Transcriptome Profiling and Expression Localization of Key Sex-Related Genes in a Socially-Controlled Hermaphroditic Clownfish, Amphiprion clarkii
Abstract
1. Introduction
2. Results
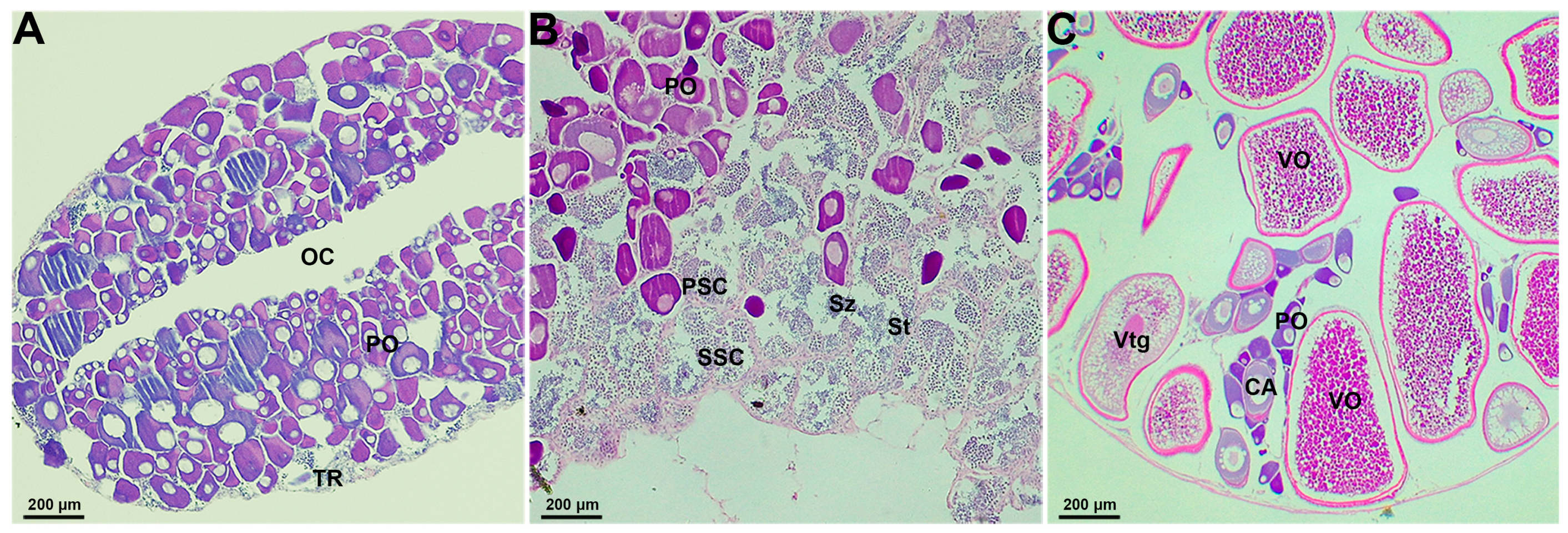
2.1. Gonadal Histological Characteristics in the Socially-Controlled Groups of A. clarkii
2.2. Sequencing, Assembly, and Preliminary Analysis of Gonadal Transcriptomes
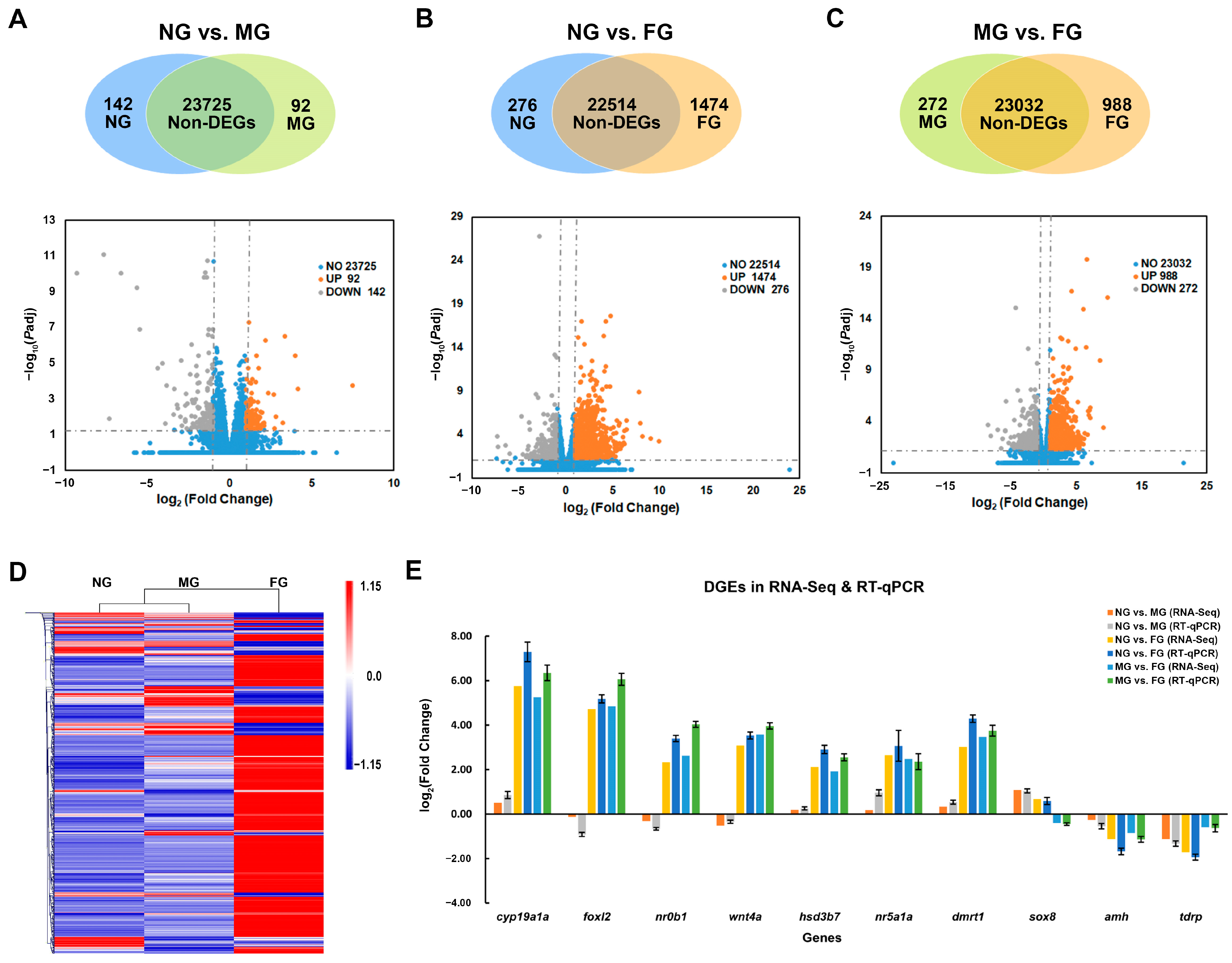
2.3. Identification and Validation of DEGs
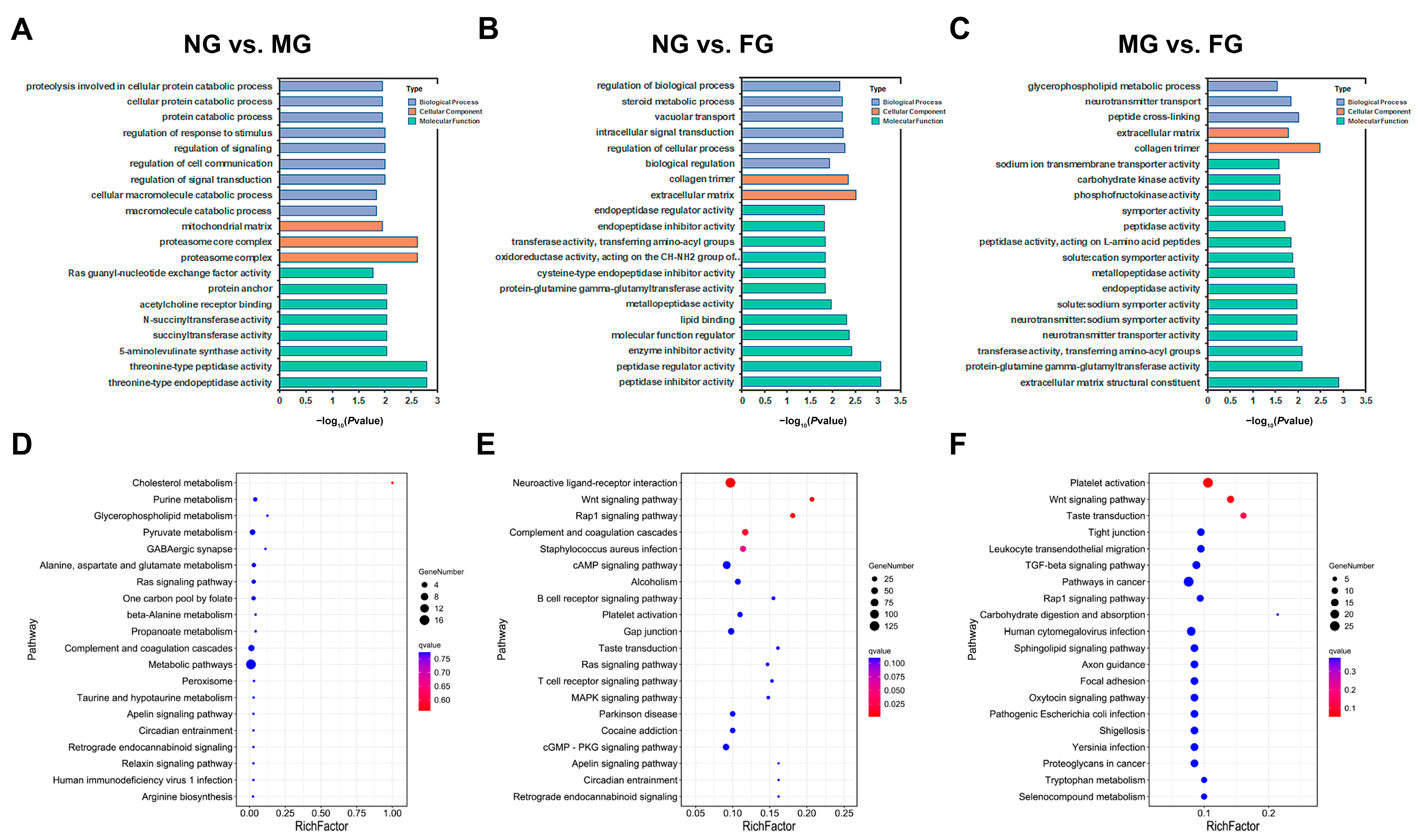
2.4. GO and KEGG Functional Enrichment of DEGs
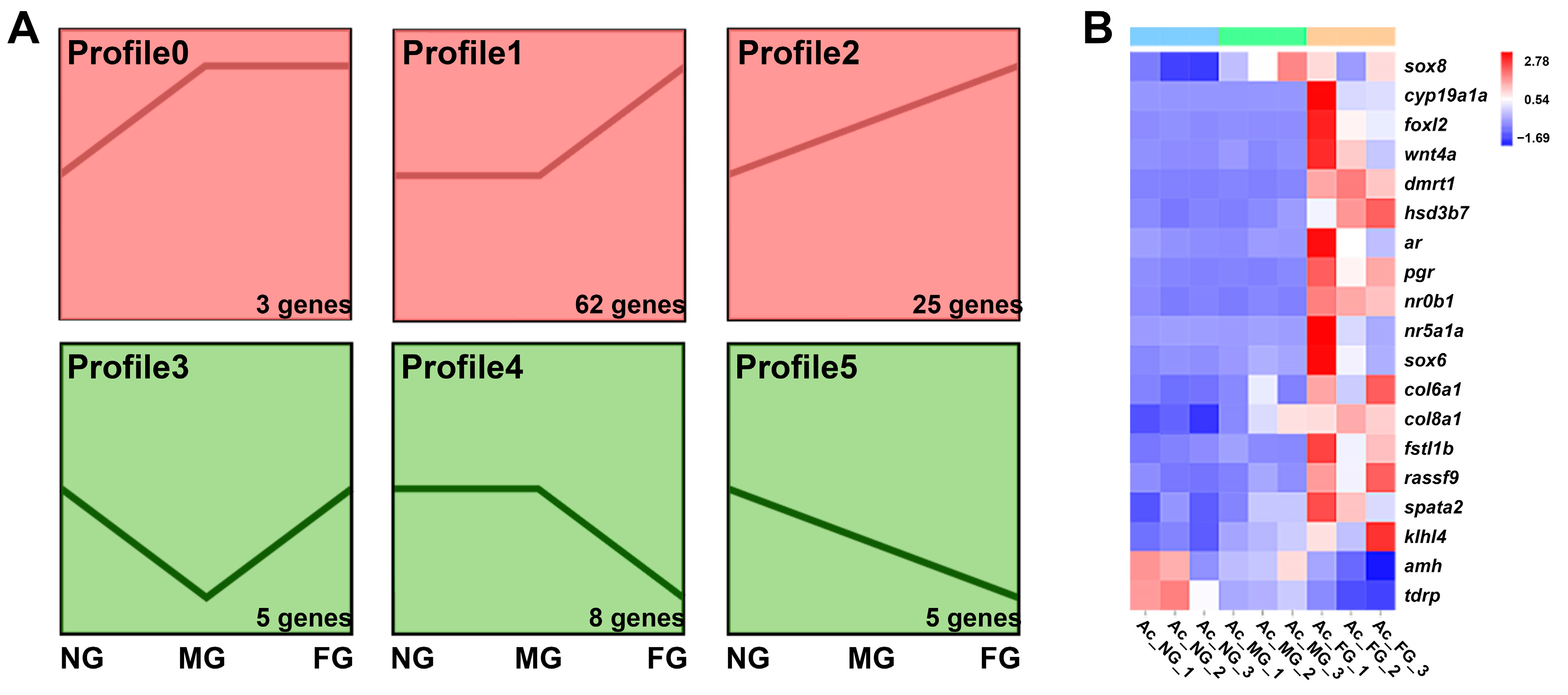
