dejeneffe, M.; Leroy, R.; Massy, M.; Mortier, C.; Bron, D.; Lagneaux, L. Mesenchymal Stem Cells Derived from CD133-Positive Cells in Mobilized Peripheral Blood and Cord Blood: Proliferation, Oct4 Expression, and Plasticity. Stem Cells 2005, 23, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, G.Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, P.; Charbord, P.; Eder, V.; Domenech, J. Multipotential Mesenchymal Stem Cells Are Mobilized into Peripheral Blood by Hypoxia. Stem Cells 2006, 24, 2202–2208. [Google Scholar] [CrossRef]
- Wang, C.-H.; Cherng, W.-J.; Yang, N.-I.; Kuo, L.-T.; Hsu, C.-M.; Yeh, H.-I.; Lan, Y.-J.; Yeh, C.-H.; Stanford, W.L. Late-Outgrowth Endothelial Cells Attenuate Intimal Hyperplasia Contributed by Mesenchymal Stem Cells after Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Speyer, C.L.; Steffes, C.P.; Tyburski, J.G.; Ram, J.L. Lipopolysaccharide Induces Relaxation in Lung Pericytes by an INOS-Independent Mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L880–L887. [Google Scholar] [CrossRef] [PubMed]
- Orange, D.E.; Yao, V.; Sawicka, K.; Fak, J.; Frank, M.O.; Parveen, S.; Blachere, N.E.; Hale, C.; Zhang, F.; Raychaudhuri, S.; et al. RNA Identification of PRIME Cells Predicting Rheumatoid Arthritis Flares. N. Engl. J. Med. 2020, 383, 218–228. [Google Scholar] [CrossRef]
- Klopp, A.H.; Spaeth, E.L.; Dembinski, J.L.; Woodward, W.A.; Munshi, A.; Meyn, R.E.; Cox, J.D.; Andreeff, M.; Marini, F.C. Tumor Irradiation Increases the Recruitment of Circulating Mesenchymal Stem Cells into the Tumor Microenvironment. Cancer Res. 2007, 67, 11687–11695. [Google Scholar] [CrossRef]
- Patry, C.; Doniga, T.; Lenz, F.; Viergutz, T.; Weiss, C.; Toenshoff, B.; Kalenka, A.; Yard, B.; Krebs, J.; Schaible, T.; et al. Increased Mobilization of Mesenchymal Stem Cells in Patients with Acute Respiratory Distress Syndrome Undergoing Extracorporeal Membrane Oxygenation. PLoS ONE 2020, 15, e0227460. [Google Scholar] [CrossRef]
- Tse, W.T.; Pendleton, J.D.; Beyer, W.M.; Egalka, M.C.; Guinan, E.C. Suppression of Allogeneic T-Cell Proliferation by Human Marrow Stromal Cells: Implications in Transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal Stem Cells Suppress Lymphocyte Proliferation In Vitro and Prolong Skin Graft Survival In Vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic Marrow Stromal Cells Are Immune Rejected by MHC Class I- and Class II-Mismatched Recipient Mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-Derived Mesenchymal Stem Cells Are Immunogenic in an Allogeneic Host and Stimulate Donor Graft Rejection in a Nonmyeloablative Setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Laroye, C.; Gibot, S.; Reppel, L.; Bensoussan, D. Concise Review: Mesenchymal Stromal/Stem Cells: A New Treatment for Sepsis and Septic Shock? Stem Cells 2017, 35, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered Mesenchymal Stem Cells Protect against Ischemic Acute Renal Failure through Differentiation-Independent Mechanisms. Am. J. Physiol. Renal Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 Receptor Antagonist Mediates the Antiinflammatory and Antifibrotic Effect of Mesenchymal Stem Cells during Lung Injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef]
- Parekkadan, B.; van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal Stem Cell-Derived Molecules Reverse Fulminant Hepatic Failure. PLoS ONE 2007, 2, e941. [Google Scholar] [CrossRef]
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R.; et al. Mesenchymal Stem Cells Effectively Modulate Pathogenic Immune Response in Experimental Autoimmune Encephalomyelitis. Ann. Neurol. 2007, 61, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Laroye, C.; Lemarié, J.; Boufenzer, A.; Labroca, P.; Cunat, L.; Alauzet, C.; Groubatch, F.; Cailac, C.; Jolly, L.; Bensoussan, D.; et al. Clinical-Grade Mesenchymal Stem Cells Derived from Umbilical Cord Improve Septic Shock in Pigs. Intensive Care Med. Exp. 2018, 6, 24. [Google Scholar] [CrossRef]
