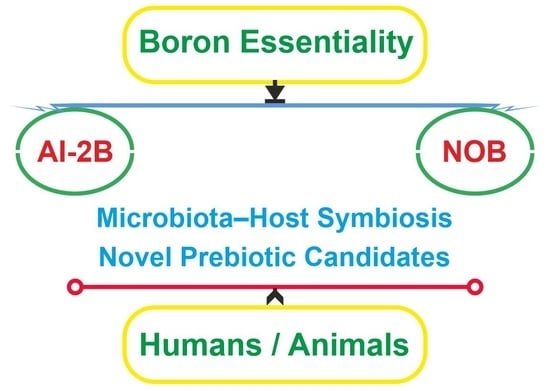
New Insights into Boron Essentiality in Humans and Animals
Abstract
1. Introduction
2. New Insights into the Essentiality of B Species in Symbiosis across Life Kingdoms
3. Is AI-2B an Essential Quorum Sensing in the Symbiosis between Microbiota and Hosts?
4. Are NOB Species Essential for Healthy Human/Animal Microbiome Symbiosis?
5. Are NOB Species Novel Prebiotic Candidates?
6. Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef]
- Scorei, R.; Cimpoiaşu, V.M. Boron enhances the thermostability of carbohydrates. Orig. Life Evol. Biosph. 2006, 36, 1–11. [Google Scholar] [CrossRef]
- Scorei, R. Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on Earth. Orig. Life Evol. Biosph. 2012, 42, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Furukawa, Y.; Kakegawa, T.; Bita, A.; Scorei, R.; Benner, S.A. Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: Borate as a multifaceted problem solver in prebiotic chemistry. Angew. Chemie Int. Ed. Engl. 2016, 55, 15816–15820. [Google Scholar] [CrossRef]
- Zumreoglu-Karan, B.; Kose, D.A. Boric acid: A simple molecule of physiologic, therapeutic and prebiotic significance. Pure Appl. Chem. 2015, 87, 155–162. [Google Scholar] [CrossRef]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araújo, W.L. Boron: More than an essential element for land plants? Front. Plant Sci. 2021, 11, 610307. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, L.; Esteban, E.; De Lorenzo, C.; Fernandez-Pascual, M.; De Felipe, M.R.; Garate, A.; Bonilla, I. Essentiality of boron for symbiotic dinitrogen fixation in pea (Pisum sativum) Rhizobium nodules. Plant Physiol. 1994, 104, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Raja, C.E.; Omine, K. Characterization of boron resistant and accumulating bacteria Lysinibacillus fusiformis M1, Bacillus cereus M2, Bacillus cereus M3, Bacillus pumilus M4 isolated from former mining site, Hokkaido, Japan. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2012, 47, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Fregoso, E.; Farfán-García, E.D.; García-Coronel, I.H.; Martínez-Herrera, E.; Alatorre, A.; Scorei, R.I.; Soriano-Ursúa, M.A. Effects of boron-containing compounds in the fungal kingdom. J. Trace Elem. Med. Biol. 2021, 65, 126714. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.D. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ. Health Perspect. 1994, 102, 35–43. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Eckhert, C.D. Boron. Adv. Nutr. 2020, 11, 461–462. [Google Scholar] [CrossRef]
- Nielsen, F.H. The justification for providing dietary guidance for the nutritional intake of boron. Biol. Trace Elem. Res. 1998, 66, 319–330. [Google Scholar] [CrossRef]
- Nielsen, F.H. Is boron nutritionally relevant? Nutr. Rev. 2008, 66, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Rowe, R.I.; Bouzan, C.; Nabili, S.; Eckhert, C.D. The response of trout and zebrafish embryos to low and high boron concentrations is U-shaped. Biol. Trace Elem. Res. 1998, 66, 261–270. [Google Scholar] [CrossRef]
- Rowe, R.I.; Eckhert, C.D. Boron is required for zebrafish embryogenesis. J. Exp. Biol. 1999, 202, 1649–1654. [Google Scholar] [CrossRef]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A. Paradoxes in the origin of life. Orig. Life Evol. Biosph. 2014, 44, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Q.; Zheng, M.; Hao, S.; Lum, J.S.; Chen, X.; Huang, X.F.; Yu, Y.; Zheng, K. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota–brain axis in diet-induced obese mice. J. Neuroinflamm. 2020, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols–microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bi, Z.; Yang, C.; Guo, Y.; Yuan, J.; Li, L.; Guo, Y. Effects of different doses of omega-3 polyunsaturated fatty acids on gut microbiota and immunity. Food Nutr. Res. 2021, 65, 6263. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef] [PubMed]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: An overview. Front. Immunol. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.M.; Nemzer, B.V.; Rangavajla, N.; Biţă, A.; Rogoveanu, O.C.; Neamţu, J.; Scorei, I.R.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; et al. The fructoborates: Part of a family of naturally occurring sugar–borate complexes—Biochemistry, physiology, and impact on human health: A review. Biol. Trace Elem. Res. 2019, 188, 11–25. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Biţă, A.; Bejenaru, L.E.; Bejenaru, C.; Croitoru, O.; Rău, G.; Rogoveanu, O.C.; Florescu, D.N.; Neamţu, J.; Scorei, I.D.; et al. Calcium fructoborate for bone and cardiovascular health. Biol. Trace Elem. Res. 2016, 172, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chang, J.S.; Hunter, J.M.; Nemzer, B.V. Identification and quantification of fructoborate ester complex using liquid chromatography coupled with Q exactive orbitrap mass spectrometry. J. Food Res. 2017, 6, 85–92. [Google Scholar] [CrossRef]
- Brown, P.H.; Hu, H. Phloem mobility of boron is species dependent: Evidence for phloem mobility in sorbitol-rich species. Ann. Bot. 1996, 77, 497–506. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J. Two chains of rhamnogalacturonan II are cross-linked by borate–diol ester bonds in higher plant cell walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef]
- Brown, P.H.; Hu, H. Phloem boron mobility in diverse plant species. Bot. Acta 1998, 111, 331–335. [Google Scholar] [CrossRef]
- Miljkovic, D.; Scorei, R.I.; Cimpoiaşu, V.M.; Scorei, I.D. Calcium fructoborate: Plant-based dietary boron for human nutrition. J. Diet. Suppl. 2009, 6, 211–226. [Google Scholar] [CrossRef]
- Scorei, I.D.; Scorei, R.I. Calcium fructoborate helps control inflammation associated with diminished bone health. Biol. Trace Elem. Res. 2013, 155, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Dinca, L.; Scorei, R. Boron in human nutrition and its regulations use. J. Nutr. Ther. 2013, 2, 22–29. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Smoum, R.; Al-Quntar, A.A.; Ali, H.A.; Pergament, I.; Srebnik, M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002, 163, 931–942. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A. Naturally occurring boron containing compounds and their biological activities. J. Nat. Prod. Resour. 2017, 3, 147–154. Available online: https://www.jacsdirectory.com/journal-of-natural-products-and-resources/articleview.php?id=40 (accessed on 13 April 2022).
