Electrochemical and Ion Transport Studies of Li+ Ion-Conducting MC-Based Biopolymer Blend Electrolytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Results
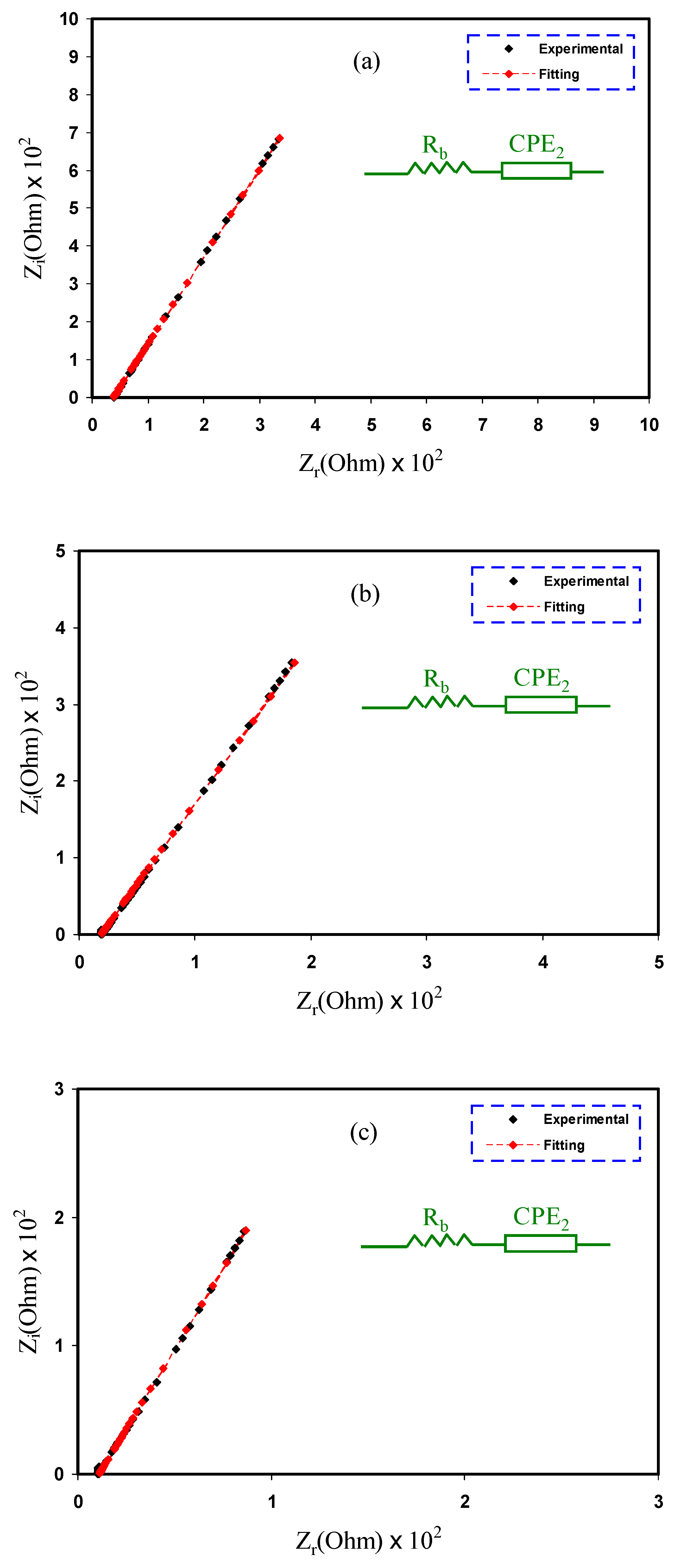
2.2. Impedance Study
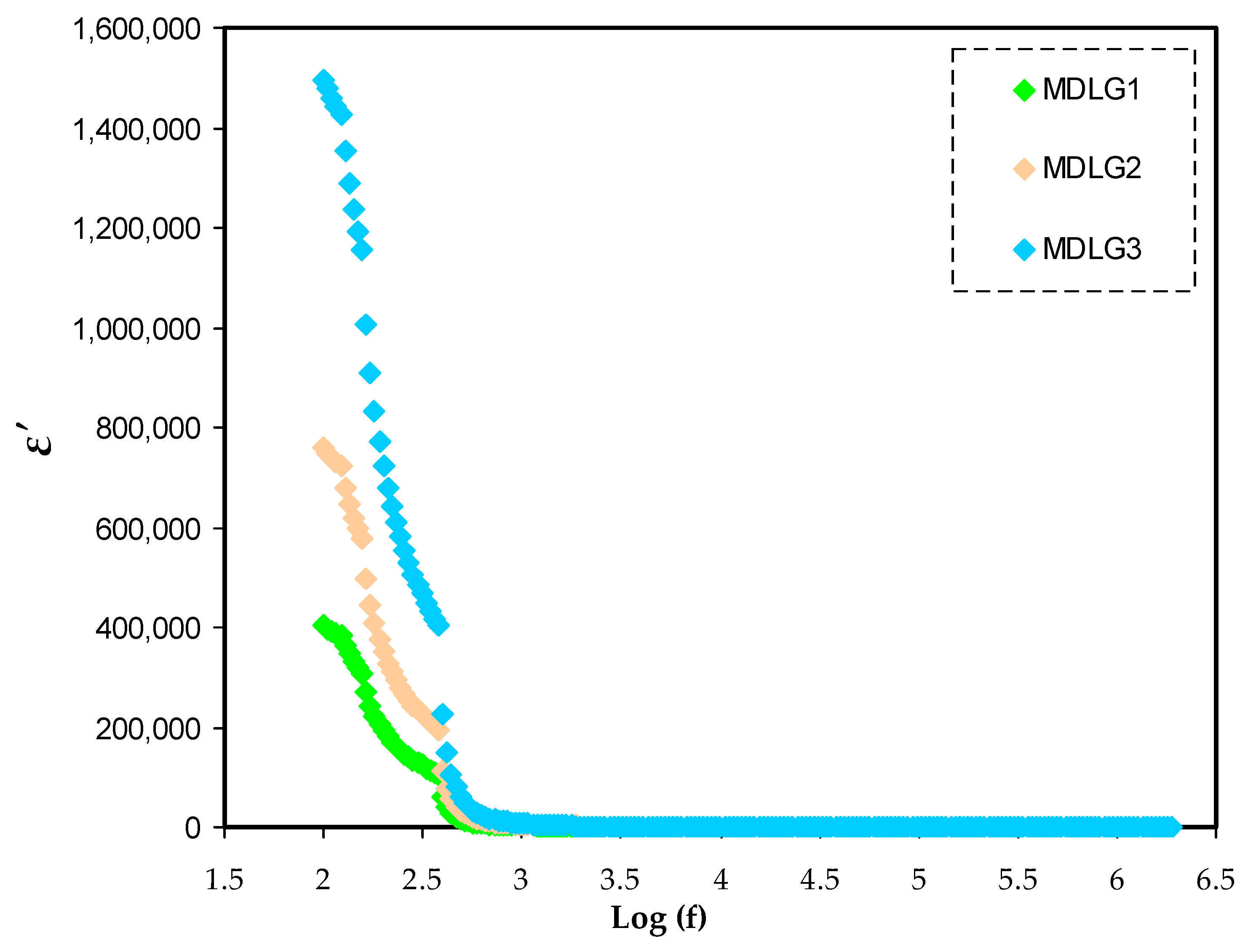
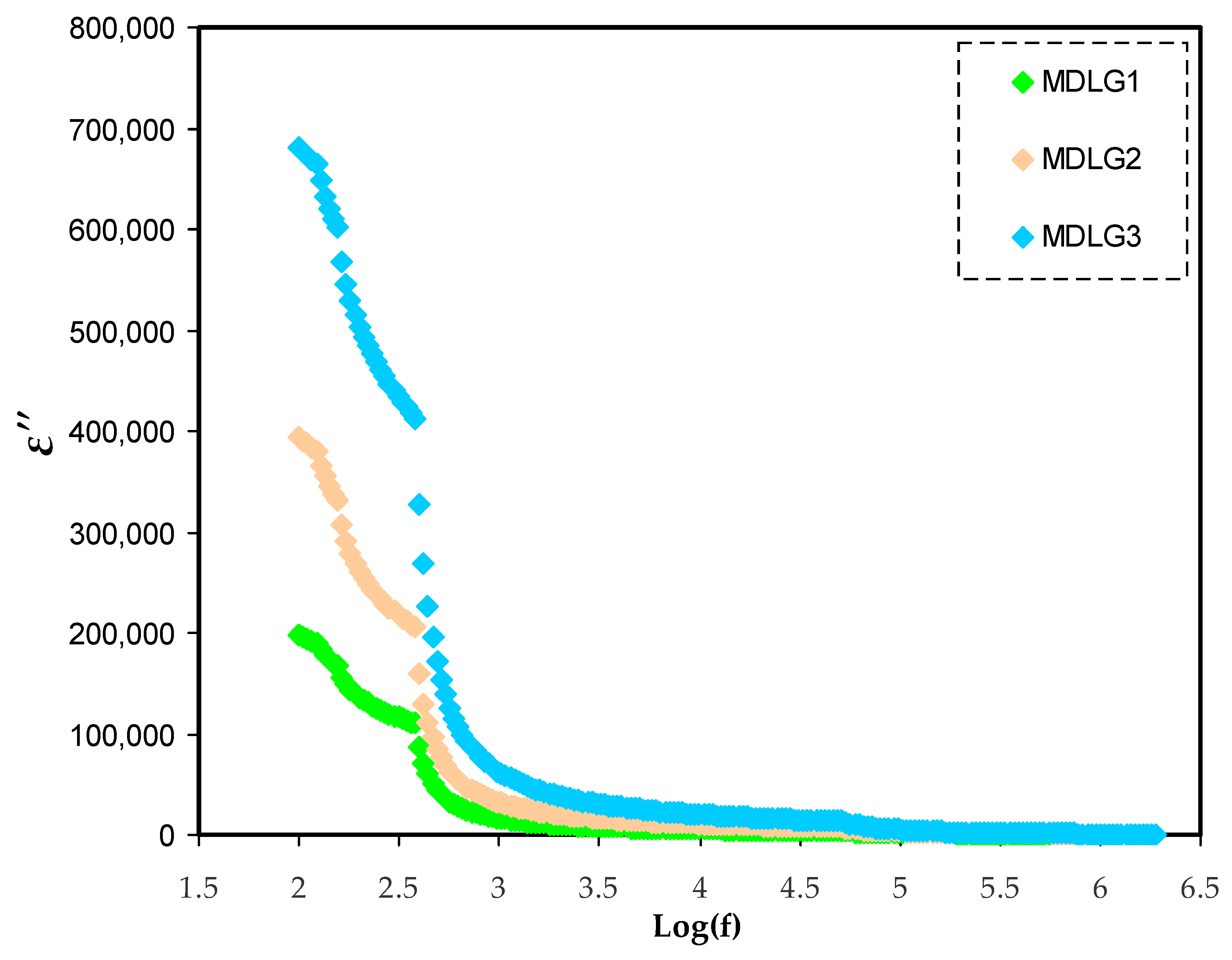
2.3. Dielectric Properties
2.4. TNM Study
3. Materials and Methods
3.1. Materials
3.2. Electrolyte Preparation
3.3. Methods of Characterizations
3.3.1. FTIR and EIS Measurements
3.3.2. TNM and LSV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadi, J.M.; Aziz, S.B.; Kadir, M.F.Z.; El-Badry, Y.A.; Ahamad, T.; Hussein, E.E.; Asnawi, A.S.F.M.; Abdullah, R.M.; Alshehri, S.M. Design of plasticized proton conducting Chitosan: Dextran based biopolymer blend electrolytes for EDLC application: Structural, impedance and electrochemical studies. Arab. J. Chem. 2021, 14, 103394. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Brza, M.A.; Kadir, M.F.Z.; Abdulwahid, R.T.; Ali Al-Asbahi, B.; Ahmed Ali Ahmed, A. Structural and energy storage behavior of ion conducting biopolymer blend electrolytes based on methylcellulose: Dextran polymers. Alex. Eng. J. 2022, 61, 9273–9285. [Google Scholar] [CrossRef]
- Alexandre, S.A.; Silva, G.G.; Santamaría, R.; Trigueiro, J.P.C.; Lavall, R.L. A highly adhesive PIL/IL gel polymer electrolyte for use in flexible solid state supercapacitors. Electrochim. Acta 2019, 299, 789–799. [Google Scholar] [CrossRef]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Ali, F. Structural, Ion Transport Parameter and Electrochemical Properties of Plasticized Polymer Composite Electrolyte Based on PVA: A Novel Approach to Fabricate High Performance EDLC Devices. Polym. Test. 2020, 91, 106813. [Google Scholar] [CrossRef]
- Nyuk, C.M.; Isa, M.I.N.M. Solid biopolymer electrolytes based on carboxymethyl cellulose for use in coin cell proton batteries. J. Sustain. Sci. Manag. 2017, 2017, 42–48. [Google Scholar]
- Salleh, N.S.; Aziz, S.B.; Aspanut, Z.; Kadir, M.F.Z. Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 2016, 22, 2157–2167. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Shukur, M.F.; Kadir, M.F.Z. Protonic cell performance employing electrolytes based on plasticized methylcellulose-potato starch-NH4NO3. Ionics 2019, 25, 559–572. [Google Scholar] [CrossRef]
- Stepniak, I.; Galinski, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisengang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors-preparation and characterization. RSC Adv. 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Preparation and characterization of phosphoric acid-doped hydroxyethyl cellulose electrolyte for use in supercapacitor. Mater. Renew. Sustain. Energy 2015, 4, 10. [Google Scholar] [CrossRef]