- Laroye, C.; Boufenzer, A.; Jolly, L.; Cunat, L.; Alauzet, C.; Merlin, J.-L.; Yguel, C.; Bensoussan, D.; Reppel, L.; Gibot, S. Bone Marrow vs Wharton’s Jelly Mesenchymal Stem Cells in Experimental Sepsis: A Comparative Study. Stem Cell Res. Ther. 2019, 10, 192. [Google Scholar] [CrossRef]
- Horak, J.; Nalos, L.; Martinkova, V.; Tegl, V.; Vistejnova, L.; Kuncova, J.; Kohoutova, M.; Jarkovska, D.; Dolejsova, M.; Benes, J.; et al. Evaluation of Mesenchymal Stem Cell Therapy for Sepsis: A Randomized Controlled Porcine Study. Front. Immunol. 2020, 11, 126. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Ding, X.-F.; Liang, H.-Y.; Zhang, X.-J.; Liu, S.-H.; Han, B.; Duan, X.-G.; Sun, T.-W. Efficacy of Mesenchymal Stem Cell Therapy for Sepsis: A Meta-Analysis of Preclinical Studies. Stem Cell Res. Ther. 2020, 11, 214. [Google Scholar] [CrossRef]
- Gennadiy, G.; Polina, M.; Elena, P.; Larisa, K.; Vera, T.; Eduard, G.; Valeriy, S. The Results of the Single Center Pilot Randomized Russian Clinical Trial of Mesenchymal Stromal Cells in Severe Neutropenic Patients with Septic Shock (RUMCESS). Int. J. Blood Res. Disord. 2018, 5, 33. [Google Scholar] [CrossRef]
- He, X.; Ai, S.; Guo, W.; Yang, Y.; Wang, Z.; Jiang, D.; Xu, X. Umbilical Cord-Derived Mesenchymal Stem (Stromal) Cells for Treatment of Severe Sepsis: Aphase 1 Clinical Trial. Transl. Res. J. Lab. Clin. Med. 2018, 199, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Perlee, D.; van Vught, L.A.; Scicluna, B.P.; Maag, A.; Lutter, R.; Kemper, E.M.; van’t Veer, C.; Punchard, M.A.; González, J.; Richard, M.P.; et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells 2018, 36, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal Stem (Stromal) Cells for Treatment of ARDS: A Phase 1 Clinical Trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical Remission of a Critically Ill COVID-19 Patient Treated by Human Umbilical Cord Mesenchymal Stem Cells: A Case Report. Medicine 2020, 99, e21429. [Google Scholar] [CrossRef]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of Acute Respiratory Distress Syndrome with Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Randomized, Placebo-Controlled Pilot Study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef]
- Yip, H.-K.; Fang, W.-F.; Li, Y.-C.; Lee, F.-Y.; Lee, C.-H.; Pei, S.-N.; Ma, M.-C.; Chen, K.-H.; Sung, P.-H.; Lee, M.S. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit. Care Med. 2020, 48, e391–e399. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L.; et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering 2020, 6, 1153–1161. [Google Scholar] [CrossRef]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with Allogeneic Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome (START Study): A Randomised Phase 2a Safety Trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Can, A.; Celikkan, F.T.; Cinar, O. Umbilical Cord Mesenchymal Stromal Cell Transplantations: A Systemic Analysis of Clinical Trials. Cytotherapy 2017, 19, 1351–1382. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.A.; Stewart, D.J.; Mei, S.H.J.; Courtman, D.; Watpool, I.; Granton, J.; Marshall, J.; Dos Santos, C.; Walley, K.R.; Winston, B.W.; et al. Cellular Immunotherapy for Septic Shock. A Phase I Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, K.; Wang, J.-P.; Dos Santos, C.; Walley, K.R.; Marshall, J.; Fergusson, D.A.; Winston, B.W.; Granton, J.; Watpool, I.; Stewart, D.J.; et al. Effects of Mesenchymal Stem Cell Treatment on Systemic Cytokine Levels in a Phase 1 Dose Escalation Safety Trial of Septic Shock Patients. Crit. Care Med. 2019, 47, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Laterre, P.