- Scorei, R.; Bita, A.; Dinca, L.; Mogosanu, D.; Rangavajla, N. Diester Chlorogenoborate Complex: Methods for Identification, Synthesis, Purification and Uses Thereof. United States Patent and Trademark Office (USPTO), Provisional Patent Application No. 63271159/10/24/2021, 2021. Available online: https://www.uspto.gov/patents/basics/types-patent-applications/provisional-application-patent (accessed on 13 April 2022).
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163–170. [Google Scholar] [CrossRef]
- Leidner, D.E. Review and theory symbiosis: An introspective retrospective. J. Assoc. Inf. Syst. 2018, 19, 552–567. [Google Scholar] [CrossRef]
- Nelson, P.G.; May, G. Coevolution between mutualists and parasites in symbiotic communities may lead to the evolution of lower virulence. Am. Nat. 2017, 190, 803–817. [Google Scholar] [CrossRef][Green Version]
- González, J.E.; Marketon, M.M. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. 2003, 67, 574–592. [Google Scholar] [CrossRef]
- Abreu, I.; Cerda, M.E.; de Nanclares, M.P.; Baena, I.; Lloret, J.; Bonilla, I.; Bolaños, L.; Reguera, M. Boron deficiency affects rhizobia cell surface polysaccharides important for suppression of plant defense mechanisms during legume recognition and for development of nitrogen-fixing symbiosis. Plant Soil 2012, 361, 385–395. [Google Scholar] [CrossRef]
- Bolaños, L.; Redondo-Nieto, M.; Bonilla, I.; Wall, L.G. Boron requirement in the Discaria trinervis (Rhamnaceae) and Frankia symbiotic relationship. Its essentiality for Frankia BCU110501 growth and nitrogen fixation. Physiol. Plant. 2002, 115, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.; Timmers, A.C.J.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Aydemir, S.; Kaya, C. Mitigation effects of mycorrhiza on boron toxicity in wheat (Triticum durum) plants. N. Z. J. Crop. Hortic. Sci. 2009, 37, 99–104. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, R.; Sun, H.; Liu, C. Alleviation of boron toxicity in plants: Mechanisms and approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2975–3015. [Google Scholar] [CrossRef]
- Simón-Grao, S.; Nieves, M.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Fernández-Zapata, J.C.; García-Sánchez, F. Arbuscular mycorrhizal symbiosis improves tolerance of Carrizo citrange to excess boron supply by reducing leaf B concentration and toxicity in the leaves and roots. Ecotoxicol. Environ. Saf. 2019, 173, 322–330. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Ruiz-Lozano, J.M. Elucidating the possible involvement of maize aquaporins in the plant boron transport and homeostasis mediated by Rhizophagus irregularis under drought stress conditions. Int. J. Mol. Sci. 2020, 21, 1748. [Google Scholar] [CrossRef]
- Bonilla, I.; Garcia-González, M.; Mateo, P. Boron requirement in cyanobacteria: Its possible role in the early evolution of photosynthetic organisms. Plant Physiol. 1990, 94, 1554–1560. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.; Mateo, P.; Bonilla, I. Boron requirement for envelope structure and function in Anabaena PCC 7119 heterocysts. J. Exp. Bot. 1991, 42, 925–929. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, I.; Redondo-Nieto, M.; El-Hamdaoui, A.; Wall, L.G.; Bolaños, L. Essentiality of boron for symbiotic nitrogen fixation in legumes and actinorhizal plants. In Boron in Plant and Animal Nutrition; Goldbach, H.E., Brown, P.H., Rerkasem, B., Thellier, M., Wimmer, M.A., Bell, R.W., Eds.; Springer: Boston, MA, USA, 2002; pp. 261–267. [Google Scholar] [CrossRef]
- Harris, W.R.; Amin, S.A.; Küpper, F.C.; Green, D.H.; Carrano, C.J. Borate binding to siderophores: Structure and stability. J. Am. Chem. Soc. 2007, 129, 12263–12271. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, G.A.; Frantellizzi, A.J.; Gaesser, K.M.; Tammariello, S.P.; Ahmed, A. Autoinducer-2 detection among commensal oral streptococci is dependent on pH and boric acid. J. Microbiol. 2016, 54, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Sperandio, V. Bacterial cell-to-cell signaling in the gastrointestinal tract. Infect. Immun. 2005, 73, 3197–3209. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, F.Y.; Pan, X.Y.; Zhang, Y.N.; Zhang, F. Boric acid was orally toxic to different instars of Blattella germanica (L.) (Blattodea: Blattellidae) and caused dysbiosis of the gut microbiota. Pestic. Biochem. Physiol. 2021, 172, 104756. [Google Scholar] [CrossRef]
- Bourgeois, A.C.; Scott, M.E.; Sabally, K.; Koski, K.G. Low dietary boron reduces parasite (Nematoda) survival and alters cytokine profiles but the infection modifies liver minerals in mice. J. Nutr. 2007, 137, 2080–2086. [Google Scholar] [CrossRef]
- Fort, D.J.; Rogers, R.L.; McLaughlin, D.W.; Sellers, C.M.; Schlekat, C.L. Impact of boron deficiency on Xenopus laevis: A summary of biological effects and potential biochemical roles. Biol. Trace Elem. Res. 2002, 90, 117–142. [Google Scholar] [CrossRef]