- Hassan, M.F.; Azimi, N.S.N.; Kamarudin, K.H.; Sheng, C.K. Solid polymer electrolytes based on starch-Magnesium Sulphate: Study on morphology and electrical conductivity. ASM Sci. J. 2018, 11, 17–28. [Google Scholar]
- Du, B.W.; Hu, S.Y.; Singh, R.; Tsai, T.T.; Lin, C.C.; Ko, F.H. Eco-friendly and biodegradable biopolymer chitosan/Y2O3 composite materials in flexible organic thin-film transistors. Materials 2017, 10, 1026. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Hemalatha, R.; Boopathi, G. Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J. Solid State Electrochem. 2018, 22, 3209–3223. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Kadir, M.F.Z. Dextran from Leuconostoc mesenteroides-doped ammonium salt-based green polymer electrolyte. Bull. Mater. Sci. 2019, 42, 57. [Google Scholar] [CrossRef]
- Nadirah, B.N.; Ong, C.C.; Saheed, M.S.M.; Yusof, Y.M.; Shukur, M.F. Structural and conductivity studies of polyacrylonitrile/methylcellulose blend based electrolytes embedded with lithium iodide. Int. J. Hydrogen Energy 2020, 45, 19590–19600. [Google Scholar] [CrossRef]
- Salman, Y.A.K.; Abdullah, O.G.; Hanna, R.R.; Aziz, S.B. Conductivity and electrical properties of chitosan-methylcellulose blend biopolymer electrolyte incorporated with lithium tetrafluoroborate. Int. J. Electrochem. Sci. 2018, 13, 3185–3199. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Coppola, L.; Nicotera, I. Composite gel polymer electrolytes based on organo-modified nanoclays: Investigation on lithium-ion transport and mechanical properties. Membranes 2018, 8, 69. [Google Scholar] [CrossRef]
- Gohel, K.; Kanchan, D.K. Ionic conductivity and relaxation studies in PVDF-HFP:PMMA-based gel polymer blend electrolyte with LiClO4 salt. J. Adv. Dielectr. 2018, 8, 1850005. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Salsamendi, M.; Nair, J.R.; Vygodskii, Y.S.; Mecerreyes, D.; Gerbaldi, C. Single-Ion Block Copoly(ionic liquid)s as Electrolytes for All-Solid State Lithium Batteries. ACS Appl. Mater. Interfaces 2016, 8, 10350–10359. [Google Scholar] [CrossRef]
- Mantravadi, R.; Chinnam, P.R.; Dikin, D.A.; Wunder, S.L. High Conductivity, High Strength Solid Electrolytes Formed by in Situ Encapsulation of Ionic Liquids in Nanofibrillar Methyl Cellulose Networks. ACS Appl. Mater. Interfaces 2016, 8, 13426–13436. [Google Scholar] [CrossRef]
- Weng, R.; Chen, L.; Lin, S.; Zhang, H.; Wu, H.; Liu, K.; Cao, S.; Huang, L. Preparation and characterization of antibacterial cellulose/chitosan nanofiltration membranes. Polymers 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.T.; Seifi-Aghjekohal, P. Sonocatalytic degradation of 2-hydroxyethyl cellulose in the presence of some nanoparticles. Ultrason. Sonochem. 2015, 26, 265–272. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Majid, S.R.; Arof, A.K. Transport studies of NH4NO3 doped methyl cellulose electrolyte. Synth. Met. 2010, 160, 1040–1044. [Google Scholar] [CrossRef]