-F.; Sánchez-García, M.; van der Poll, T.; de la Rosa, O.; Cadogan, K.-A.; Lombardo, E.; François, B. A Phase Ib/IIa, Randomised, Double-Blind, Multicentre Trial to Assess the Safety and Efficacy of Expanded Cx611 Allogeneic Adipose-Derived Stem Cells (EASCs) for the Treatment of Patients with Community-Acquired Bacterial Pneumonia Admitted to the Intensive Care Unit. BMC Pulm. Med. 2020, 20, 309. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal Stromal Cell-Derived Exosomes Attenuate Myocardial Ischaemia-Reperfusion Injury through MiR-182-Regulated Macrophage Polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Shahir, M.; Mahmoud Hashemi, S.; Asadirad, A.; Varahram, M.; Kazempour-Dizaji, M.; Folkerts, G.; Garssen, J.; Adcock, I.; Mortaz, E. Effect of Mesenchymal Stem Cell-derived Exosomes on the Induction of Mouse Tolerogenic Dendritic Cells. J. Cell. Physiol. 2020, 235, 7043–7055. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-Derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-Derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal. CCS 2016, 14, 2. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Krishnan, A.; Muthusamy, S.; Fernandez, F.B.; Kasoju, N. Mesenchymal Stem Cell-Derived Extracellular Vesicles in the Management of COVID19-Associated Lung Injury: A Review on Publications, Clinical Trials and Patent Landscape. Tissue Eng. Regen. Med. 2022, 19, 659–673. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.-L. Sepsis Biomarkers: A Review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Carrigan, S.D.; Scott, G.; Tabrizian, M. Toward Resolving the Challenges of Sepsis Diagnosis. Clin. Chem. 2004, 50, 1301–1314. [Google Scholar] [CrossRef]
- Póvoa, P. C-Reactive Protein: A Valuable Marker of Sepsis. Intensive Care Med. 2002, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kibe, S.; Adams, K.; Barlow, G. Diagnostic and Prognostic Biomarkers of Sepsis in Critical Care. J. Antimicrob. Chemother. 2011, 66 (Suppl. S2), ii33–ii40. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Huang, T.; Cui, W.; Shen, H. Diagnostic Value of the Soluble Triggering Receptor Expressed on Myeloid Cells-1 in Bacterial Infection: A Meta-Analysis. Intensive Care Med. 2009, 35, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.J.M.L. Neutrophil CD64: A Diagnostic Marker for Infection and Sepsis. Clin. Chem. Lab. Med. 2009, 47, 903–916. [Google Scholar] [CrossRef]
- Yin, W.-P.; Li, J.-B.; Zheng, X.-F.; An, L.; Shao, H.; Li, C.-S. Effect of Neutrophil CD64 for Diagnosing Sepsis in Emergency Department. World J. Emerg. Med. 2020, 11, 79–86. [Google Scholar] [CrossRef]
- Pizzolato, E.; Ulla, M.; Galluzzo, C.; Lucchiari, M.; Manetta, T.; Lupia, E.; Mengozzi, G.; Battista, S. Role of Presepsin for the Evaluation of Sepsis in the Emergency Department. Clin. Chem. Lab. Med. 2014, 52, 1395–1400. [Google Scholar] [CrossRef]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and Prognostic Value of Presepsin in the Management of Sepsis in the Emergency Department: A Multicenter Prospective Study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef]
- Samraj, R.S.; Zingarelli, B.; Wong, H.R. Role of Biomarkers in Sepsis Care. Shock 2013, 40, 358–365. [Google Scholar] [CrossRef]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. SuPAR: The Molecular Crystal Ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Aliu-Bejta, A.; Atelj, A.; Kurshumliu, M.; Dreshaj, S.; Baršić, B. Presepsin Values as Markers of Severity of Sepsis. Int. J. Infect. Dis. IJID 2020, 95, 1–7. [Google Scholar] [CrossRef]