- Evariste, L.; Flahaut, E.; Baratange, C.; Barret, M.; Mouchet, F.; Pinelli, E.; Galibert, A.M.; Soula, B.; Gauthier, L. Ecotoxicological assessment of commercial boron nitride nanotubes toward Xenopus laevis tadpoles and host-associated gut microbiota. Nanotoxicology 2021, 15, 35–51. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Trindade-Silva, A.E.; Hanora, A.; Han, A.W.; Flores, M.S.; Vizzoni, V.; Schrago, C.G.; Soares, C.A.; Concepcion, G.P.; Distel, D.L.; et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl. Acad. Sci. USA 2013, 110, E295–E304. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Adhikari, B.; Pontin, K.P.; Latorre, J.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Merino-Guzman, R.; Méndez-Albores, A.; Kwon, Y.M.; et al. Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella enteritidis: Proof of concept. Res. Vet. Sci. 2019, 123, 7–13. [Google Scholar] [CrossRef]
- Sizmaz, O.; Koksal, B.H.; Yildiz, G. Rumen microbial fermentation, protozoan abundance and boron availability in yearling rams fed diets with different boron concentrations. J. Anim. Feed Sci. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Miyamoto, S.; Sutoh, M.; Shiomoto, A.; Yamazaki, S.; Nishimura, K.; Yonezawa, C.; Matsue, H.; Hoshi, M. Determination of boron in animal materials by reactor neutron induced prompt gamma-ray analysis. J. Radioanal. Nucl. Chem. 2000, 244, 307–309. [Google Scholar] [CrossRef]
- Sun, P.P.; Luo, Y.; Wu, X.T.; Ansari, A.R.; Wang, J.; Yang, K.L.; Xiao, K.; Peng, K.M. Effects of supplemental boron on intestinal proliferation and apoptosis in African ostrich chicks (Efectos del boro suplementario sobre la proliferación intestinal y apoptosis en polluelos de avestruz africana). Int. J. Morphol. 2016, 34, 830–835. [Google Scholar] [CrossRef]
- Mitruţ, I.; Cojocaru, M.O.; Scorei, I.R.; Biţă, A.; Mogoşanu, G.D.; Popescu, M.; Olimid, D.A.; Manolea, H.O. Preclinical and histological study of boron-containing compounds hydrogels on experimental model of periodontal disease. Rom. J. Morphol. Embryol. 2021, 62, 219–226. [Google Scholar] [CrossRef]
- Kuru, R.; Balan, G.; Yilmaz, S.; Taslı, P.N.; Akyuz, S.; Yarat, A.; Sahin, F. The level of two trace elements in carious, non-carious, primary, and permanent teeth. Eur. Oral Res. 2020, 54, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Macelline, S.P.; Wickramasuriya, S.S.; Shin, T.K.; Kim, E.; Son, H.C.; Heo, J.M. Moderate dietary boron supplementation improved growth performance, crude protein digestibility and diarrhea index in weaner pigs regardless to the sanitary condition. Anim. Biosci. 2022, 35, 434–443. [Google Scholar] [CrossRef]
- Bargiel, P.; Szczuko, M.; Stachowska, L.; Prowans, P.; Czapla, N.; Markowska, M.; Petriczko, J.; Kledzik, J.; Jędrzejczyk-Kledzik, A.; Palma, J.; et al. Microbiome metabolites and thyroid dysfunction. J. Clin. Med. 2021, 10, 3609. [Google Scholar] [CrossRef] [PubMed]
- Kuru, R.; Yilmaz, S.; Balan, G.; Tuzuner, B.A.; Tasli, P.N.; Akyuz, S.; Yener Ozturk, F.; Altuntas, Y.; Yarat, A.; Sahin, F. Boron-rich diet may regulate blood lipid profile and prevent obesity: A nondrug and self-controlled clinical trial. J. Trace Elem. Med. Biol. 2019, 54, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Ratjen, I.; Enderle, J.; Seidel, U.; Rimbach, G.; Lieb, W. Plasma boron concentrations in the general population: A cross-sectional analysis of cardio-metabolic and dietary correlates. Eur. J. Nutr. 2022, 61, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Hastings, J.W. Quorum sensing on a global scale: Massive numbers of bioluminescent bacteria make milky seas. Appl. Environ. Microbiol. 2006, 72, 2295–2297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, L.; Luo, Y. Bacterial quorum-sensing systems and their role in intestinal bacteria–host crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.T.; Xavier, K.B.; Campagna, S.R.; Taga, M.E.; Semmelhack, M.F.; Bassler, B.L.; Hughson, F.M. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 2004, 15, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Valastyan, J.S.; Bassler, B.L. A host-produced autoinducer-2 mimic activates bacterial quorum sensing. Cell Host Microbe 2016, 19, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Christiaen, S.E.A.; O’Connell Motherway, M.; Bottacini, F.; Lanigan, N.; Casey, P.G.; Huys, G.; Nelis, H.J.; van Sinderen, D.; Coenye, T. Autoinducer-2 plays a crucial role in gut colonization and probiotic functionality of Bifidobacterium breve UCC2003. PLoS ONE 2014, 9, e98111. [Google Scholar] [CrossRef] [PubMed]
- Bivar Xavier, K. Bacterial interspecies quorum sensing in the mammalian gut microbiota. C. R. Biol. 2018, 341, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.S.; Xie, L.W.; Cai, S.; Xu, J.Y.; Zhou, H.; Tang, L.F.; Yang, C.; Fang, S.; Li, M.; Tian, Y. Dysbiosis of gut microbiota is associated with the progression of radiation-induced intestinal injury and is alleviated by oral compound probiotics in mouse model. Front. Cell. Infect. Microbiol. 2021, 11, 717636. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Li, L.Q.; Yang, T.; She, X.; Ai, Q.; Wang, Z.L. Autoinducer-2 may be a new biomarker for monitoring neonatal necrotizing enterocolitis. Front. Cell. Infect. Microbiol. 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.D.; Durst, R.W. Chemistry and biology of boron. Biofactors 1992, 3, 229–239. Available online: https://pubmed.ncbi.nlm.nih.gov/1605832/ (accessed on 13 April 2022).