- Pinotti, A.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Study on microstructure and physical properties of composite films based on chitosan and methylcellulose. Food Hydrocoll. 2007, 21, 66–72. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics 2017, 23, 1137–1154. [Google Scholar] [CrossRef]
- Kadir, M.; Hamsan, M. Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics 2018, 24, 2379–2398. [Google Scholar] [CrossRef]
- Yang, P.; Liu, L.; Li, L.; Hou, J.; Xu, Y.; Ren, X.; An, M.; Li, N. Gel polymer electrolyte based on polyvinylidenefluoride-co- hexafluoropropylene and ionic liquid for lithium ion battery. Electrochim. Acta 2014, 115, 454–460. [Google Scholar] [CrossRef]
- Amran, N.N.A.; Manan, N.S.A.; Kadir, M.F.Z. The effect of LiCF3SO3 on the complexation with potato starch-chitosan blend polymer electrolytes. Ionics 2016, 22, 1647–1658. [Google Scholar] [CrossRef]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef]
- Dumitra, M.; Meltze, V. Characterization of electron beam irradiated collagen-polyvinylpyrrolidone (PVP) and collagen-dextran (DEX) blends. Dig. J. Nanomater. Biostruct. 2011, 6, 1793–1803. [Google Scholar]
- Shukur, M.F.; Kadir, M.F.Z. Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 2015, 21, 111–124. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Brevik, I.; Hafiz, M.H.; Asnawi, A.S.; Yusof, Y.M.; Abdulwahid, R.T.; Kadir, M.F.Z. Blending and characteristics of electrochemical double-layer capacitor device assembled from plasticized proton ion conducting chitosan:Dextran:NH4PF6 polymer electrolytes. Polymers 2020, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Ndruru, S.T.C.L.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Preparation and characterization of biopolymer electrolyte membranes based on liclo4-complexed methyl cellulose as lithium-ion battery separator. J. Eng. Technol. Sci. 2020, 52, 28–50. [Google Scholar] [CrossRef]
- Hafiza, M.N.; Isa, M.I.N. Correlation between structural, ion transport and ionic conductivity of plasticized 2-hydroxyethyl cellulose based solid biopolymer electrolyte. J. Memb. Sci. 2020, 597, 117176. [Google Scholar] [CrossRef]
- Aziz, S.B.; Dannoun, E.M.A.; Murad, A.R.; Mahmoud, K.H.; Brza, M.A.; Nofal, M.M.; Elsayed, K.A.; Abdullah, S.N.; Hadi, J.M.; Kadir, M.F.Z. Influence of scan rate on CV Pattern: Electrical and electrochemical properties of plasticized Methylcellulose: Dextran (MC:Dex) proton conducting polymer electrolytes. Alex. Eng. J. 2022, 61, 5919–5937. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; MNofal, M.; Karim, W.O.; Brevik, I.; Brza, M.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F.Z. Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes. Polymers 2020, 12, 1411. [Google Scholar] [CrossRef]