- Larsen, F.F.; Petersen, J.A. Novel Biomarkers for Sepsis: A Narrative Review. Eur. J. Intern. Med. 2017, 45, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Becchi, C.; Pillozzi, S.; Fabbri, L.P.; Al Malyan, M.; Cacciapuoti, C.; Della Bella, C.; Nucera, M.; Masselli, M.; Boncinelli, S.; Arcangeli, A.; et al. The Increase of Endothelial Progenitor Cells in the Peripheral Blood: A New Parameter for Detecting Onset and Severity of Sepsis. Int. J. Immunopathol. Pharmacol. 2008, 21, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Rafat, N.; Hanusch, C.; Brinkkoetter, P.T.; Schulte, J.; Brade, J.; Zijlstra, J.G.; van der Woude, F.J.; van Ackern, K.; Yard, B.A.; Beck, G.C. Increased Circulating Endothelial Progenitor Cells in Septic Patients: Correlation with Survival. Crit. Care Med. 2007, 35, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Schmidt, J.; Hüttner, H.; Tschaikowsky, K. The Natural Elimination Rate of Procalcitonin in Patients with Normal and Impaired Renal Function. Intensive Care Med. 2000, 26 (Suppl. S2), S212–S216. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a Diagnostic Marker for Sepsis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Nylen, E.S.; Langer, I.; Schlatter, M.; Becker, K.L.; Keller, U.; Müller, B. In Vitro and in Vivo Calcitonin I Gene Expression in Parenchymal Cells: A Novel Product of Human Adipose Tissue. Endocrinology 2003, 144, 5578–5584. [Google Scholar] [CrossRef]
- Brodská, H.; Malíčková, K.; Adámková, V.; Benáková, H.; Šťastná, M.M.; Zima, T. Significantly Higher Procalcitonin Levels Could Differentiate Gram-Negative Sepsis from Gram-Positive and Fungal Sepsis. Clin. Exp. Med. 2013, 13, 165–170. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in Sepsis and Systemic Inflammation: A Harmful Biomarker and a Therapeutic Target. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef]
- Delsing, C.E.; Gresnigt, M.S.; Leentjens, J.; Preijers, F.; Frager, F.A.; Kox, M.; Monneret, G.; Venet, F.; Bleeker-Rovers, C.P.; van de Veerdonk, F.L.; et al. Interferon-Gamma as Adjunctive Immunotherapy for Invasive Fungal Infections: A Case Series. BMC Infect. Dis. 2014, 14, 166. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Zulewski, H.; Keller, U.; Müller, B. Autocrine/Paracrine Role of Inflammation-Mediated Calcitonin Gene-Related Peptide and Adrenomedullin Expression in Human Adipose Tissue. Endocrinology 2005, 146, 2699–2708. [Google Scholar] [CrossRef]
- Tan, K.H.; Tan, S.S.; Sze, S.K.; Lee, W.K.R.; Ng, M.J.; Lim, S.K. Plasma Biomarker Discovery in Preeclampsia Using a Novel Differential Isolation Technology for Circulating Extracellular Vesicles. Am. J. Obstet. Gynecol. 2014, 211, 380.e1–380.e13. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Lim, S.K. Membrane Lipids Define Small Extracellular Vesicle Subtypes Secreted by Mesenchymal Stromal Cells. J. Lipid Res. 2019, 60, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.V.; Mattick, J.S. The Rise of Regulatory RNA. Nat. Rev. Genet. 2014, 15, 423–437. [Google Scholar] [CrossRef]
- Brudecki, L.; Ferguson, D.A.; McCall, C.E.; El Gazzar, M. Mitogen-Activated Protein Kinase Phosphatase 1 Disrupts Proinflammatory Protein Synthesis in Endotoxin-Adapted Monocytes. Clin. Vaccine Immunol. CVI 2013, 20, 1396–1404. [Google Scholar] [CrossRef]
- Precone, V.; Stornaiuolo, G.; Amato, A.; Brancaccio, G.; Nardiello, S.; Gaeta, G.B. Different Changes in Mitochondrial Apoptotic Pathway in Lymphocytes and Granulocytes in Cirrhotic Patients with Sepsis. Liver Int. 2013, 33, 834–842. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.-J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of MiR-155 and MiR-125b Levels Following Lipopolysaccharide/TNF-Alpha Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Ho, P.-C.; Chang, K.-C.; Chuang, Y.-S.; Wei, L.-N. Cholesterol Regulation of Receptor-Interacting Protein 140 via MicroRNA-33 in Inflammatory Cytokine Production. FASEB J. 2011, 25, 1758–1766. [Google Scholar] [CrossRef]
- How, C.-K.; Hou, S.-K.; Shih, H.-C.; Huang, M.-S.; Chiou, S.-H.; Lee, C.-H.; Juan, C.-C. Expression Profile of MicroRNAs in Gram-Negative Bacterial Sepsis. Shock 2015, 43, 121–127. [Google Scholar] [CrossRef]