- Ascenso, O.S.; Marques, J.C.; Santos, A.R.; Xavier, K.B.; Ventura, M.R.; Maycock, C.D. An efficient synthesis of the precursor of AI-2, the signalling molecule for inter-species quorum sensing. Bioorg. Med. Chem. 2011, 19, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Grimm, V.; Riedel, C.U. AI-2 to the rescue against antibiotic-induced intestinal dysbiosis? Trends Microbiol. 2015, 23, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Pesce, C.; Oshone, R.; Hurst, S.G., 4th; Kleiner, V.A.; Tisa, L.S. Stable transformation of the actinobacteria Frankia spp. Appl. Environ. Microbiol. 2016, 85, e00957-19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Fujiwara, T. Mechanism of boron tolerance in soil bacteria. Can. J. Microbiol. 2010, 56, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Yokota, A.; Fujiwara, T. A novel highly boron tolerant bacterium, Bacillus boroniphilus sp. nov., isolated from soil, that requires boron for its growth. Extremophiles 2007, 11, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.; Williams, P. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface 2009, 6, 959–978. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—Mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond just bacteria: Functional biomes in the gut ecosystem including virome, mycobiome, archaeome and helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Versalovic, J. Biochemical features of beneficial microbes: Foundations for therapeutic microbiology. Microbiol. Spectr. 2017, 5, BAD-0012-2016. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.J.; Bassler, B.L. Interspecies communication in bacteria. J. Clin. Investig. 2003, 112, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Liu, X.; Wang, Z.; Jiang, M.; Wang, R.; Xie, L.; Liu, Q.; Xie, X.; Shang, D.; et al. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nat. Commun. 2020, 11, 5371. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015, 10, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.; Ahmed, A.M.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A., Jr.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Tondt, J.; Yancy, W.S.; Westman, E.C. Application of nutrient essentiality criteria to dietary carbohydrates. Nutr. Res. Rev. 2020, 33, 260–270. [Google Scholar] [CrossRef]
- Dupre, J.N.; Keenan, M.J.; Hegsted, M.; Brudevold, A.M. Effects of dietary boron in rats fed a vitamin D-deficient diet. Environ. Health Perspect. 1994, 102, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.N.; Brown, P.H. Techniques for boron determination and their application to the analysis of plant and soil samples. Plant Soil 1997, 193, 15–33. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Chilloux, J.; Neves, A.L.; Boulangé, C.L.; Dumas, M.E. The microbial–mammalian metabolic axis: A critical symbiotic relationship. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef] [PubMed]
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.A.; Schott, E.M.; Gill, S.R.; Zuscik, M.J. The gut microbiome–joint connection: Implications in osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Wouthuyzen-Bakker, M.; Friedrich, A.W.; Parvizi, J. The relation between the gut microbiome and osteoarthritis: A systematic review of literature. PLoS ONE 2021, 16, e0261353. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Newnham, R.E. Essentiality of boron for healthy bones and joints. Environ. Health Perspect. 1994, 102, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The role of microbiome in brain development and neurodegenerative diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex differences in gut microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Prejac, J.; Skalny, A.A.; Grabeklis, A.R.; Uzun, S.; Mimica, N.; Momčilović, B. Assessing the boron nutritional status by analyzing its cummulative frequency distribution in the hair and whole blood. J. Trace Elem. Med. Biol. 2018, 45, 50–56. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The human gut microbiome in health and disease. Integr. Med. (Encinitas) 2014, 13, 17–22. Available online: https://pubmed.ncbi.nlm.nih.gov/26770121/ (accessed on 13 April 2022).