- Shukur, M.F.; Azmi, M.S.; Zawawi, S.M.M.; Majid, N.A.; Illias, H.A.; Kadir, M.F.Z. Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys. Scr. 2013, 2013, 014049. [Google Scholar] [CrossRef]
- Mejenom, A.A.; Hafiza, M.N.; Isa, M.I.N. X-Ray diffraction and infrared spectroscopic analysis of solid biopolymer electrolytes based on dual blend carboxymethyl cellulose-chitosan doped with ammonium bromide. ASM Sci. J. 2018, 11, 37–46. [Google Scholar]
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hassan, A.N.; Ahmad, H.A.; Faidhalla, Y.A.; Kadir MF, Z. Structural, thermal, morphological and optical properties of PEO filled with biosynthesized Ag nanoparticles: New insights to band gap study. Results Phys. 2019, 13, 102220. [Google Scholar] [CrossRef]
- Pistorius, A.M.A.; DeGrip, W.J. Deconvolution as a tool to remove fringes from an FT-IR spectrum. Vib. Spectrosc. 2004, 36, 89–95. [Google Scholar] [CrossRef]
- Ramlli, M.A.; Bashirah, N.A.A.; Isa, M.I.N. Ionic Conductivity and Structural Analysis of 2-hyroxyethyl Cellulose Doped with Glycolic Acid Solid Biopolymer Electrolytes for Solid Proton Battery. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 440, p. 012038. [Google Scholar] [CrossRef]
- Xi, J.; Bai, Y.; Qiu, X.; Zhu, W.; Chen, L.; Tang, X. Conductivities and transport properties of microporous molecular sieves doped composite polymer electrolyte used for lithium polymer battery. New J. Chem. 2005, 29, 1454–1460. [Google Scholar] [CrossRef]
- Abarna, S.; Hirankumar, G. Electrical, dielectric and electrochemical studies on new Li ion conducting solid polymer electrolytes based on polyethylene glycol p-tert-octylphenyl ether. Polym. Sci. Ser. A 2017, 59, 660–668. [Google Scholar] [CrossRef]
- Aniskari, N.A.B.; Isa, M.I.N.M. The effect of ionic charge carriers in 2-hydroxyethyl cellulose solid biopolymer electrolytes doped glycolic acid via FTIR-deconvolution technique. J. Sustain. Sci. Manag. 2017, 12, 71–79. [Google Scholar]
- Kumar, M.; Tiwari, T.; Chauhan, J.K.; Srivastava, N. Erratum: UnderstanDing the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped Zwitterionic polymer system (Materials Research Express (2013) 1 (045003)). Mater. Res. Express 2014, 1, 045003. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Khairul, W.M.; Isa, M.I.N. Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J. Non. Cryst. Solids 2012, 358, 1104–1112. [Google Scholar] [CrossRef]