2.5. Screening and Profiling Analysis of Sex-Related Genes
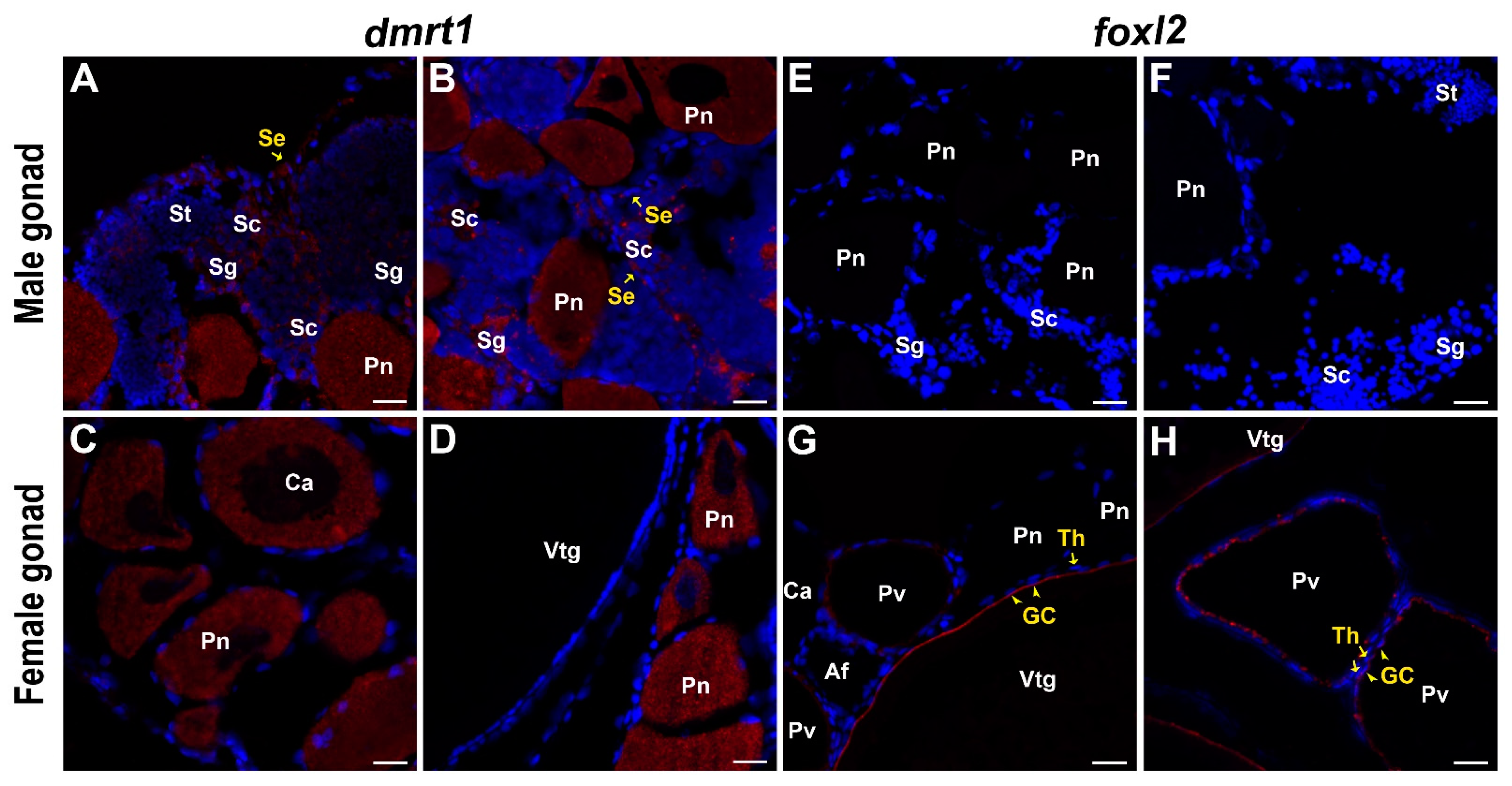
2.6. Molecular Characterization and Expression Pattern of dmrt1
2.7. Molecular Characterization and Expression Pattern of foxl2
3. Discussion
3.1. Transcriptome Analysis Revealed Similar Gene Expression Profiles between Nonbreeders and Males, Which Supported by Gonadal Histology
3.2. The Importance of Ovarian Steroidogenesis and the Central Role of Aromatase in the Female Sex Differentiation of Clownfish
3.3. Sox8 and Amh Are Potential Key Factors That May Regulate Male Gonad Development in Clownfish
3.4. The Special Expression Pattern of dmrt1 Implies Its Potential Function in Oocytes of Clownfish
3.5. The Higher Expression Levels of ar and nr0b1 in Females Imply That Androgens Might Be Closely Related to the Social Status Maintenance of Clownfish
3.6. Novel Function of Candidate Genes in the Context of Sexual Development
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Gonadal Histological Analysis
4.3. cDNA Library Construction and Sequencing
4.4. Quality Control and Transcriptome Assembly
4.5. Identification of DEGs
4.6. Quantitative Real-Time PCR (RT-qPCR) Verification
4.7. Sex-Related Gene Sequence Confirmation and In Situ Hybridization (ISH) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality; Routledge: London, UK, 2019. [Google Scholar]
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Tree of Sex Consortium. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899. [Google Scholar] [CrossRef] [PubMed]
- De Mitcheson, Y.S.; Liu, M. Functional hermaphroditism in teleosts. Fish Fish. 2008, 9, 1–43. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: http://www.fishbase.org (accessed on 12 March 2022).