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef]
- Wang, X.; Xia, N.; Liu, L. Boronic acid-based approach for separation and immobilization of glycoproteins and its application in sensing. Int. J. Mol. Sci. 2013, 14, 20890–20912. [Google Scholar] [CrossRef]
- Redondo-Nieto, M.; Reguera, M.; Bonilla, I.; Bolaños, L. Boron dependent membrane glycoproteins in symbiosome development and nodule organogenesis: A model for a common role of boron in organogenesis. Plant Signal. Behav. 2008, 3, 298–300. [Google Scholar] [CrossRef][Green Version]
- Popov, T.; Angelieva, R. The mechanism of action of boron on the organism of warm blooded animals (entry, distribution and elimination). Gig. Sanit. 1969, 34, 78–81. Available online: https://pubmed.ncbi.nlm.nih.gov/5348992/ (accessed on 13 April 2022). [PubMed]
- Baspinar, N.; Basoglu, A.; Semacan, A.; Gulersoy, E. Short term effects of dietary boron on mineral status in dairy cows. Int. J. Environ. Agric. Res. 2017, 3, 65–70. Available online: https://ijoear.com/assets/articles_menuscripts/file/IJOEAR-NOV-2017-2.pdf (accessed on 13 April 2022). [CrossRef]
- Newnham, R. Discovering the cure for arthritis. Nutr. Health 2004, 17, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Scorei, R.; Cimpoiasu, V.M.; Iordachescu, D. In vitro evaluation of the antioxidant activity of calcium fructoborate. Biol. Trace Elem. Res. 2005, 107, 127–134. [Google Scholar] [CrossRef]
- Scorei, R.; Ciubar, R.; Iancu, C.; Mitran, V.; Cimpean, A.; Iordachescu, D. In vitro effects of calcium fructoborate on fMLP-stimulated human neutrophil granulocytes. Biol. Trace Elem. Res. 2007, 118, 27–37. [Google Scholar] [CrossRef]
- Scorei, R.I.; Ciofrangeanu, C.; Ion, R.; Cimpean, A.; Galateanu, B.; Mitran, V.; Iordachescu, D. In vitro effects of calcium fructoborate upon production of inflammatory mediators by LPS-stimulated RAW 264.7 macrophages. Biol. Trace Elem. Res. 2010, 135, 334–344. [Google Scholar] [CrossRef]
- Scorei, R.I.; Rotaru, P. Calcium fructoborate—Potential anti-inflammatory agent. Biol. Trace Elem. Res. 2011, 143, 1223–1238. [Google Scholar] [CrossRef]
- Scorei, R.; Mitrut, P.; Petrisor, I.; Scorei, I. A double-blind, placebo-controlled pilot study to evaluate the effect of calcium fructoborate on systemic inflammation and dyslipidemia markers for middle-aged people with primary osteoarthritis. Biol. Trace Elem. Res. 2011, 144, 253–263. [Google Scholar] [CrossRef][Green Version]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Mogoantă, L.; Bejenaru, C.; Mogoşanu, G.D.; Bejenaru, L.E. Natural and semisynthetic candidate molecules for COVID-19 prophylaxis and treatment. Rom. J. Morphol. Embryol. 2020, 61, 321–334. [Google Scholar] [CrossRef]
- Scorei, I.R.; Biţă, A.; Mogoşanu, G.D. Letter to the Editor: Boron enhances the antiviral activity of the curcumin against SARS-CoV-2. Rom. J. Morphol. Embryol. 2020, 61, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Văruţ, R.M.; Melinte, P.R.; Pîrvu, A.S.; Gîngu, O.; Sima, G.; Oancea, C.N.; Teişanu, A.C.; Drăgoi, G.; Biţă, A.; Manolea, H.O.; et al. Calcium fructoborate coating of titanium–hydroxyapatite implants by chemisorption deposition improves implant osseointegration in the femur of New Zealand White rabbit experimental model. Rom. J. Morphol. Embryol. 2020, 61, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Criste, R.D.; Grossu, D.V.; Scorei, R.; Duca, R.C.; Ciurascu, C.; Mitrut, M. Investigations on the effect of the supplemental VETABOR, boron-enriched concentrate added to broiler diets, on breast meat fatty acids profile. In Proceedings of the XVIIth European Symposium on the Quality of Poultry Meat, Doorwerth, The Netherlands, 23–26 May 2005; pp. 165–169. Available online: https://www.cabi.org/Uploads/animal-science/worlds-poultry-science-association/WPSA-the-netherlands-2005/96.pdf (accessed on 13 April 2022).
- Criste, R.D.; Grossu, D.V.; Scorei, R.; Duca, R.C.; Mitrut, M.; Ciurascu, C. New investigations on the effect of the dietary boron on broilers and layers; boron and food quality. Arch. Zootech. 2005, 8, 65–78. Available online: https://www.researchgate.net/publication/261027124_New_investigations_on_the_effect_of_the_dietary_boron_on_broilers_and_layers_boron_and_food_quality (accessed on 13 April 2022).
- Untea, A.; Panaite, T.; Criste, R.D. Effect of the dietary calcium fructoborate given to weaned piglets on calcium balance. Arch. Zootech. 2008, 11, 61–68. Available online: https://www.ibna.ro/arhiva/AZ%2011-3/AZ11-3%2006_Arabela.pdf (accessed on 13 April 2022).
- Scorei, I.R. Calcium fructoborate: Plant-based dietary boron as potential medicine for cancer therapy. Front. Biosci. (Schol. Ed.) 2011, 3, 205–215. [Google Scholar] [CrossRef]
- Taranu, I.; Marin, D.E.; Manda, G.; Motiu, M.; Neagoe, I.; Tabuc, C.; Stancu, M.; Olteanu, M. Assessment of the potential of a boron–fructose additive in counteracting the toxic effect of Fusarium mycotoxins. Br. J. Nutr. 2011, 106, 398–407. [Google Scholar] [CrossRef]
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Scorei, R.; Bita, A.; Dinca, L.; Mogosanu, D.; Rangavajla, N. Methods for extracting high content of diester cholorenoborate complex from green coffee beans and uses thereof. United States Patent and Trademark Office (USPTO), Provisional Patent Application No. 63326931/04/04/2022, 2022. Available online: https://www.uspto.gov/patents/basics/types-patent-applications/provisional-application-patent (accessed on 13 April 2022).