- Fonseca, C.P.; Cavalcante, F.; Amaral, F.A.; Souza, C.A.Z.; Neves, S. Thermal and conduction properties of a PCL-biodegradable gel polymer electrolyte with LiClO4, LiF3CSO3, and LiBF4 salts. Int. J. Electrochem. Sci. 2007, 2, 52–63. [Google Scholar]
- Misenan, M.; Khiar, A. Conductivity, Dielectric And Modulus Studies of Methylcellulose-NH 4 TF Polymer. Eurasian J. Biol. Chem. Sci. J. 2018, 1, 59–62. [Google Scholar]
- Teo, L.P.; Buraidah, M.H.; Nor, A.F.M.; Majid, S.R. Conductivity and dielectric studies of Li2SnO3. Ionics 2012, 18, 655–665. [Google Scholar] [CrossRef]
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z. Employing of Trukhan model to estimate ion transport parameters in PVA based solid polymer electrolyte. Polymers 2019, 11, 1694. [Google Scholar] [CrossRef]
- Lee, D.K.; Allcock, H.R. The effects of cations and anions on the ionic conductivity of poly[bis(2-(2-methoxyethoxy)ethoxy)phosphazene] doped with lithium and magnesium salts of trifluoromethanesulfonate and bis(trifluoromethanesulfonyl)imidate. Solid State Ionics 2010, 181, 1721–1726. [Google Scholar] [CrossRef]
- Awasthi, P.; Das, S. Reduced electrode polarization at electrode and analyte interface in impedance spectroscopy using carbon paste and paper. Rev. Sci. Instrum. 2019, 90, 124103. [Google Scholar] [CrossRef]
- Marf, A.S.; Aziz, S.B.; Abdullah, R.M. Plasticized H+ ion-conducting PVA:CS-based polymer blend electrolytes for energy storage EDLC application. J. Mater. Sci. Mater. Electron. 2020, 31, 18554–18568. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ali, F.; Anuar, H.; Ahamad, T.; Kareem, W.O.; Brza, M.A.; Kadir, M.F.Z.; Abu Ali, O.A.; Saleh, D.I.; Asnawi, A.S.F.M.; et al. Structural and electrochemical studies of proton conducting biopolymer blend electrolytes based on MC:Dextran for EDLC device application with high energy density. Alex. Eng. J. 2022, 61, 3985–3997. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hadi, J.M.; Elham, E.M.; Abdulwahid, R.T.; Saeed, S.R.; Marf, A.S.; Karim, W.O.; Kadir, M.F.Z. The study of plasticized amorphous biopolymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan with high ion conductivity for energy storage electrical double-layer capacitors (EDLC) device application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef]
- Uǧuz, H.; Goyal, A.; Meenpal, T.; Selesnick, I.W.; Baraniuk, R.G.; Kingsbury, N.G.; Haiter Lenin, A.; Mary Vasanthi, S.; Jayasree, T.; Adam, M.; et al. ce pte d M us pt. J. Phys. Energy 2020, 2, 1–31. [Google Scholar]
- Al-Omari, A.N.; Lear, K.L. Dielectric characteristics of spin-coated dielectric films using on-wafer parallel-plate capacitors at microwave frequencies. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 1151–1161. [Google Scholar] [CrossRef]
- Park, S.J.; Yoon, S.A.N.; Ahn, Y.H. Dielectric constant measurements of thin films and liquids using terahertz metamaterials. RSC Adv. 2016, 6, 69381–69386. [Google Scholar] [CrossRef]