- Miura, S.; Komatsu, T.; Higa, M.; Bhandari, R.K.; Nakamura, S.; Nakamura, M. Gonadal sex differentiation in protandrous anemone fish, Amphiprion clarkii. Fish Physiol. Biochem. 2003, 28, 165–166. [Google Scholar] [CrossRef]
- Nakamura, M.; Miura, S.; Nozu, R.; Kobayashi, Y. Opposite-directional sex change in functional female protandrous anemonefish, Amphiprion clarkii: Effect of aromatase inhibitor on the ovarian tissue. Zool. Lett. 2015, 1, 30. [Google Scholar] [CrossRef][Green Version]
- Fricke, H.; Fricke, S. Monogamy and sex change by aggressive dominance in coral reef fish. Nature 1977, 266, 830–832. [Google Scholar] [CrossRef]
- Ochi, H. Mating behavior and sex change of the anemonefish, Amphiprion clarkii, in the temperate waters of southern Japan. Environ. Biol. Fishes 1989, 26, 257–275. [Google Scholar] [CrossRef]
- Mitchell, J.S. Social correlates of reproductive success in false clown anemonefish: Subordinate group members do not pay-to-stay. Evol. Ecol. Res. 2003, 5, 89–104. [Google Scholar]
- Buston, P.M.; Cant, M.A. A new perspective on size hierarchies in nature: Patterns, causes, and consequences. Oecologia 2006, 149, 362–372. [Google Scholar] [CrossRef]
- Buston, P. Size and growth modification in clownfish. Nature 2003, 424, 145–146. [Google Scholar] [CrossRef]
- Hattori, A.; Yanagisawa, Y. Life-history pathways in relation to gonadal sex differentiation in the anemonefish, Amphiprion clarkii, in temperate waters of Japan. Environ. Biol. Fishes 1991, 31, 139–155. [Google Scholar] [CrossRef]
- Ochi, H. Acquisition of breeding space by nonbreeders in the anemonefish Amphiprion clarkii in temperate waters of southern Japan. Ethology 1989, 83, 279–294. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A.; et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin-I, T.; Kohara, Y.; Kuroki, Y.; Toyoda, A.; Fujiyama, A.; et al. Co-option of Sox3 as the male determining factor on the y chromosome in the fish Oryzias dancena. Nat. Commun. 2014, 5, 4157. [Google Scholar] [CrossRef]
- Myosho, T.; Otake, H.; Masuyama, H.; Matsuda, M.; Kuroki, Y.; Fujiyama, A.; Naruse, K.; Hamaguchi, S.; Sakaizumi, M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 2012, 191, 163–170. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Kamiya, T.; Kai, W.; Tasumi, S.; Oka, A.; Matsunaga, T.; Mizuno, N.; Fujita, M.; Suetake, H.; Suzuki, S.; Hosoya, S.; et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 2012, 8, e1002798. [Google Scholar] [CrossRef]
- Koyama, T.; Nakamoto, M.; Morishima, K.; Yamashita, R.; Yamashita, T.; Sasaki, K.; Kuruma, Y.; Mizuno, N.; Suzuki, M.; Okada, Y.; et al. A SNP in a steroidogenic enzyme is associated with phenotypic sex in Seriola Fishes. Curr. Biol. 2019, 29, 1901–1909. [Google Scholar] [CrossRef]
- Casas, L.; Saborido-Rey, F. Environmental cues and mechanisms underpinning sex change in Fish. Sex. Dev. 2021, 15, 108–121. [Google Scholar] [CrossRef]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef] [PubMed]
- Bruslé-Sicard, S.; Reinboth, R. Protandric hermaphrodite peculiarities in Amphiprion frenatus Brevoort (Teleostei, Pomacentridae). J. Fish Biol. 1990, 36, 383–390. [Google Scholar] [CrossRef]
- Nakamura, M.; Mariko, T.; Nagahama, Y. Ultrastructure and in vitro steroidogenesis of the gonads in the protandrous anemonefish Amphiprion frenatus. Jpn. J. Ichthyol. 1994, 41, 47–56. [Google Scholar]
- Fricke, H.W. Social control of sex: Field experiments with the anemonefish Amphiprion bicinctus. Z. Für Tierpsychol. 1983, 61, 71–77. [Google Scholar] [CrossRef]
- Mills, S.C.; O’Donnell, J.L.; Bernardi, G.; Beldade, R. Natural endocrine profiles of the group-living skunk anemonefish Amphiprion akallopisos in relation to their size-based dominance hierarchy. J. Fish Biol. 2018, 92, 773–789. [Google Scholar] [CrossRef]
- Godwin, J.R.; Thomas, P. Sex change and steroid profiles in the protandrous anemonefish Amphiprion melanopus (Pomacentridae, Teleostei). Gen. Comp. Endocrinol. 1993, 91, 144–157. [Google Scholar] [CrossRef]
- Fricke, H.W. Mating system, resource defence and sex change in the anemonefish Amphiprion akallopisos. Z. Für Tierpsychol. 1979, 50, 313–326. [Google Scholar] [CrossRef]
- Madhu, R.; Madhu, K.; Venugopal, K.M. Sex change of hatchery produced Amphiprion ocellaris: Influence of mating system removal on gonad maturation and nesting success. J. Mar. Biol. Assoc. India 2010, 52, 62–69. [Google Scholar]
- Zhang, H.; Zhang, Y.; Guo, Y.; Zhang, X.; Wang, Q.; Liu, X.; Lin, H. Kiss2 but not kiss1 is involved in the regulation of social stress on the gonad development in yellowtail clownfish, Amphiprion clarkii. Gen. Comp. Endocrinol. 2020, 298, 113551. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Horiguchi, R.; Miura, S.; Nakamura, M. Sex- and tissue-specific expression of P450 aromatase (cyp19a1a) in the yellowtail clownfish, Amphiprion clarkii. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 237–244. [Google Scholar] [CrossRef]