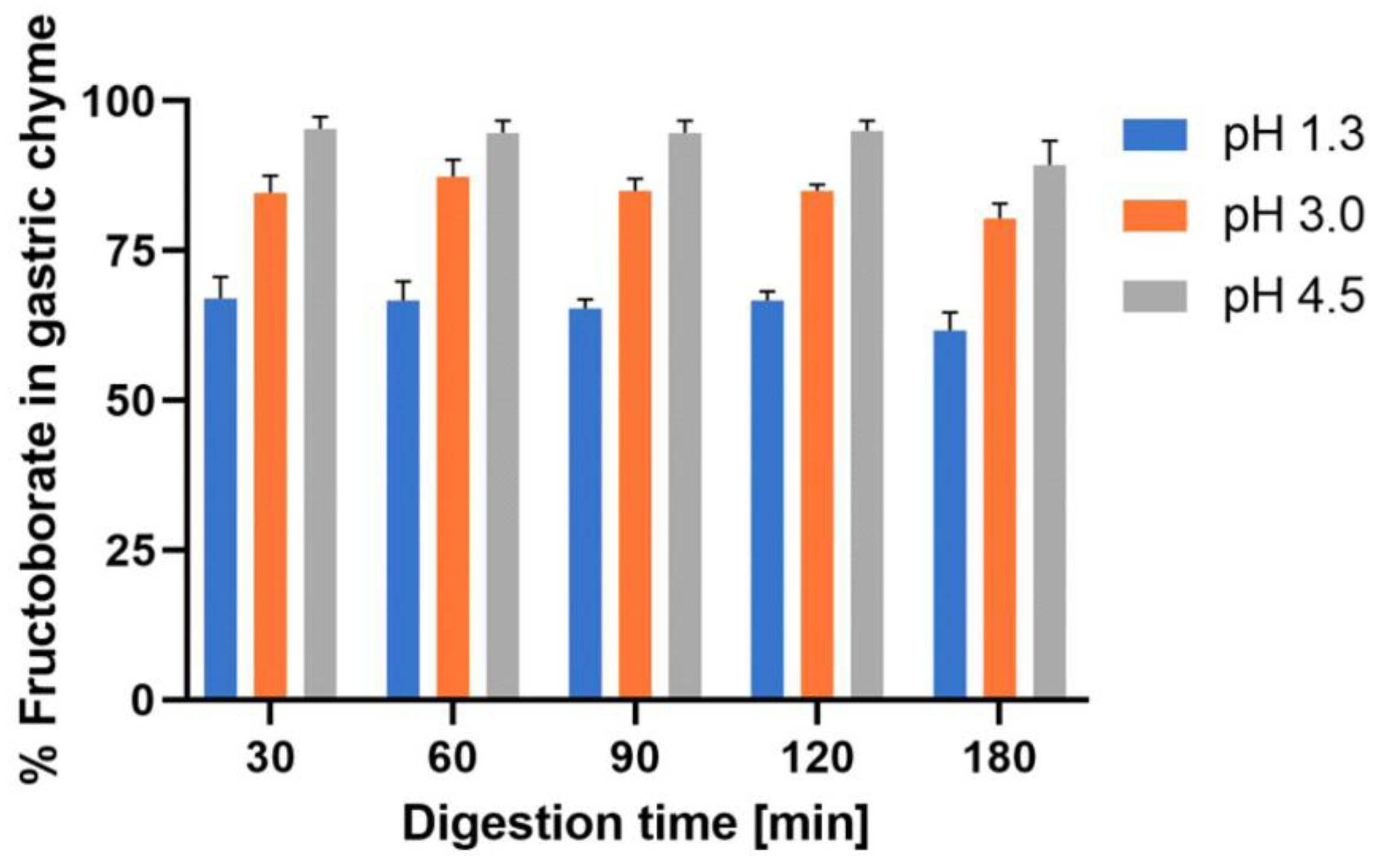
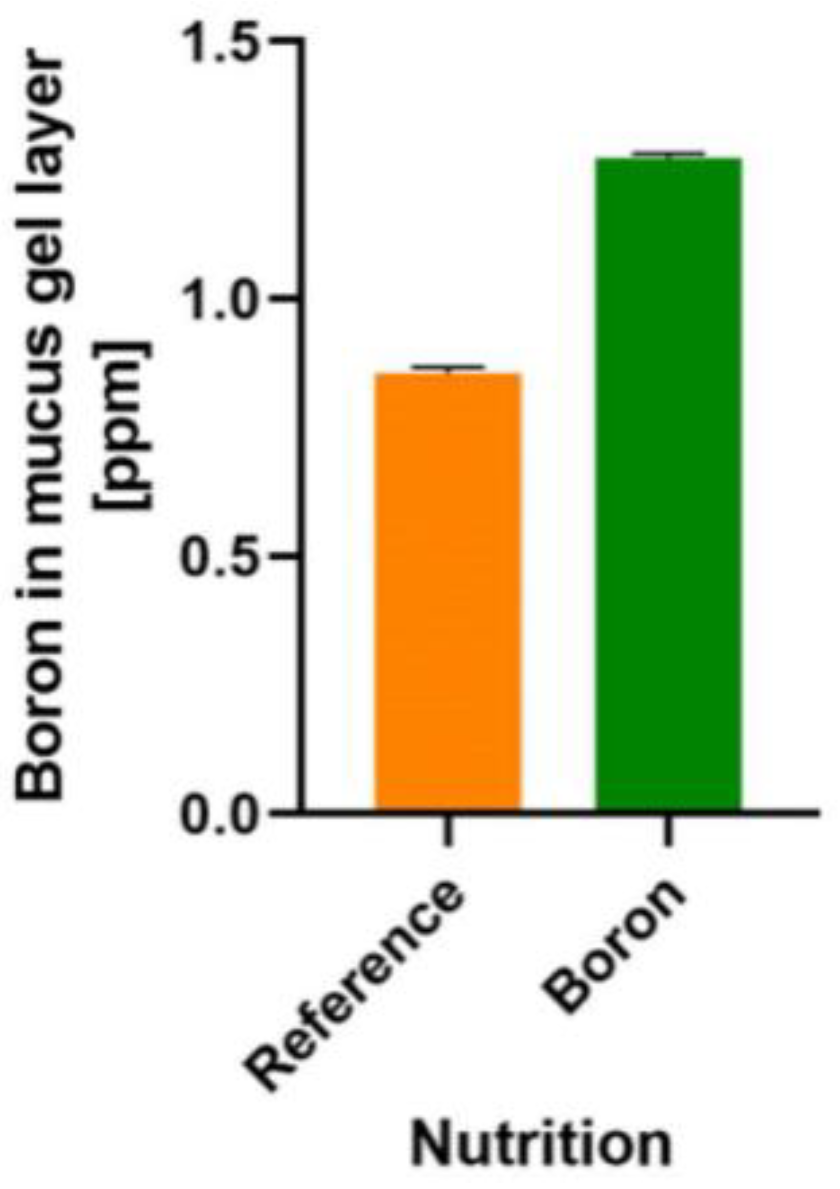
- Ferrua, M.J.; Singh, R.P. Human gastric simulator (Riddet model). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 61–71. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.; Dave, A.; Singh, H. In vitro digestion of soymilk using a human gastric simulator: Impact of structural changes on kinetics of release of proteins and lipids. Food Hydrocoll. 2021, 111, 106235. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Carrière, F.; Moreau, H.; Raphel, V.; Laugier, R.; Benicourt, C.; Junien, J.L.; Verger, R. Purification and biochemical characterization of dog gastric lipase. Eur. J. Biochem. 1991, 202, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract—Influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Trinh, P.; Zaneveld, J.R.; Safranek, S.; Rabinowitz, P.M. One health relationships between human, animal, and environmental microbiomes: A mini-review. Front. Public Health 2018, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Schmidt, M.; Vu, B.; Essmann, M.; Larsen, B. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J. Antimicrob. Chemother. 2009, 63, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Brotman, R.M. Making inroads into improving treatment of bacterial vaginosis—Striving for long-term cure. BMC Infect. Dis. 2015, 15, 292. [Google Scholar] [CrossRef]
- De Seta, F.; Leonardi, L. Compositions containing boric acid and a mixture of Lactobacillus. World Intellectual Property Organization (WIPO), International Patent WO 2015/173693 A1. 2015. Available online: https://patentimages.storage.googleapis.com/ce/b6/32/3bb205af4bcf7d/WO2015173693A1.pdf (accessed on 13 April 2022).
- Routray, I.; Ali, S. Boron induces lymphocyte proliferation and modulates the priming effects of lipopolysaccharide on macrophages. PLoS ONE 2016, 11, e0150607. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef]
- Hussain, S.A.; Abood, S.J.; Gorial, F.I. The adjuvant use of calcium fructoborate and borax with etanercept in patients with rheumatoid arthritis: Pilot study. J. Intercult. Ethnopharmacol. 2016, 6, 58–64. [Google Scholar] [CrossRef]