- Anderson, L.; Jacob, M. Microwave characterization of a novel, environmentally friendly, plasma polymerized organic thin film. Phys. Procedia 2011, 14, 87–90. [Google Scholar] [CrossRef]
- Aziz, S.B.; Mamand, S.M.; Saed, S.R.; Abdullah, R.M.; Hussein, S.A. New Method for the Development of Plasmonic Metal-Semiconductor Interface Layer: Polymer Composites with Reduced Energy Band Gap. J. Nanomater. 2017, 2017, 060803. [Google Scholar] [CrossRef]
- Aziz, S.B.; Kadir, M.F.Z.; Hamsan, M.H.; Woo, H.J.; Brza, M.A. Development of Polymer Blends Based on PVA:POZ with Low Dielectric Constant for Microelectronic Applications. Sci. Rep. 2019, 9, 13163. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Saeed, S.R.; Brza, M.A.; Abdulwahid, R.T.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z.; Muzakir, S.K. Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes 2020, 10, 363. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Hamsan, M.H.; Abdulwahid, R.T.; Kadir, M.F.Z.; Ghareeb, H.O. Role of nano-capacitor on dielectric constant enhancement in PEO:NH4SCN:xCeO2 polymer nano-composites: Electrical and electrochemical properties. J. Mater. Res. Technol. 2020, 9, 9283–9294. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Ghareeb, H.O.; Hussein, S.A. Electrochemical Impedance study of Proton Conducting Polymer Electrolytes based on PVC Doped with Thiocyanate and Plasticized with Glycerol. Int. J. Electrochem. Sci. 2020, 15, 4671–4683. [Google Scholar] [CrossRef]
- Tamilselvi, P.; Hema, M. Structural, thermal, vibrational, and electrochemical behavior of lithium ion conducting solid polymer electrolyte based on poly(vinyl alcohol)/poly(vinylidene fluoride) blend. Polym. Sci. Ser. A 2016, 58, 776–784. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mohamed, P.A.; Kadir MF, Z.; Hamsan, M.H.; Abdulwahid, R.T.; Woo, H.J. Increase of metallic silver nanoparticles in Chitosan:AgNt based polymer electrolytes incorporated with alumina filler. Results Phys. 2019, 13, 102326. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x−1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30–46. [Google Scholar] [CrossRef]
- Khiar, A.S.A.; Anuar, M.R.S.; Parid, M.A.M. Effect of 1-ethyl-3-methylimidazolium nitrate on the electrical properties of starch/chitosan blend polymer electrolyte. Mater. Sci. Forum 2016, 846, 510–516. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Abidin, Z.H.Z. Optical and Electrical Characteristics of Silver Ion Conducting Nanocomposite Solid Polymer Electrolytes Based on Chitosan. J. Electron. Mater. 2017, 46, 6119–6130. [Google Scholar] [CrossRef]