- Casas, L.; Saborido-Rey, F.; Ryu, T.; Michell, C.; Ravasi, T.; Irigoien, X. Sex change in clownfish: Molecular insights from transcriptome analysis. Sci. Rep. 2016, 6, 35461. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Saenz-Agudelo, P.; Irigoien, X. High-throughput sequencing and linkage mapping of a clownfish genome provide insights on the distribution of molecular players involved in sex change. Sci. Rep. 2018, 8, 4073. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lin, B.; Li, G.; Chen, H.; Liu, M. Sequencing and transcriptome analysis for reproduction-related genes identification and SSRs discovery in sequential hermaphrodite Amphiprion ocellaris. Turk. J. Fish. Aquat. Sci. 2018, 19, 1049–1059. [Google Scholar]
- Li, Y.; Huang, J.; Liu, Z.; Zhou, Y.; Xia, B.; Wang, Y.; Kang, Y.; Wang, J. Transcriptome analysis provides insights into hepatic responses to moderate heat stress in the rainbow trout (Oncorhynchus mykiss). Gene 2017, 619, 1–9. [Google Scholar] [CrossRef]
- Martin, J.A.; Wang, Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011, 12, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Austin, C.M.; Hammer, M.P.; Lee, Y.P.; Croft, L.J.; Gan, H.M. Finding Nemo: Hybrid assembly with Oxford Nanopore and Illumina reads greatly improves the clownfish (Amphiprion ocellaris) genome assembly. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szczesniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Liu, H.; Lamm, M.S.; Rutherford, K.; Black, M.A.; Godwin, J.R.; Gemmell, N.J. Large-scale transcriptome sequencing reveals novel expression patterns for key sex-related genes in a sex-changing fish. Biol. Sex Differerces 2015, 6, 26. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, J.; Zhang, Z.; Huang, Y.; Ruan, Z.; Chen, S.; Zhu, F.; You, X.; Jia, C.; Meng, Q.; et al. A comparative transcriptomic study on developmental gonads provides novel insights into sex change in the protandrous black porgy (Acanthopagrus schlegelii). Genomics 2019, 111, 277–283. [Google Scholar] [CrossRef]
- Böhne, A.; Sengstag, T.; Salzburger, W. Comparative transcriptomics in East African cichlids reveals sex- and species-specific expression and new candidates for sex differentiation in fishes. Genome Biol. Evol. 2014, 6, 2567–2585. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Feng, B.; Zhang, Z.; Wang, R.; Tang, L.; Li, W.; Li, Q.; Piferrer, F.; Shao, C. Transcriptome of Gonads From High Temperature Induced Sex Reversal During Sex Determination and Differentiation in Chinese Tongue Sole, Cynoglossus semilaevis. Front. Genet. 2019, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.; Zhou, Q.; Bachtrog, D. Sex-biased transcriptome evolution in Drosophila. Genome Biol. Evol. 2012, 4, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H.; Parsch, J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 2007, 8, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; Carney, G.E.; Mo, Q.; Vannucci, M.; Jones, A.G. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genom. 2009, 10, 579. [Google Scholar] [CrossRef]
- Khoo, M.L.; Das, S.K.; Ghaffar, M.A. Growth pattern, diet and reproductive biology of the clownfish Amphiprion ocellaris in waters of Pulau Tioman, Malaysia. Egypt. J. Aquat. Res. 2018, 44, 233–239. [Google Scholar] [CrossRef]
- Miura, S.; Horiguchi, R.; Nakamura, M. Immunohistochemical evidence for 11beta-hydroxylase (P45011beta) and androgen production in the gonad during sex differentiation and in adults in the protandrous anemonefish Amphiprion clarkii. Zool. Sci. 2008, 25, 212–219. [Google Scholar] [CrossRef]
- Hattori, A. Socially controlled growth and size-dependent sex change in the anemonefish Amphiprion frenatus in Okinawa, Japan. Jpn. J. Ichthyol. 1991, 38, 165–177. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Piferrer, F.; Blázquez, M. Aromatase distribution and regulation in fish. Fish Physiol. Biochem. 2005, 31, 215–226. [Google Scholar] [CrossRef]
- Siegfried, K.R. In search of determinants: Gene expression during gonadal sex differentiation. J. Fish Biol. 2010, 76, 1879–1902. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, L.; Li, Z.; Gui, J.F. Expression pattern, cellular localization and promoter activity analysis of ovarian aromatase (Cyp19a1a) in protogynous hermaphrodite red-spotted grouper. Mol. Cell. Endocrinol. 2009, 307, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Kobayashi, T.; Zhou, L.Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.; Nagahama, Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yamaguchi, S.; Hirai, T.; Kitano, T. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 2007, 359, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Auguste, A.; Bessiere, L.; Vanet, A.; Todeschini, A.L.; Veitia, R.A. FOXL2: A central transcription factor of the ovary. J. Mol. Endocrinol. 2014, 52, R17–R33. [Google Scholar] [CrossRef]