- Rogoveanu, O.C.; Mogoşanu, G.D.; Bejenaru, C.; Bejenaru, L.E.; Croitoru, O.; Neamţu, J.; Pietrzkowski, Z.; Reyes-Izquierdo, T.; Biţă, A.; Scorei, I.D.; et al. Effects of calcium fructoborate on levels of C-reactive protein, total cholesterol, low-density lipoprotein, triglycerides, IL-1β, IL-6, and MCP-1: A double-blind, placebo-controlled clinical study. Biol. Trace Elem. Res. 2015, 163, 124–131. [Google Scholar] [CrossRef][Green Version]
- Becker, R.A.; Bykov, Y.V. Boron preparations in psychiatry and neurology: Their rise, fall and renewed of interest. Acta Biomed. Scient. 2018, 3, 85–100. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Apdik, H.; Bayrak, O.F.; Gulluoglu, S.; Tuysuz, E.C.; Gusev, O.; Rizvanov, A.A.; Nikerel, E.; Şahin, F. A new hope for obesity management: Boron inhibits adipogenesis in progenitor cells through the Wnt/β-catenin pathway. Metabolism 2017, 69, 130–142. [Google Scholar] [CrossRef]
- Coban, F.K.; Liman, R.; Cigerci, I.H.; Ince, S.; Hazman, O.; Bozkurt, M.F. The antioxidant effect of boron on oxidative stress and DNA damage in diabetic rats. Fresenius Environ. Bull. 2015, 24, 4059–4066. Available online: https://www.researchgate.net/publication/286186112_The_antioxidant_effect_of_boron_on_oxidative_stress_and_DNA_damage_in_diabetic_rats (accessed on 13 April 2022).
- Hernandez, V.; Crépin, T.; Palencia, A.; Cusack, S.; Akama, T.; Baker, S.J.; Bu, W.; Feng, L.; Freund, Y.R.; Liu, L.; et al. Discovery of a novel class of boron-based antibacterials with activity against Gram-negative bacteria. Antimicrob. Agents Chemother. 2013, 57, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Meacham, S.L. Growing evidence for human health benefits of boron. J. Evid. Based Complement. Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- MacGillivray, P.C.; Fraser, M.S. Boric acid poisoning in infancy arising from the treatment of napkin rash. Arch. Dis. Child. 1953, 28, 484–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zan, R.; Hubbezoglu, I.; Ozdemir, A.K.; Tunc, T.; Sumer, Z.; Alici, O. Antibacterial effect of different concentration of boric acid against Enterococcus faecalis biofilms in root canal. Marmara Dent. J. 2013, 1, 76–80. Available online: https://dergipark.org.tr/en/pub/marudj/issue/17589/184279 (accessed on 13 April 2022).