- Aziz, S.B.; Asnawi, A.S.F.M.; Abdulwahid, R.T.; Ghareeb, H.O.; Alshehri, S.M.; Ahamad, T.; Hadi, J.M.; Kadir, M.F.Z. Design of potassium ion conducting PVA based polymer electrolyte with improved ion transport properties for EDLC device application. J. Mater. Res. Technol. 2021, 13, 933–946. [Google Scholar] [CrossRef]
- Nofal, M.M.; Aziz, S.B.; Brza, M.A.; Abdullah, S.N.; Dannoun, E.M.A.; Hadi, J.M.; Murad, A.R.; Al-Saeedi, S.I.; Kadir, M.F.Z. Studies of Circuit Design, Structural, Relaxation and Potential Stability of Polymer Blend Electrolyte Membranes Based on PVA:MC Impregnated with NH4I Salt. Membranes 2022, 12, 284. [Google Scholar] [CrossRef]
- Nofal, M.M.; Hadi, J.M.; Aziz, S.B.; Brza, M.A.; Asnawi, A.S.F.M.; Dannoun, E.M.A.; Abdullah, A.M.; Kadir, M.F.Z. A Study of Methylcellulose Based Polymer Electrolyte Impregnated with Potassium Ion Conducting Carrier: Impedance, EEC Modeling, FTIR, Dielectric, and Device Characteristics. Materials 2021, 14, 4859. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Nofal, M.M.; Hussein, S.A.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z.; Woo, H.J. Electrical, Dielectric Property and Electrochemical Performances of Plasticized Silver Ion-Conducting Chitosan-Based Polymer Nanocomposites. Membranes 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Dannoun, E.M.A.; Hamsan, M.H.; Ghareeb, H.O.; Nofal, M.M.; Karim, W.O.; Asnawi, A.S.F.M.; Hadi, J.M.; Kadir, M.F.Z.A. A Polymer Blend Electrolyte Based on CS with Enhanced Ion Transport and Electrochemical Properties for Electrical Double Layer Capacitor Applications. Polymers 2021, 13, 930. [Google Scholar] [CrossRef] [PubMed]
- Shuhaimi, N.E.A.; Alias, N.A.; Majid, S.R.; Arof, A.K. Electrical Double Layer Capacitor With Proton Conducting Κ-Carrageenan–Chitosan Electrolytes. Funct. Mater. Lett. 2009, 1, 195–201. [Google Scholar] [CrossRef]
- Mazuki, N.; Majeed, A.P.P.A.; Samsudin, A.S. Study on electrochemical properties of CMC-PVA doped NH4Br based solid polymer electrolytes system as application for EDLC. J. Polym. Res. 2020, 27, 135. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Kadir, M.F.Z.; Dannoun, E.M.A.; Brza, M.A.; Hadi, J.M.; Abdullah, R.M. Bio-Based Plasticized PVA Based Polymer Blend Electrolytes for Energy Storage EDLC Devices: Ion Transport Parameters and Electrochemical Properties. Materials 2021, 14, 1994. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Abdulwahid, R.T.; Kadir, M.F.Z.; Hadi, J.M.; Hessien, M.M.; Kareem, W.O.; Dannoun, E.M.A.; Saeed, S.R. Impedance, FTIR and transport properties of plasticized proton conducting biopolymer electrolyte based on chitosan for electrochemical device application. Results Phys. 2021, 29, 104770. [Google Scholar] [CrossRef]