- Yao, H.H.; Matzuk, M.M.; Jorgez, C.J.; Menke, D.B.; Page, D.C.; Swain, A.; Capel, B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev. Dyn. 2004, 230, 210–215. [Google Scholar] [CrossRef]
- Milla, S.; Wang, N.; Mandiki, S.N.M.; Kestemont, P. Corticosteroids: Friends or foes of teleost fish reproduction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Takada, S.; Kennedy, C.L.; Scott, G.; Harada, S.; Ray, M.K.; Dai, Q.; Wilhelm, D.; de Kretser, D.M.; Eddy, E.M.; et al. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev. Biol. 2008, 316, 359–370. [Google Scholar] [CrossRef]
- Xia, X.; Zhao, J.; Du, Q.; Chang, Z. cDNA cloning and expression analysis of two distinct Sox8 genes in Paramisgurnus dabryanus (Cypriniformes). J. Genet. 2010, 89, 183–192. [Google Scholar] [CrossRef]
- Xia, X.; Zhao, J.; Du, Q.; Chang, Z. Isolation and expression of two distinct Sox8 genes in mudloach (Misgurnus anguillicaudatus). Biochem. Genet. 2011, 49, 161–176. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Li, X.; Ni, F.; Sun, M.; Zhang, Q.; Yu, H.; Wang, X. The evolution and possible role of two Sox8 genes during sex differentiation in Japanese flounder (Paralichthys olivaceus). Mol Reprod Dev. 2019, 86, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish--Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Todd, E.V.; Goikoetxea, A.; Ortega-Recalde, O.; Hore, T.A. Natural sex change in fish. Curr. Top. Dev. Biol. 2019, 134, 71–117. [Google Scholar]
- Skaar, K.S.; Nóbrega, R.H.; Magaraki, A.; Olsen, L.C.; Schulz, R.W.; Male, R. Proteolytically activated, recombinant anti-müllerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology 2011, 152, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wilken, J.; Phillips, N.B.; Narendra, U.; Chan, G.; Stratton, S.M.; Kent, S.B.; Weiss, M.A. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 2000, 14, 1750–1764. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Chiu, P.C.; Lin, C.J.; Lyu, Y.S.; Lan, D.S.; Chang, C.F. Testicular dmrt1 is involved in the sexual fate of the ovotestis in the protandrous black porgy. Biol. Reprod. 2012, 86, 41. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, H.; Huang, X.; Gao, S.; Yu, H.; Zhou, R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem. Biophys. Res. Commun. 2005, 330, 950–957. [Google Scholar] [CrossRef]
- Johnsen, H.; Seppola, M.; Torgersen, J.S.; Delghandi, M.; Andersen, Ø. Sexually dimorphic expression of dmrt1 in immature and mature Atlantic cod (Gadus morhua L.). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 197–205. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Shui, Y.; Gao, S.; Yu, H.; Cheng, H.; Zhou, R. Multiple alternative splicing and differential expression of dmrt1 during gonad transformation of the rice field eel. Biol. Reprod. 2005, 73, 1017–1024. [Google Scholar] [CrossRef]
- Guan, G.; Kobayashi, T.; Nagahama, Y. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the Tilapia (Oreochromis niloticus). Biochem. Biophys. Res. Commun. 2000, 272, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Cao, X.; Wang, H.; Lin, L.; Ding, S. Gonadal structure and expression localization of sex-related genes in the hermaphroditic grouper Epinephelus akaara (Perciformes: Epinephelidae). Aquaculture 2021, 542, 736902. [Google Scholar] [CrossRef]
- Gill, A.; Jamnongjit, M.; Hammes, S.R. Androgens promote maturation and signaling in mouse oocytes independent of transcription: A release of inhibition model for mammalian oocyte meiosis. Mol. Endocrinol. 2004, 18, 97–104. [Google Scholar] [CrossRef]
- Alward, B.A.; Laud, V.A.; Skalnik, C.J.; York, R.A.; Juntti, S.A.; Fernald, R.D. Modular genetic control of social status in a cichlid fish. Proc. Natl. Acad. Sci. USA 2020, 117, 28167–28174. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.R.; Liley, N.R. Androgen control of social status in males of a wild population of stoplight parrotfish, Sparisoma viride (Scaridae). Horm. Behav. 1991, 25, 1–18. [Google Scholar] [CrossRef]
- Lalli, E.; Melner, M.H.; Stocco, D.M.; Sassone-Corsi, P. DAX-1 blocks steroid production at multiple levels. Endocrinology 1998, 139, 4237–4243. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jeffs, B.; Ito, M.; Achermann, J.C.; Richard, N.Y.; Hales, D.B.; Jameson, J.L. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc. Natl. Acad. Sci. USA 2001, 98, 7988–7993. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Maltese, W.A. Death pathways triggered by activated Ras in cancer cells. Front. Biosci. A J. Virtual Libr. 2011, 16, 1693–1713. [Google Scholar] [CrossRef]