- Ho, J.; Chan, H.; Wong, S.H.; Wang, M.H.T.; Yu, J.; Xiao, Z.; Liu, X.; Choi, G.; Leung, C.C.H.; Wong, W.T.; et al. The Involvement of Regulatory Non-Coding RNAs in Sepsis: A Systematic Review. Crit. Care 2016, 20, 383. [Google Scholar] [CrossRef] [PubMed]
| Indications | MSC Type | Animal Model | Results | References |
|---|---|---|---|---|
| ARDS | BM-MSCs | Murine model | MSCs-mediated inhibition of TNF-alpha, IL-1alpha, and IL1RN mRNA in lung, IL1RN protein in bronchoalveolar lavage (BAL) fluid, and trafficking of lymphocytes and neutrophils into the lung. | Ortiz et al. [184] |
| Ischemia/reperfusion Acute Renal Failure | BM-MSCs | Murine model | Beneficial effects of MSCs characterized by a reduction of the expression of proinflammatory cytokines and an up-regulation of anti-inflammatory cytokines primarily mediated via complex paracrine actions and not by their differentiation into target cells. | Tögel et al. [183] |
| Fulminant Hepatic Failure (FHF) | BM-MSCs | Murine model | MSCs can provide a significant survival benefit in rats undergoing FHF. The authors observed a cell mass-dependent reduction in mortality that was abolished at high cell numbers indicating a therapeutic window. Histopathological analysis of liver tissue after MSC treatment showed dramatic reduction of panlobular leukocytic infiltrates, hepatocellular death, and bile duct duplication. | Parekkadan et al. [185] |
| Experimental Autoimmune Encephalomyelitis (EAE) | BM-MSCs | Murine model | Immunoregulatory properties of MSCs interfere with the autoimmune attack during EAE inducing an in vivo state of T-cell unresponsiveness occurring within secondary lymphoid organs. | Zappia et al. [102] |
| Autoimmune Encephalomyelitis | UC-MSCs | Murine model | MSC-treated mice showed a significantly milder disease and fewer relapses compared to control mice related to a lower number of inflammatory infiltrates, a reduced demyelination and axonal loss. In vivo, PLP-specific T-cell response and antibody titers were significantly lower in MSC-treated mice. | Gerdoni et al. [186] |
| Sepsis and septic shock | AT-MSCs | Murine model | MSCs-immunomodulatory capacities decrease tissue inflammation by regulating cytokine homeostasis and decreasing the traffic of immune cells into organs. They own antibacterial capacities mediated by direct action on the bacterial load through secreting antibacterial peptides and by indirect action through increasing the phagocytic activity of macrophages and neutrophils. MSC infusion reduced organ failure and mortality associated with sepsis and septic shock. | Laroye et al. [182] |
| Septic shock | BM-MSCs | Porcine model | UC-MSCs infusion reduced peritonitis-associated hypotension, hyperlactatemia, and multiple organ failure. Cardiovascular failure was attenuated, as attested by a better mean arterial pressure and reduced lactatemia, despite lower norepinephrine requirements. UC-MSCs improved survival (60% survival vs. 0% at 24 h). | Laroye et al. [187] |
| Sepsis | BM-MSCs and WJ-MSCs | Porcine model | MSCs regulated leukocytes trafficking and reduced organ dysfunction. WJ-MSCs improved bacterial clearance and survival. | Laroye et al. [188] |
| Sepsis | BM-MSCs | Porcine model | BM-MSCs IV administration was well-tolerated. MSCs were not capable of reversing sepsis-induced disturbances in multiple biological, organ, and cellular systems. | Horak et al. [189] |
| Sepsis | Various types of MSCs | Various animal models | There was a statistically significant association between MSC therapy and lower mortality in sepsis animal models, supporting the potential therapeutic effect of MSC treatment in future clinical trials. | Sun et al. [190] |
| Indications | Study phase or type | MSC type | Patients, n | Dose | Results | References |
|---|---|---|---|---|---|---|