- Böckenfeld, N.; Willeke, M.; Pires, J.; Anouti, M.; Balducci, A. On the Use of Lithium Iron Phosphate in Combination with Protic Ionic Liquid-Based Electrolytes. J. Electrochem. Soc. 2013, 160, A559–A563. [Google Scholar] [CrossRef]

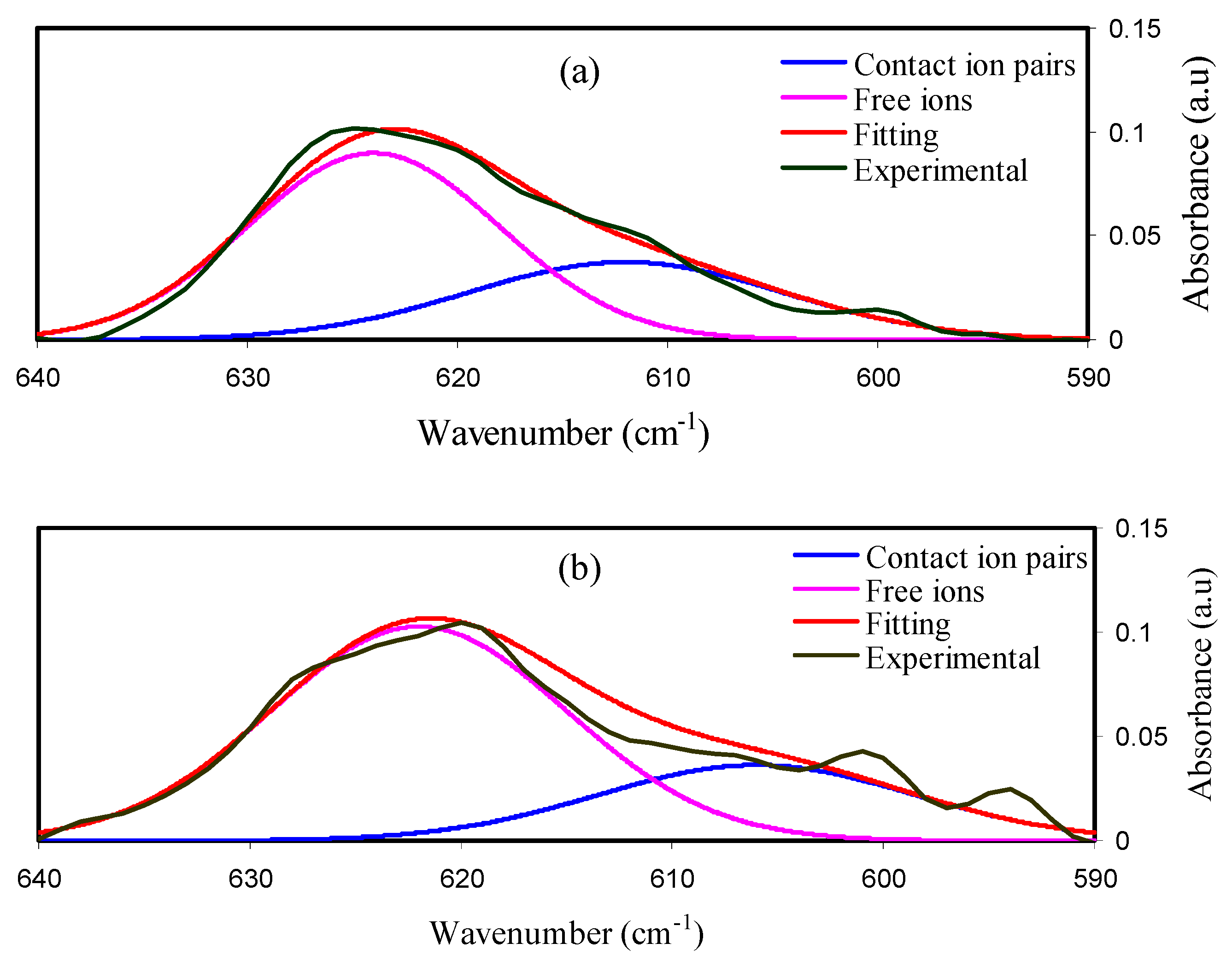
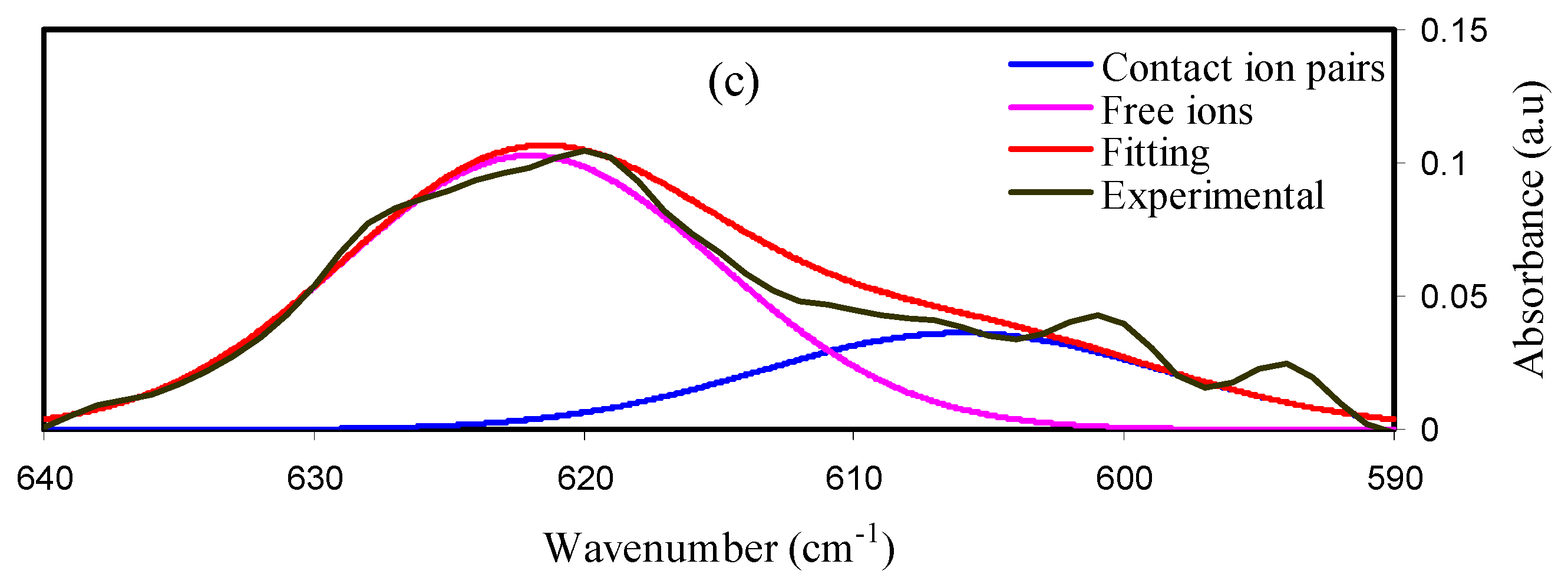


| Sample | FI% | CIP% |
|---|---|---|
| MDLG1 | 65.85% | 34.14% |
| MDLG2 | 72.58% | 27.42% |
| MDLG3 | 76.95% | 23.05% |
| Glycerol % | n (cm−3) | µ (cm2 V−1 s) | D (cm2 s−1) |
|---|---|---|---|
| MDLG1 | 2.32 × 1022 | 4.0 × 10−8 | 1.04 × 10−9 |
| MDLG2 | 5.92 × 1022 | 1.09 × 10−7 | 2.83 × 10−9 |
| MDLG3 | 1.13 × 1023 | 1.10 × 10−7 | 2.86 × 10−9 |
| Sample | K (F−1) | CPE (F) | Rb (Ohm) | Conductivity (S cm−1) |
|---|---|---|---|---|
| MDLG1 | 5.21 × 104 | 1.92 × 10−5 | 3.80 × 101 | 3.93 × 10−4 |
| MDLG2 | 2.45 × 104 | 4.08 × 10−5 | 1.89 × 101 | 8.16 × 10−4 |
| MDLG3 | 1.59 × 104 | 6.29 × 10−5 | 1.10 × 101 | 1.45 × 10−3 |
| Sample | D (cm2 s−1) | µ (cm2 V−1 s) | n (cm−3) |
|---|---|---|---|
| MDLG1 | 1.72 × 10−7 | 6.71 × 10−6 | 3.65 × 1020 |
| MDLG2 | 1.88 × 10−7 | 7.33 × 10−6 | 6.95 × 1020 |
| MDLG3 | 2.64 × 10−7 | 1.03 × 10−5 | 8.80 × 1020 |
| Sample Code | MC (g) | Dex (g) | LiClO4 (g) | Glycerol (g) | Glycerol wt.% |
|---|---|---|---|---|---|
| MDLG1 | 0.6 | 0.4 | 0.666 | 0.271 | 14 |
| MDLG2 | 0.6 | 0.4 | 0.666 | 0.647 | 28 |
| MDLG3 | 0.6 | 0.4 | 0.666 | 1.206 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dannoun, E.M.A.; Aziz, S.B.; Brza, M.A.; Al-Saeedi, S.I.; Nofal, M.M.; Mishra, K.; Abdullah, R.M.; Karim, W.O.; Hadi, J.M. Electrochemical and Ion Transport Studies of Li+ Ion-Conducting MC-Based Biopolymer Blend Electrolytes. Int. J. Mol. Sci. 2022, 23, 9152. https://doi.org/10.3390/ijms23169152
Dannoun EMA, Aziz SB, Brza MA, Al-Saeedi SI, Nofal MM, Mishra K, Abdullah RM, Karim WO, Hadi JM. Electrochemical and Ion Transport Studies of Li+ Ion-Conducting MC-Based Biopolymer Blend Electrolytes. International Journal of Molecular Sciences. 2022; 23(16):9152. https://doi.org/10.3390/ijms23169152
Chicago/Turabian StyleDannoun, Elham M. A., Shujahadeen B. Aziz, Mohamad A. Brza, Sameerah I. Al-Saeedi, Muaffaq M. Nofal, Kuldeep Mishra, Ranjdar M. Abdullah, Wrya O. Karim, and Jihad M. Hadi. 2022. "Electrochemical and Ion Transport Studies of Li+ Ion-Conducting MC-Based Biopolymer Blend Electrolytes" International Journal of Molecular Sciences 23, no. 16: 9152. https://doi.org/10.3390/ijms23169152
APA StyleDannoun, E. M. A., Aziz, S. B., Brza, M. A., Al-Saeedi, S. I., Nofal, M. M., Mishra, K., Abdullah, R. M., Karim, W. O., & Hadi, J. M. (2022). Electrochemical and Ion Transport Studies of Li+ Ion-Conducting MC-Based Biopolymer Blend Electrolytes. International Journal of Molecular Sciences, 23(16), 9152. https://doi.org/10.3390/ijms23169152