- Sayin, Z.; Ucan, U.S.; Sakmanoglu, A. Antibacterial and antibiofilm effects of boron on different bacteria. Biol. Trace Elem. Res. 2016, 173, 241–246. [Google Scholar] [CrossRef]
- Kremer, D.; Pieters, T.T.; Verhaar, M.C.; Berger, S.P.; Bakker, S.J.L.; van Zuilen, A.D.; Joles, J.A.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am. J. Transplant. 2021, 21, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Vandeputte, D.; Joossens, M. Effects of low and high FODMAP diets on human gastrointestinal microbiota composition in adults with intestinal diseases: A systematic review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef] [PubMed]
- Pirouz, F.; Najafpour, G.; Jahanshahi, M.; Sharifzadeh Baei, M. Biodistribution of calcium fructoborate as a targeting agent for boron neutron capture therapy in an experimental model of MDA-MB-231 breast cancer cells. Biocatal. Agric. Biotechnol. 2019, 22, 101389. [Google Scholar] [CrossRef]
- Pirouz, F.; Najafpour, G.; Jahanshahi, M.; Sharifzadeh Baei, M. Plant-based calcium fructoborate as boron-carrying nanoparticles for neutron cancer therapy. Int. J. Eng. Trans. A Basics 2019, 32, 460–466. [Google Scholar] [CrossRef]
- Thibeault, S.A.; Fay, C.C.; Sauti, G.; Kang, J.H.; Park, C. Radiation shielding materials containing hydrogen, boron and nitrogen. World Intellectual Properties Organization (WIPO), International Publication No. WO 2013/074134 A1. 2013. Available online: https://patentimages.storage.googleapis.com/20/21/9c/973fbd372c482a/WO2013074134A1.pdf (accessed on 13 April 2022).
- Evcin, A.; Çelen, Y.Y.; Çiçek Bezir, N.; Ersoy, B. Use of ilmenite and boron waste as a radiation shielding panel. AIP Conf. Proc. 2018, 2042, 020001. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A. The potential use of boron containing resources for protection against nuclear radiation. Radiat. Phys. Chem. 2021, 188, 109601. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron chemistry for medical applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.S.; Zhu, Y.; Xie, H.; Hosmane, N.S.; Zhang, Y. Organoboron compounds: Effective antibacterial and antiparasitic agents. Molecules 2021, 26, 3309. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, J.; Hosmane, N.S.; Zhang, Y. Chapter 1—Introduction: Basic concept of boron and its physical and chemical properties. In Fundamentals and Applications of Boron Chemistry, 1st ed.; Zhu, Y., Hosmane, N.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 1–57. [Google Scholar] [CrossRef]
- Arora, S.; Chahal, D.S. Effect of soil properties on boron adsorption and release in arid and semi-arid benchmark soils. Commun. Soil Sci. Plant Anal. 2010, 41, 2532–2544. [Google Scholar] [CrossRef]
- Akkurt, İ.; Çanakciı, H.; Mavi, B.; Günoğlu, K. Natural radioactivity of boron added clay samples. AIP Conf. Proc. 2011, 1400, 512–517. [Google Scholar] [CrossRef]
- Moraetis, D.; Al Lamki, M.; Muhammad, D.; Yaroubi, S.; Al Batashi, H.; Pracejus, B. Boron content and sources in Tertiary aquifers in the Sultanate of Oman. In Proceedings of the 19th EGU General Assembly, Vienna, Austria, 23–28 April 2017; Available online: https://ui.adsabs.harvard.edu/abs/2017EGUGA..19.3180M/abstract (accessed on 13 April 2022).
- Bilgici Cengiz, G.; Öztanriöver, E. Analysis of natural radioactivity levels in soil samples and dose assessment for Digor district, Kars, Turkey. Caucasian J. Sci. 2018, 5, 30–39. Available online: https://dergipark.org.tr/en/pub/cjo/issue/38939/424149 (accessed on 13 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. https://doi.org/10.3390/ijms23169147
Biţă A, Scorei IR, Bălşeanu TA, Ciocîlteu MV, Bejenaru C, Radu A, Bejenaru LE, Rău G, Mogoşanu GD, Neamţu J, et al. New Insights into Boron Essentiality in Humans and Animals. International Journal of Molecular Sciences. 2022; 23(16):9147. https://doi.org/10.3390/ijms23169147
Chicago/Turabian StyleBiţă, Andrei, Ion Romulus Scorei, Tudor Adrian Bălşeanu, Maria Viorica Ciocîlteu, Cornelia Bejenaru, Antonia Radu, Ludovic Everard Bejenaru, Gabriela Rău, George Dan Mogoşanu, Johny Neamţu, and et al. 2022. "New Insights into Boron Essentiality in Humans and Animals" International Journal of Molecular Sciences 23, no. 16: 9147. https://doi.org/10.3390/ijms23169147
APA StyleBiţă, A., Scorei, I. R., Bălşeanu, T. A., Ciocîlteu, M. V., Bejenaru, C., Radu, A., Bejenaru, L. E., Rău, G., Mogoşanu, G. D., Neamţu, J., & Benner, S. A. (2022). New Insights into Boron Essentiality in Humans and Animals. International Journal of Molecular Sciences, 23(16), 9147. https://doi.org/10.3390/ijms23169147