| ARDS | Phase I trial | AT-MSCs | Control: 6 Experimental: 6 | 1 × 106 cells/kg | Safety and feasibility of an AT-MSCs single infusion in treatment of ARDS | Zheng et al. [197] |
| ARDS | Phase I trial | BM-MSCs | 9 | 1, 5 and 10 × 106 cells/kg | A single infusion of allogeneic BM-MSCs is well tolerated in patients with moderate to severe ARDS | Wilson et al. [194] |
| ARDS | Phase I trial | UC-MSCs | 9 | 1, 5 and 10 × 106 cells/kg | Safety of a single infusion of UC-MSCs | Yip et al. [198] |
| H7N9-ARDS | Phase I trial | MB-MSCs | Control: 44 Experimental: 17 | 1 × 106 cells/kg in 3 or 4 injections | No harmful effects observed | Chen et al. [199] |
| SARS-CoV-2 ARDS | Phase I trial | UC-MSCs | 1 (Case report) | 50 × 106 cells/kg × 3 injections | Good tolerance of allogenic UC-MSCs | Liang et al. [195] |
| SARS-CoV-2 ARDS | Case report | UC-MSCs | Control: 3 Experimental: 7 | 1 × 106 cells/kg | No adverse effects observed | Leng et al. [196] |
| ARDS | Phase I | BM-MSCs | Control: 20 Experimental: 40 | 10 × 106 cells/kg | Safety of MSCs infusion | Matthay et al. [200] |
| Various critical illness conditions | Meta-analysis | UC-MSCs | 93 peer-reviewed full articles or abstracts | various | No long-term adverse effects, tumor formation or cell rejection founded | Can et al. [201] |
| Septic shock | Phase I | BM-MSCs | Control: 21 Experimental: 9 | 0.5, 1 and 3 × 106 cells/kg | Infusion of freshly cultured allogenic BM-MSCs up to 3 × 106 cells/kg seems safe | Mcintyre et al. [202] |
| Severe sepsis | Phase I | UC-MSCs | Control: 15 Historical case-matched: 15 | 1, 2 and 3 × 106 cells/kg | No infusion-associated serious events or treatment-related adverse events | He et al. [192] |
| LPS-mediated sepsis (LPS at 2 ng/kg) 1 h after MSC infusion) | Phase I | AT-MSCs | 32 (healthy subjects) | 0.25, 1 and 4 × 106 cells/kg | IV infusion of AT-MSCs at a dose of 4.106 cells/kg is well tolerated and associated with various procoagulant, pro and anti-inflammatory effects | Perlee et al. [193] |
| Septic shock | Phase I | BM-MSCs | Control: 21 Historical case-matched group: 9 | 0.3, 1 and 3 × 106 cells/kg | Safe response characterized by the absence of elevation of plasma-cytokine levels | Schlosser et al. [203] |
| ARDS (the START study) | Phase IIa | BM-MSCs | Control: 20 Experimental: 40 | 10 × 106 cells/kg | Significant decrease of Angiopoietin-2, a marker of endothelial dysfunction. No survival improvement | Matthay et al. [200] |
| Septic shock in severe neutropenic patients (the RUMCESS study) | Phase II | BM-MSCs | Control: 15 Experimental: 15 | 1 × 106 cells/kg | Good tolerance and safety of MSCs infusion in neutropenic patients. A faster hemodynamic stabilization, vasopressor with- drawal, attenuation of respiratory failure and shortening of the neutropenia duration period | Gennadiy et al. [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, M.; Bedoui, Y.; Vagner, D.; Raffray, L.; Ah-Pine, F.; Doray, B.; Gasque, P. Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2022, 23, 9274. https://doi.org/10.3390/ijms23169274
Daniel M, Bedoui Y, Vagner D, Raffray L, Ah-Pine F, Doray B, Gasque P. Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs). International Journal of Molecular Sciences. 2022; 23(16):9274. https://doi.org/10.3390/ijms23169274
Chicago/Turabian StyleDaniel, Matthieu, Yosra Bedoui, Damien Vagner, Loïc Raffray, Franck Ah-Pine, Bérénice Doray, and Philippe Gasque. 2022. "Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs)" International Journal of Molecular Sciences 23, no. 16: 9274. https://doi.org/10.3390/ijms23169274
APA StyleDaniel, M., Bedoui, Y., Vagner, D., Raffray, L., Ah-Pine, F., Doray, B., & Gasque, P. (2022). Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs). International Journal of Molecular Sciences, 23(16), 9274. https://doi.org/10.3390/ijms23169274