- Fan, H.Y.; Shimada, M.; Liu, Z.; Cahill, N.; Noma, N.; Wu, Y.; Gossen, J.; Richards, J.S. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 2008, 135, 2127–2137. [Google Scholar] [CrossRef]
- Kougioumtzi, A.; Tsaparas, P.; Magklara, A. Deep sequencing reveals new aspects of progesterone receptor signaling in breast cancer cells. PLoS ONE 2014, 9, e98404. [Google Scholar] [CrossRef]
- Joo, S.; Oh, S.H.; Sittadjody, S.; Opara, E.C.; Jackson, J.D.; Lee, S.J.; Yoo, J.J.; Atala, A. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed. Mater. 2016, 11, 065009. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.N.; Drummond-Barbosa, D. Maintenance of proper germline stem cell number requires adipocyte collagen in adult Drosophila females. Genetics 2018, 209, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, N. Sox6, jack of all trades: A versatile regulatory protein in vertebrate development. Dev. Dyn. 2011, 240, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Large-scale temporal gene expression profiling during gonadal differentiation and early gametogenesis in rainbow trout. Biol. Reprod. 2005, 73, 959–966. [Google Scholar] [CrossRef]
- Vizziano, D.; Randuineau, G.; Baron, D.; Cauty, C.; Guiguen, Y. Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Dev. Dyn. 2007, 236, 2198–2206. [Google Scholar] [CrossRef]
- Charlier, C.; Montfort, J.; Chabrol, O.; Brisard, D.; Nguyen, T.; Le Cam, A.; Richard-Parpaillon, L.; Moreews, F.; Pontarotti, P.; Uzbekova, S.; et al. Oocyte-somatic cells interactions, lessons from evolution. BMC Genom. 2012, 13, 560. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, H.; Zhou, W.; Zhang, Z.; Yang, Z.; Lu, Y.; Lu, B.; Wang, X.; Ding, Q.; Hu, R. Molecular cloning of a novel nuclear factor, TDRP1, in spermatogenic cells of testis and its relationship with spermatogenesis. Biochem. Biophys. Res. Commun. 2010, 394, 29–35. [Google Scholar] [CrossRef]
- Rolland, J.; Silvestro, D.; Litsios, G.; Faye, L.; Salamin, N. Clownfishes evolution below and above the species level. Proc. R. Soc. B 2018, 285, 20171796. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.G.; Armbruster, P.A. Comparative performance of transcriptome assembly methods for non-model organisms. BMC Genom. 2016, 17, 523. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression of RNA-Seq data at the gene level-the DESeq package. Heidelb. Ger. Eur. Mol. Biol. Lab. 2012, 10, f1000research. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Samples | Raw Reads (×106) | Clean Reads (×106) | Q20 (%) | Q30 (%) | GC (%) | Total Map (%) | Exon (%) | Intron (%) | Intergenic (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ac_NG_1 | 68.15 | 66.85 | 98.44 | 95.48 | 50.01 | 70.77 | 73.49 | 4.71 | 21.81 |
| Ac_NG_2 | 69.56 | 68.47 | 98.32 | 95.16 | 50.09 | 70.67 | 72.42 | 4.73 | 22.86 |
| Ac_NG_3 | 61.47 | 60.27 | 98.16 | 94.83 | 49.92 | 69.31 | 71.76 | 4.97 | 23.27 |
| Ac_MG_1 | 55.75 | 54.81 | 98.28 | 95.03 | 50.51 | 71.00 | 72.86 | 4.83 | 22.31 |
| Ac_MG_2 | 59.84 | 58.46 | 98.14 | 94.75 | 49.94 | 71.26 | 75.04 | 4.41 | 20.56 |
| Ac_MG_3 | 60.32 | 59.24 | 98.24 | 94.99 | 49.90 | 71.88 | 75.62 | 4.36 | 20.02 |
| Ac_FG_1 | 61.39 | 59.89 | 98.32 | 95.16 | 49.62 | 74.39 | 79.70 | 3.97 | 16.33 |
| Ac_FG_2 | 53.66 | 52.60 | 98.14 | 94.84 | 49.89 | 70.00 | 72.11 | 4.65 | 23.25 |
| Ac_FG_3 | 51.66 | 50.70 | 98.26 | 95.00 | 49.11 | 72.57 | 78.28 | 4.25 | 17.47 |
| Total | 541.80 | 531.29 | / | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Qu, M.; Tang, W.; Liu, S.; Ding, S. Transcriptome Profiling and Expression Localization of Key Sex-Related Genes in a Socially-Controlled Hermaphroditic Clownfish, Amphiprion clarkii. Int. J. Mol. Sci. 2022, 23, 9085. https://doi.org/10.3390/ijms23169085
Wang H, Qu M, Tang W, Liu S, Ding S. Transcriptome Profiling and Expression Localization of Key Sex-Related Genes in a Socially-Controlled Hermaphroditic Clownfish, Amphiprion clarkii. International Journal of Molecular Sciences. 2022; 23(16):9085. https://doi.org/10.3390/ijms23169085
Chicago/Turabian StyleWang, Huan, Meng Qu, Wei Tang, Shufang Liu, and Shaoxiong Ding. 2022. "Transcriptome Profiling and Expression Localization of Key Sex-Related Genes in a Socially-Controlled Hermaphroditic Clownfish, Amphiprion clarkii" International Journal of Molecular Sciences 23, no. 16: 9085. https://doi.org/10.3390/ijms23169085
APA StyleWang, H., Qu, M., Tang, W., Liu, S., & Ding, S. (2022). Transcriptome Profiling and Expression Localization of Key Sex-Related Genes in a Socially-Controlled Hermaphroditic Clownfish, Amphiprion clarkii. International Journal of Molecular Sciences, 23(16), 9085. https://doi.org/10.3390/ijms23169085





